SUMMARY
Type 1 interferon (IFN) inhibits the release of HIV-1 virus particles via poorly defined mechanisms. Here, we show that IFNα induces retention of viral particles on the surface of fibroblasts, T cells, or primary lymphocytes infected with HIV-1 lacking the Vpu protein. Retained particles are tethered to cell surfaces, can be endocytosed, appear fully assembled, exhibit mature morphology, and can be detached by protease. Strikingly, expression of the HIV-1 Vpu protein attenuates the ability of human cells to adhere to, and thereby retain, nascent HIV-1 particles upon IFNα treatment. Vpu also counteracts the IFNα-induced retention of virus-like particles assembled from the Ebola virus matrix protein. Furthermore, levels of IFNα that suppress replication of Vpu-defective HIV-1 have little effect on wild-type HIV-1. Thus, we propose that HIV-1 expresses Vpu to counteract an IFNα-induced, general host defense that inhibits dissemination of enveloped virions from the surface of infected cells.
INTRODUCTION
Type 1 interferons (IFNs) are a major component of the innate immune response that limits systemic dissemination of viral infections and drives adaptive responses required for viral clearance and protection (Stetson and Medzhitov, 2006). The expression and release of IFNs from virus-infected cells is triggered by recognition of extra- and intracellular molecular patterns such as double-stranded RNA and aberrant glycosylation resulting from viral replication. The IFNα family (released mainly by lymphoid dendritic cells) and IFNβ (released from most somatic cells) bind to IFNα/β receptors and induce the expression of IFN-regulated genes via STAT-dependent signaling (van Boxel-Dezaire et al., 2006). In addition to enhancing the presentation and recognition of viral antigens (Stetson and Medzhitov, 2006), some type 1 IFN-induced genes directly inhibit viral replication (Samuel, 2001). For example, the GTPases MxA and MxB block replication of several negative-strand RNA viruses, possibly by effecting sub-cellular trafficking of viral components (Haller and Kochs, 2002), while 2′5′oligoadenylate synthase and RNaseL target viral RNAs for destruction (Samuel, 2001). While the mechanisms of action of many IFN-induced antiviral proteins are unknown, the broad spectrum of some activities should make them difficult for viruses to avoid via simple sequence variation. This provides an evolutionary impetus for viruses to acquire completely new functions that neutralize IFN-induced cellular defenses.
Retrovirus restriction factors, such as APOBEC3G and TRIM5α (Bieniasz, 2004; Goff, 2004), that target HIV-1 can be IFN-regulated genes (Asaoka et al., 2005; Peng et al., 2006; Tanaka et al., 2006), suggesting that these mediators of intrinsic immunity to retroviruses are specialized extensions of the wider innate antiviral defense. Moreover, some clinical observations (Levy et al., 2003; Siegal et al., 2001) suggest that IFNα may inhibit HIV-1 replication in vivo. While IFNα can suppress HIV-1 replication in cultured T cells and macrophages (Poli et al., 1989), the precise mechanism by which replication is inhibited is not clear. Although some reports suggest IFNα-induced blocks in HIV-1 reverse transcription and gene expression (Shirazi and Pitha, 1992, 1993), the most commonly observed effect of IFNα on HIV-1 replication is inhibition of the release of progeny virions from infected cells (Gottlinger et al., 1991; Kornbluth et al., 1989; Poli et al., 1989). γ-retrovirus and β-retrovirus release can also be inhibited by IFNα (Bilello et al., 1982; Chatterjee and Hunter, 1987). Microscopic studies of IFNα-treated infected T cells, macrophages, and cell lines indicate that HIV-1 particles can accumulate prominently at the plasma membrane and in undefined intracellular vacuoles (Biswas et al., 1992; Kornbluth et al., 1989; Smith et al., 1991).
Viral protein U (Vpu), a small integral membrane protein encoded by HIV-1 and its closest relatives (Hout et al., 2004), can facilitate HIV-1 particle release from some cells, and its absence can result in HIV-1 particle accumulation within and on infected cells (Gottlinger et al., 1993; Klimkait et al., 1990). Importantly, Vpu appears to overcome a host activity that blocks retrovirus particle release in some restrictive human cell types (Varthakavi et al.,2003), Recently, we showed that Vpu-defective HIV-1 particles accumulated prominently within late endosomes of restrictive cells and, particularly when their internalization was prevented, on cell surfaces (Neil et al., 2006). Kinetic and other analyses of HIV-1 Gag accumulation in nonpermissive cells suggest that, in the absence of Vpu, HIV-1 Gag initially targets the plasma membrane, assembles, and then is reinternalized as fully assembled virions (Harila et al., 2006; Neil et al., 2006). Moreover, HIV-1 particles that accumulate on restrictive cell surfaces were fully mature and could be removed by protease treatment, suggesting that a host protein tethers nascent HIV-1 virions to the surface of restrictive cells.
Given the superficial morphological similarities associated with the blocks to HIV-1 particle release observed in the absence of Vpu or upon treatment of infected cells with IFNα, we explored whether these blocks were directly related. Significantly, IFNα treatment of fibroblast and T cell lines, in which Vpu is not ordinarily required for efficient HIV-1 release, induced the retention of particles via a protease-sensitive tether, as well as their accumulation of in late endosomes. Notably, these effects were much more pronounced, and sometimes only evident, when Vpu was absent. Furthermore, Vpu deletion caused HIV-1 replication in T cells to become more sensitive to inhibition by IFNα. In addition, IFNα blocked the release of enveloped particles assembled using an unrelated viral structural protein. Thus, these studies suggest that HIV-1 acquired the Vpu protein at least in part to overcome an IFN-induced host defense mechanism that tethers nascent virions to infected cells and prevents their dissemination.
RESULTS
Type 1 IFN Induces a Block in Vpu-Defective HIV-1 Release
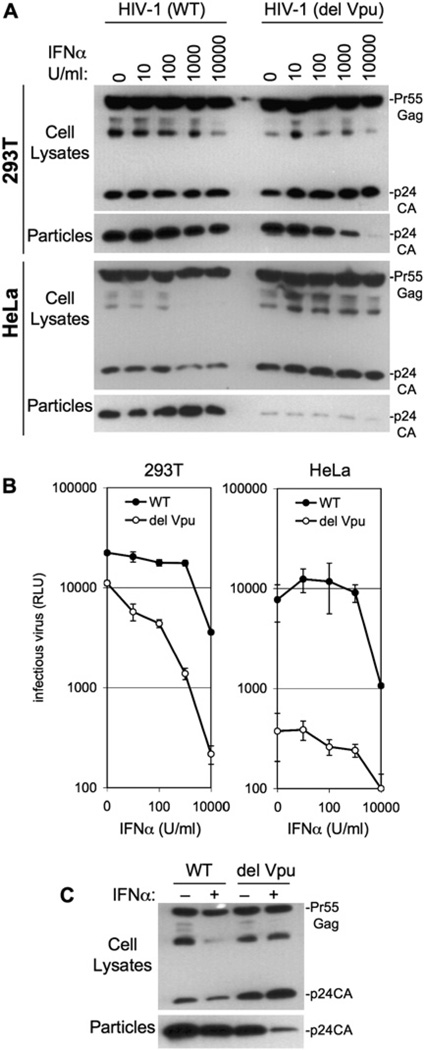
To test the notion that IFNα-induced and Vpu mutation-induced blocks in HIV-1 release were related, we compared the properties of an HIV-1 strain, NL4-3 [HIV-1(WT)], with a Vpu-deleted counterpart [HIV-1(del Vpu)]. 293T and HeLa cells were infected with mixed vesicular stomatitis virus glycoprotein (VSV-G) pseudotypes of each virus, and, thereafter, HIV-1 protein expression and particle yield were determined using western blot and infectivity assays (Figure 1). Since neither cell type expresses CD4, this procedure resulted in a single cycle of HIV-1 replication. As expected, and despite equivalent levels of Gag expression, the release of HIV-1(del Vpu) particles from HeLa cells was severely reduced as compared to HIV-1(WT) particles, and increased levels of processed p24 protein remained associated with the cells (Figure 1A). In contrast, approximately equivalent levels of HIV-1(WT) and HIV-1 (del Vpu) particles were released by infected 293T cells. However, while the release of HIV-1(del Vpu) particles from 293T cells was increasingly impaired upon treatment with increasing doses of IFNα, the release of HIV-1(WT) was only marginally affected by IFNα treatment. Indeed, IFNα-treated 293T cells exhibited a phenotype that resembled that of untreated HeLa cells, where HIV-1 release was highly dependent on Vpu. Notably, inefficient HIV-1 (del Vpu) release from HeLa cells was not further inhibited by IFNα. Measurements of infectious particle release by 293T and HeLa cells were largely concordant with these results. Specifically, 10–1000 U/ml IFNα induced an ∼10-fold reduction in the yield of infectious HIV-1(del Vpu) particles, specifically from 293T cells, while infectious HIV-1(WT) particle yield was unaffected at the same doses of IFNα. At very high concentrations of IFNα (10,000 U/ml), the yield of infectious virions was reduced in both cell lines, irrespective of Vpu expression (Figure 1B). To demonstrate formally that Vpu was responsible for the IFNα resistance of HIV-1(WT), 293T cells were transfected with HIV-1(WT) and HIV-1(del Vpu) proviral plasmids, and a plasmid that would express Vpu in trans. Again, the generation of infectious HIV-1(del Vpu) particles was impaired by IFNa, with little effect on HIV-1(WT). However, expression of Vpu in trans caused HIV-1(del Vpu) to be resistant to the inhibitory effect of IFNα (Figure S1 in the Supplemental Data available with this article online).
Figure 1. IFNα Specifically Blocks the Release of Vpu-Defective HIV-1 Particles.
(A) 293T or HeLa cells were infected with VSV-G-pseudotyped HIV-1(WT) or HIV-1(del Vpu) at an moi of 0.2 and then treated with the indicated dose of IFNα. Extracellular particles and cell lysates were harvested and analyzed by western blotting using an anti-p24CA monoclonal antibody.
(B) Same as (A) except that infectious virions in culture supernatants were measured using HeLa-TZM indicator cells and a chemiluminescent assay and expressed in relative light units (RLU). Error bars indicate the standard deviations of the mean.
(C) Jurkat T cells were infected as in (A) with VSV-G-pseudotyped, envelope-defective mutants of HIV-1(WT) or HIV-1(del Vpu) and were left untreated (−) or were treated with 1000 U/ml IFNα (+).
To determine whether a similar effect could be observed in cells that more closely resemble natural targets of HIV-1 infection, CD4+ Jurkat T cells were infected with VSV-G-pseudotyped HIV-1(WT) and HIV-1(del Vpu). In this case, envelope-defective HIV-1 constructs were used to prevent multiple rounds of replication, or cell fusion. A marginal effect of Vpu on the release of HIV-1 particles from untreated Jurkat cells was observed (Figure 1C). However, IFNα treatment selectively decreased the release of HIV-1(del Vpu) particles and also increased in the level of cell-associated processed p24. Taken together, these results demonstrate that the requirement for Vpu can be induced by IFNα treatment of cells that normally do not restrict Vpu-defective HIV-1 particle release, leading to a phenotype that resembles that observed in restrictive cells (e.g., HeLa), where the requirement for Vpu is constitutive.
Vpu Suppression of the IFNα-Induced Block in HIV-1 Release Is Cell Type Specific
Another cell line in which Vpu was shown previously not to be required for efficient HIV-1 particle release is the African green monkey (AGM) cell line COS-7. IFNα again dramatically inhibited the release of HIV-1 particles from COS-7 cells without affecting viral Gag protein expression, or processing (Figure 2A). However, in this case both HIV-1(del Vpu) and HIV-1(WT) were similarly affected, indicating that Vpu was unable to counteract the IFNα-induced block in this cell type. Nonetheless, in the same experiment, Vpu clearly reversed an IFN-induced block to HIV-1 released in 293T cells (Figure 2A). Measurements of infectious HIV-1(WT) and HIV-1(del Vpu) virion release gave essentially the same result, in that infectious particle production from COS-7 cells was inhibited up to 100-fold by IFNα, irrespective of the presence or absence of Vpu, in contrast to 293T cells (Figure 2B). Thus, the ability of Vpu to counteract the IFNα-induced inhibition of HIV-1 release was cell type specific, and Vpu failed to function in at least one cell line from a nonhuman species.
Figure 2. HIV-1 Vpu Antagonizes IFNα-Induced Inhibition of HIV-1 Release in Human 293T Cells but Not in African Green Monkey COS Cells.
(A) 293T cells (left panels) or COS-7 cells (right panels) were infected with VSV-G-pseudotyped HIV-1(WT) or HIV-1(del Vpu) at an moi of 0.2 and then treated with the indicated dose of IFNα. Extracellular particles and cell lysates were analyzed by western blotting using an anti-p24CA monoclonal antibody.
(B) Same as (A), except that infectious virions in culture supernatants were measured using HeLa-TZM indicator cells, as in Figure 1. Error bars indicate the standard deviations of the mean.
IFNα Induces Accumulation of HIV-1 Particles on Cell Surfaces and in Endosomes
Previously, we showed that HIV-1(del Vpu) particles accumulate in HeLa cell endosomes, as a result of endocytosis of fully assembled particles that were retained on the plasma membrane. To determine whether IFNα treatment of 293T cells induced a similar outcome, we infected 293T cells (Figure 3A) and HeLa cells (Figure 3B) with fluorescent derivatives of HIV-1(WT) and HIV-1(del Vpu) that carry a fluorescent protein (YFP) inserted into the stalk region of the matrix domain of Gag (MA-YFP) (Jouvenet et al., 2006). The proportion of cells in which visible internal accumulations of MA-YFP could be seen (examples are shown in Figures 3A and 3B) was then determined (Figures 3C). In both 293T and HeLa cells, MA-YFP was observed as a diffuse cytoplasmic fluorescent signal, with accumulations of greater fluorescent intensity at the plasma membrane in all infected cells. In addition, intense intracellular accumulations of MA-YFP were observed, but this was a rare finding in 293T cells and occurred in <10% of cells infected with HIV-1/MA-YFP(WT) (Figures 3A and 3C). Notably, IFNα did not change the distribution of the HIV-1/MA-YFP(WT) signal. Moreover, 293T cells infected with HIV-1/MA-YFP (del Vpu) exhibited a similar pattern of YFP fluorescence; only a small minority (<10%) of cells exhibited intracellular accumulations (Figures 3A and 3C). In marked contrast, the number of HIV-1/MA-YFP(del Vpu) infected cells that displayed intense intracellular accumulations was increased by IFNα treatment, to ∼40% of all infected cells (Figures 3A and 3C). As expected from our previous studies (Neil et al., 2006), intracellular accumulations of MA-YFP were observed in only a small fraction of HeLa cells infected with HIV-1/MA-YFP(WT), while prominent intracellular accumulations were observed in ∼60% of HeLa cells infected with HIV-1/MA-YFP(del Vpu) (Figures 3B and 3C). Notably, IFNα had no additional effect on intracellular MA-YFP accumulation in HeLa cells (Figure 3C).
Figure 3. Nascent Vpu-Defective HIV-1 Particles Accumulate in Endosomes of IFNα-Treated 293T Cells.
(A) 293T cells were infected with VSV-G-pseudotyped HIV-1/MA-YFP(WT) or HIV-1/MA-YFP(del Vpu) encoding a modified matrix protein with YFP inserted into the stalk region. Cells were treated with 1000 U/ml IFNα where indicated and examined by deconvolution microcopy. Two examples are shown for each condition.
(B) A single representative example of HeLa cells infected with VSV-G-pseudotyped HIV-1/MA-YFP(WT) or HIV-1/MA-YFP(del Vpu), as indicated.
(C) The proportion of cells with intense MA-YFP accumulation at the plasma membrane (PM) only, or both at internal sites and plasma membrane (Internal + PM), was quantified for 293T cells or HeLa cells, as indicated following infection with HIV-1/MA-YFP(WT) or HIV-1/MA-YFP(del Vpu). Cells were otherwise untreated, or were treated with 1000 U/ml IFNα. For each data point, between 60 and 100 individual cells were evaluated.
(D and E) HIV-1/MA-YFP(del Vpu)-infected, IFNα-treated (1000 U/ml) 293T cells were immunostained using antibodies against human CD63 (D) or EEA-1 (E). Arrows indicate occasional MA-YFP puncta apparently colocalizing with EEA-1+ early endosomes. Scale bars represent 10 µm, except in the lower panels of (E), where they indicate 1 µm.
To determine the nature of IFN-induced intracellular accumulations of MA-YFP, 293T cells infected with HIV-1/ MA-YFP(del Vpu) were stained with antibodies to the early endosome marker EEA-1 or the late endosomal marker CD63. As can be seen in Figures 3D and 3E, there was prominent colocalization of MA-YFP with CD63+ late endosomes and occasional colocalization of MA-YFP with EEA1+ early endosomes in IFNα-treated 293T cells. This IFNα-induced pattern of HIV-1 Gag localization is remarkably similar to that previously observed in HeLa cells, where Vpu-defective HIV-1 particles are constitutively retained on the cell surface and then endocytosed (Neil et al., 2006). Thus, this finding suggested that the IFNα-induced block results in particle retention, internalization, and accumulation in endosomes.
Electron microscopic imaging was used to characterize the nature of the IFNα-induced virus release defect in greater detail. These analyses were performed using COS-7 cells transfected with the HIV-1(WT) or HIV-1(del Vpu) proviral constructs and then treated with IFNα (Figure 4)because the flat shape and large cytoplasmic volume of these cells was optimal for visualizing both internal and cell surface-associated virions. As was the case with infected COS-7 cells (Figure 2), both HIV-1(WT) and HIV-1 (del Vpu) were efficiently released by transfected but otherwise untreated COS-7 cells. However, particle release was strongly inhibited by IFNα treatment,as measured by either western blot or infectivity assays (Figure S2).
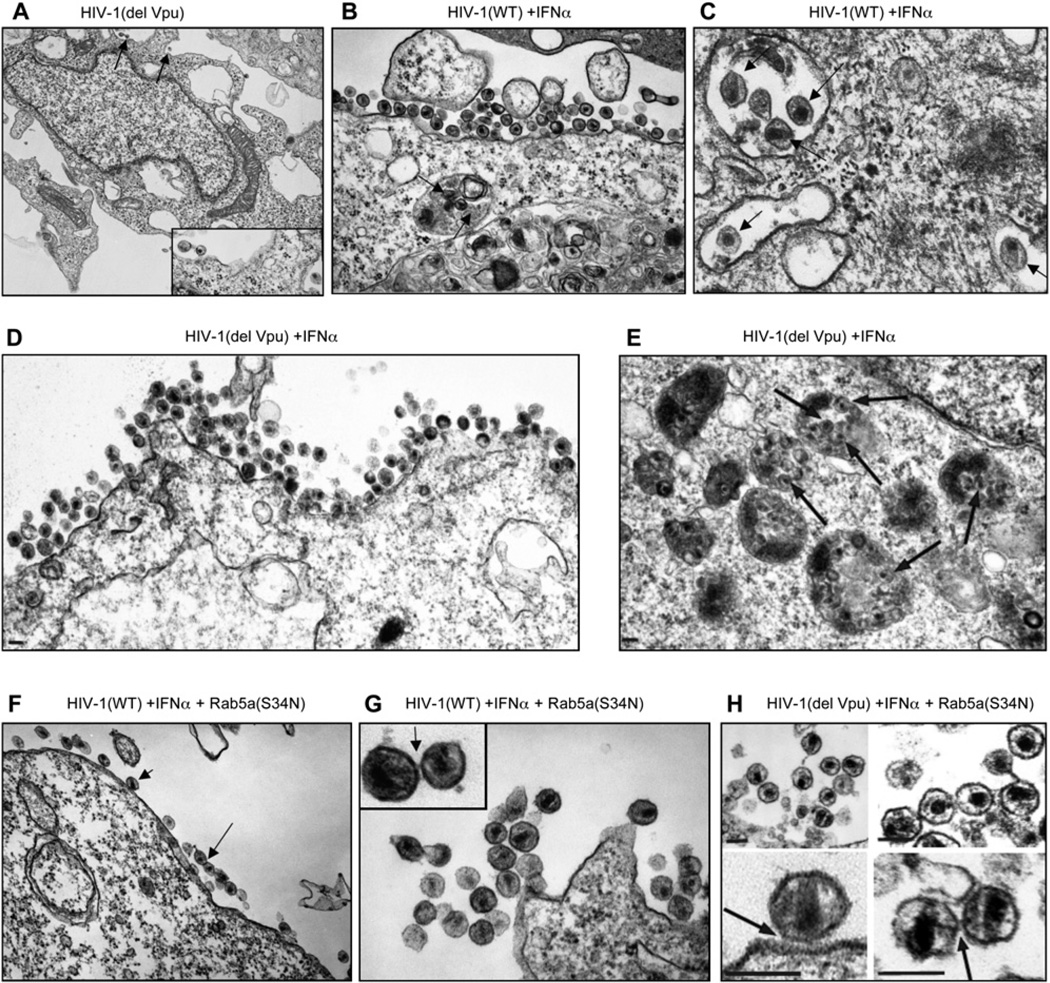
Figure 4. Thin-Sectioned Electron Microscopic Images of Cells and Virions under Conditions Where Release Was Inhibited by IFNα.
(A) Overview of a COS cell transfected with the HIV-1(del Vpu) proviral plasmid, but otherwise untreated. Arrows indicate examples of cell-associated virions (expanded view is shown in inset).
(B) Accumulations of mature HIV-1(WT) virions apparently adhered to the surface of COS-7 cells upon treatment with 500 U/ml IFNα. Note that analogous clusters were not seen in the absence of IFNα treatment. Arrows indicate virions within the lumen of endosomal compartments.
(C) Expanded image of HIV-1(WT) particles within the endosomal compartments of an IFNα-treated cell. Individual mature viral particles are highlighted.
(D) Image showing surface accumulations of mature HIV-1(del Vpu) virions apparently adhered to the surface of COS-7 cells treated with 500 U/ml IFNα.
(E) Image showing HIV-1(del Vpu) particles within the endosomal compartments of an IFNα-treated cell. Individual mature viral particles are highlighted with arrows.
(F) Surface accumulations of HIV-1(WT) mature virions adhered to the cell surface or to each other on an IFNα-treated cell. Note that the accumulation of virus within endosomal compartments was not observed in cells expressing the endocytosis inhibitor Rab5a(S34N).
(G and H) Expanded images of surface-associated HIV-1(WT) virions (G) or HIV-1(del Vpu) virions (H) in cells treated with IFNα and expressing Rab5a(S34N). Arrows highlight sites where virions appear to be adhered to the cell surface or to one another via external ‘‘tethers.’’ Scale bars in all panels represent 100 nm.
Thin-section EM images of virus-producing, but otherwise untreated, COS-7 cells revealed only rare examples of cell-associated virions, whether or not Vpu was expressed (Figure 4A). In marked contrast, IFNα induced the striking accumulation of large numbers of mature particles associated with the cell surface and intracellular compartments (Figures 4B–4E). Although single particles or small clusters of particles were occasionally observed in association with the cell surface in the absence of IFNα treatment, such large-scale surface clustering of virions was never seen in untreated control cells (Figure 4A). Indeed, we estimated that there was an approximately 100-fold greater density of mature particles on the surface of IFNα-treated cells compared to control cells (Figures 4A, 4B, and 4D). We therefore conclude that HIV-1 virions bud and mature normally in the presence of IFNα, but then remain aberrantly tethered to the outer cell surface.
IFNα treatment also caused the prominent accumulation of mature virions in internal cellular compartments (Figures 4C and 4E), which are likely endosomes, based upon the fluorescence colocalization experiments described above. Consistent with this assignment, the virion-containing compartments frequently (but not always) contained small vesicles and other membranous structures and therefore resembled late endosomal multi-vesicular bodies (Figure 4E). Significantly, the accumulation of virion-containing endosomes was dependent on IFNα treatment, as intracellular virions were rarely observed in control producer cells that were not IFNα treated (Figure 4A). Presumably because COS-7 cells are not responsive to Vpu, these observations were essentially indistinguishable when HIV-1(WT) (Figures 4B and 4C) or HIV-1(del Vpu) (Figures 4D and 4E) was used.
The accumulation of virion-containing endosomes required active endocytosis, because internal virions were absent from IFNα-treated cells in which HIV-1 WT or HIV-1(del Vpu) was coexpressed with Rab5a(S34N), whose expression inhibits uptake of endocytic cargo (Bucci et al., 1992; Li and Stahl, 1993; Stenmark et al., 1994). Importantly, Rab5a expression in itself did not affect virion release (Figure S2) or the appearance of rare virions on cells that were not treated with IFNα (data not shown). The phenotypic difference induced by Rab5a (S34N) expression in IFNα-treated cells was highly significant, as virion-containing endosomes were observed in most (>75%) of the IFNα-treated cells in which endocytosis was not blocked and where internal endosomes/ vacuolar structures were visible. In contrast, endosomal virions were never observed when endocytosis was blocked by Rab5a(S34N) expression. Nonetheless, the abundant appearance of mature virions clustered on the surface of IFNα-treated cells was maintained whether or not Rab5a(S34N) was coexpressed (Figures 4F–4H).
Expanded EM images revealed that cell-associated virions remained closely juxtaposed to the cell surface and to one another (Figures 4G and 4H). These ‘‘tethered’’ virions were fully mature and had complete, intact outer membranes, implying that tethering interactions were mediated by molecules on the cell/virion exterior. Indeed, such external linkages sometimes appeared visible in the thin-sectioned EM images of tethered particles (Figures 4G and 4H, arrows). Thus, the block to virus release observed upon IFNα treatment appears to be mediated by a cell surface molecule(s) that causes mature HIV-1 virions to adhere to the cell surface and to one another.
IFNα Induces Protease-Sensitive Tethering of Nascent HIV-1 Particles
In HeLa cells, where the requirement for Vpu in HIV-1 release is constitutive, Vpu-defective viruses remain adhered to infected cell surfaces in a form that is morphologically similar to that shown in Figure 4, where particle release was inhibited by IFNα. Particles that are constitutively retained by HeLa cells due to the absence of Vpu can be liberated by the protease subtilisin, particularly when their endocytosis is blocked (Neil et al., 2006). Protease-induced release of mature particles distinguished Vpu-defective HIV-1 from late budding domain mutants that remain cell associated, even upon protease treatment, because in that case, the cell and virion membranes are continuous (Neil et al., 2006). To determine whether IFNα treatment led to protein-mediated tethering of mature particles to infected cells, 293T cells, Jurkat cells, or primary peripheral blood mononuclear cells (PBMCs) were infected with HIV-1(WT) or HIV-1(del Vpu) and cultured with or without IFNα. Envelope-defective, VSV-G-pseudotyped, viral constructs were used for these experiments to remove any confounding effects of gp120:CD4 interactions that might be affected by Vpu, and to restrict the assay to a single round of virus replication. Constitutively released particles were harvested from the culture supernatant, as were particles liberated from cells either by buffer washing or by protease ‘‘stripping.’’ In 293T cells infected with HIV-1(WT) (Figure 5A), most of the HIV-1 particles that could be recovered were constitutively released, and only a small proportion could be recovered from cell surfaces by protease stripping. This was also true when HIV-1(WT)-infected 293T cells were treated with IFNα. When 293T cells were infected with HIV-1(del Vpu), most particles were, again, constitutively released. However, in contrast to HIV-1(WT), when HIV-1(del Vpu)-infected 293T cells were treated with IFNα, a relatively minor fraction of particles were constitutively released, and instead, most particles were recovered from cell surfaces by protease stripping (Figure 5A).
Figure 5. Protease-Sensitive Tethers Retain Nascent Particles on the Surfaces of IFNα-Treated Cells.
293T cells (A), Jurkat cells (B),orprimary PBMCs (C) were infected with VSV-G-pseudotyped, envelope-defective HIV-1(WT) or HIV-1(del Vpu) and cultured in the presence or absence of 1000 U/ml IFNα. Cell lysates and extracellular particles that were either constitutively released into the culture supernatant, or released following buffer or subtilis in stripping, were pelleted and analyzed by western blotting.
Analogous experiments, done using Jurkat T cells and PBMCs, yielded similar, but even more striking results (Figures 5B and 5C). In Jurkat cells infected with HIV-1 (WT), a minor fraction of particles were retained on cell surfaces in a form that could be liberated by protease treatment, and this fraction was slightly increased by IFNα treatment (Figure 5B). In HIV-1(del Vpu)-infected Jurkat cells, the majority of particles were retained on cell surfaces, but some particles were constitutively released. Notably, HIV-1(del Vpu) particle release was dramatically inhibited by IFNα. Indeed, almost no particles were released by IFNα-treated, HIV-1(del Vpu)-infected cells, and instead virtually all of the extracellular particles that could be recovered required protease stripping from the cell surface (Figure 5B). In PBMCs, the effects of IFNα and Vpu deletion were less dramatic than in Jurkat cells, perhaps because PBMCs contain a mixture of cell types. Nevertheless, IFNα had no detectable effect on HIV-1(WT) particle release but clearly inhibited constitutive HIV-1(del Vpu) release. Moreover, IFNα increased the proportion of HIV-1(del Vpu) particles that were recovered only after protease stripping (Figure 5C). Notably, in both Jurkat cells, and particularly PBMCs, protease stripping removed most,andsometimes all,of the detectablemature p24 capsid protein from cells, while the level of cell-associated Gag precursor was unaffected (Figures 5B and 5C). This suggests that the majority of the cell-associated mature p24 protein was in the form of particles attached to the surface by a protease-sensitive tether. Notably, both Vpu deletion and IFNα treatment increased the amount of fully processed p24, presumably representing mature virus, that was retained by cells, and could therefore only be recovered in particulate form from cells by protease stripping.
Overall, in cells that exhibit no constitutive requirement (293T) or a partial requirement (Jurkat cells and PBMCs) for Vpu in particle release, IFNα treatment selectively increased the retention of Vpu-defective, mature particles linked to the cell by a protease-sensitive tether. While the precise nature of the phenotype associated with 293T cells, Jurkat cells, and PBMCs varied slightly, in all cases Vpu clearly antagonized the ability of cells to retain particles in response to IFNα treatment.
HIV-1 Replication Is Hypersensitive to IFNα in the Absence of Vpu
Since IFNα selectively inhibited HIV-1(del Vpu) particle release from fibroblast and lymphocyte cells, we asked whether Vpu deletion sensitizes HIV-1 replication to inhibition by IFNα. In Jurkat T cells, treatment with 100 U/ml IFNα had only a minor effect on HIV-1(WT) replication, as assessed by accumulation of infectious virions in culture supernatants (Figure 6A). Moreover, HIV-1(del Vpu) replicated only slightly less efficiently than HIV-1(WT), peaking at about the same time after inoculation, but at a level that was a few-fold reduced compared to HIV-1(WT), in agreement with previous studies (Strebel et al., 1988). Strikingly, addition of 100 U/ml IFNα to infected Jurkat cells 1 day after inoculation severely inhibited subsequent HIV-1(del Vpu) replication, causing a 50- to 100-fold reduction in the level of HIV-1(del Vpu) accumulation at 7–9 days after inoculation when virus replication peaked in the untreated cultures (Figure 6A). Similar results were obtained using primary PBMCs from two donors as targets (Figure 6B). In both cases IFNα delayed the replication of HIV-1(WT), although similar yields of infectious virions were eventually obtained approximately 4–6 days later. HIV-1(del Vpu) replicated less efficiently than HIV-1(WT) in PBMCs, but replication nevertheless remained quite robust (Figure 6B). Importantly, however, addition of 100 U/ml IFNα almost completely blocked replication of HIV-1(del Vpu) in PBMCs from both donors.
Figure 6. Replication of Vpu-Defective HIV-1 in Human CD4+ T Cells Is Hypersensitive to IFNα.
CD4+ Jurkat T cells (A) or PBMCs from two donors (B) were infected with HIV-1(WT) or HIV-1(del Vpu) and treated with 100 U/ml IFNα 24 hr after infection. Supernatant sampleswere harvested at the indicated times thereafter, and infectious virus yield was measured using HeLa-TZM cells and expressed in relative light units (RLU).
Effect of IFNα and Vpu on the Release of a Nonretroviral Enveloped Virus-like Particle
Vpu is able to stimulate the release of retroviral particles that share minimal protein sequence homology (Gottlinger et al., 1993). This raises the possibility that IFNα-mediated tethering simply involves host molecule-mediated adhesion of virion and host cell membrane bilayers to each and does not require any retrovirus-specific molecules. Therefore, we next determined whether the release of an unrelated, nonretroviral virus-like particle could be inhibited by IFNα and stimulated by HIV-1 Vpu. We used the Ebola virus VP40 matrix protein because, like retroviral Gag proteins, the expression of this protein alone can generate extracellular particles that resemble authentic virions. However, other than short sequence motifs that are used to engage cellular machinery required for budding through the plasma membrane (Martin-Serrano et al., 2001), retroviral Gag and filovirus matrix proteins do not share any sequence or structural homology.
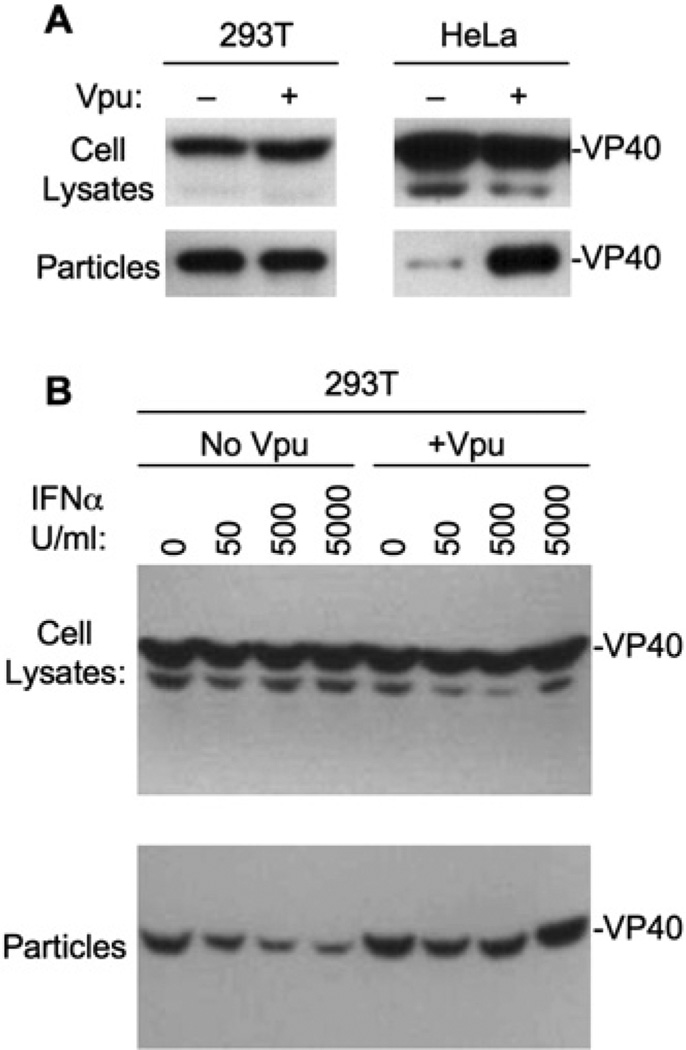
Ebola VP40 virus-like particle release from 293T cells was unaffected by the presence of coexpressed Vpu (Figure 7A). In contrast, Ebola VP40 particle release from HeLa cells was inefficient but was markedly enhanced by coexpression of HIV-1 Vpu in trans. Additionally, treatment of Ebola VP40-expressing 293T cells with IFNα caused a dose-dependent decrease in virus-like particle release without affecting VP40 expression (Figure 7B). Notably, the ability of IFNα to inhibit Ebola VP40 particle release was abolished when Vpu was expressed in trans. Thus, the ability of IFNα to inhibit enveloped particle release extends beyond retroviruses, as does the ability of Vpu to counteract IFNα-induced inhibition and stimulate particle release.
Figure 7. Vpu-Stimulated Ebola Virus-like Particle Release from HeLa Cells and IFNα-Treated 293T Cells.
(A) 293T cells (left panels) and HeLa cells (right panels) were trans-fected with a plasmid expressing HA-tagged Ebola virus VP40, and HIV-1 Vpu as indicated. Cell and virus-like particle lysates were analyzed by western blotting.
(B) Same as (A) except that 293T cells were transfected with a plasmid expressing HA-tagged Ebola virus VP40, in the presence or absence of coexpressed HIV-1 Vpu and IFNα treatment, as indicated.
DISCUSSION
While a large number of gene products, some of which are presumed antiviral factors, are known to be upregulated as part of the innate immune response to viral infection, little is known about how innate antiviral factors actually work. The data presented herein strongly suggest that a component of the type 1 interferon-induced response causes tethering of nascent HIV-1 particles to the surface of infected, virus-producing cells. In principle, this could be a very effective way to attenuate a viral infection in vivo and inhibit the dissemination of virus particles within a host. Importantly, the ability of IFNα to inhibit particle release was observed at levels of interferon that are encountered systemically in HIV-1-infected individuals (Christeff et al., 2002).
Previously, work on the HIV-1 Vpu protein has indicated that cells can be categorized into two basic types, namely, restrictive cells, such as HeLa, where Vpu is required for efficient HIV-1 release, or permissive cells, such as COS, HOS, or 293T, where HIV-1 release is not affected by Vpu (Neil et al., 2006; Varthakavi et al., 2003). Heterokaryon experiments suggested that the difference between restrictive and nonrestrictive cells is the presence or absence of a Vpu-targeted restriction factor (Varthakavi et al., 2003). The results presented herein suggest that the putative restriction factor(s) is a component of the type 1 interferon-induced innate immune response. Specifically, IFNα treatment of several nonrestrictive or weakly restrictive human cell types, including natural HIV-1 target cells, induced a particle retention phenotype that very closely resembles that observed in restrictive cells, i.e., a block to the production of new particles that could be overcome by the Vpu protein. This block was manifested as an accumulation of mature particles on cell surfaces, via a protease-sensitive tether, and in endosomes, presumably following endocytic uptake. Previous work has shown that other virion-cell interactions that can occur during virion production, i.e., those mediated by CD4 and gp120, can also induce particle retention. However, in that case the effect could be ameliorated by Nef- or Vpu-mediated CD4 downregulation (Lama et al., 1999; Ross et al., 1999). Importantly, the majority of experiments described herein were done in cells that lack CD4, or with HIV-1 clones that lack an envelope gene, excluding gp120:CD4 interactions as mediators of this effect. While the identity(ies) of molecule(s) that are responsible for virion retention are unknown, IFNα appears to endow cells and/or virions with an adhesive property that inhibits their dissociation from one another. As such, IFN-induced cell adhesion molecules, for example, would be reasonable candidates for factors that induce virion retention. The finding that virions, visualized by electron microscopy, appeared to be tethered to each other as well as to cell surfaces, also suggests that the molecule(s) involved in tethering are incorporated into virions.
Vpu can enhance the release of diverse retroviruses from restrictive cells (Gottlinger et al., 1993), implying that tethering-based restriction acts on retroviruses in general, and potentially, on other enveloped viruses that bud from the plasma membrane. Moreover, in HIV-2 and some SIV isolates that lack Vpu, an analogous function in virion release maps to the envelope gene (Abada et al., 2005), suggesting that antagonism of an IFN-induced block to virion release is a conserved feature of primate lentiviruses. Additionally, we found that Vpu can enhance the release of a very different type of virus-like particle, generated by Ebola VP40. This suggests that the tethering mechanism is broadly specific and perhaps is the result of promiscuous incorporation of adhesive molecules into the envelopes of virions as they bud through the plasma membrane.
A conceptually related type of inhibition of enveloped particle release occurs with influenza viruses, where cell surface sialic acid can be bound by virion hemagglutinin unless it is destroyed by the viral neuraminidase (Wagner et al., 2002). However, in that case, sialic acid is constitutively and universally expressed. Conversely, the apparent inhibitor of HIV-1 and Ebola virus-like particle release is IFNα induced in most cells. Moreover, the inhibitor(s) may be variable in sensitivity to Vpu, because activity could be counteracted by Vpu in several human cells, while Vpu was unable to counteract IFN-induced inhibition of HIV-1 release in AGM COS cells. It is intriguing that other antiretroviral molecules such as TRIM5α and APOBEC3G exhibit marked species-specific variation in sequence and properties as a result of apparently strong positive selection during primate evolution. In the latter case, the HIV-1 countermeasure (the Vif protein) is fully capable of counteracting the human variant of APOBEC3G but is inactive against most nonhuman primate variants. Our findings hint at the possibility that an analogous situation exists for Vpu and its target protein(s).
Some of the findings described herein differ, at least superficially, from earlier studies in which a number of investigators have reported significant effects of IFNα on the release and replication of what were presumed to be wild-type HIV-1 strains. For example, several investigators reported that IFNα inhibited the release of HIV-1 particles into supernatants of infected cultures (Gottlinger et al., 1991; Kornbluth et al., 1989; Poli et al., 1989) and induced accumulation of HIV-1 particles on the surfaces (and within) chronically infected T cells, macrophages, and macrophage-like cells (Biswas et al., 1992; Kornbluth et al., 1989; Smith et al., 1991). It is possible, however, that at least some of these earlier studies employed Vpu-defective viral strains, since several commonly used laboratory-adapted HIV-1 strains, particularly clones derived from the IIIB/LAV/Bru/Lai isolate used in several studies (Biswas et al., 1992; Gottlinger et al., 1993; Poli et al., 1989; Smith et al., 1991), harbor this lesion. Indeed, Vpu mutations appear positively selected under culture conditions that favor direct cell-to-cell transmission (Gummuluru et al., 2000), a finding that is entirely consistent with the notion that the absence of Vpu favors retention of completely formed, infectious virions on cell surfaces. It is also possible that high levels of IFNα may inhibit release, even in the presence of Vpu, perhaps by overwhelming the capacity of Vpu to counteract inhibitors. However, under normal conditions, efficient, Vpu-stimulated virion release is likely to be important for an effective spreading infection. The replication of an HIV-1 mutant lacking a functional Vpu gene was hypersensitive to inhibition by IFNα, suggesting that the phenomenon of IFNα-induced tethering is biologically relevant, and that at least one role of the HIV-1 Vpu protein is to mitigate the antiviral effects of type 1 IFN.
One recent report suggested a different mechanism for IFNα-induced inhibition of HIV-1 release, namely that the IFN-induced ubiquitin homolog ISG-15 blocks HIV-1 assembly by disrupting the interactions between Gag and Tsg101 (Okumura et al., 2006). Those results are difficult to reconcile with the IFN-induced, mature virion retention phenotype described herein. Specifically, disruption of Gag:Tsg101 interactions causes a phenotype in which immature virions containing partially processed Gag molecules remain tethered to the cell due to a failure of cellular and viral membrane scission (Demirov et al., 2002; Garrus et al., 2001; Gottlinger et al., 1991; Martin-Serrano et al., 2001). This ‘‘late budding’’ phenotype is very different from the IFNα block described here, where the viral and host cell membranes are discontinuous and the virions are mature.Moreover,particles arrestedat a latebudding stage cannot be released by protease treatment (Neil et al., 2006), while virions whose retention is induced by IFNα treatment can be liberated by protease. Finally, we found that IFNα morepotently affected HIV-1(delVpu) than HIV(WT) release, even though both viruses should be equally sensitive to disruption of the Tsg101:Gag interaction.
Cells employ a variety of IFN-induced gene products to retard replication of many viruses, but the mechanisms by which many of these factors exert their effects is unknown. Retention of nascent enveloped virions by adherence to the surface of cells via an active, IFN-induced, tethering mechanism that does not appear to require a specific virion component could, in principle, be a quite effective general way for the innate immune response to inhibit dissemination of a wide range of enveloped viruses.
EXPERIMENTAL PROCEDURES
Cells and Viruses
293T, HeLa, COS-7, HeLa-TZM, and Jurkat T cells were maintained using standard procedures. Human PBMCs were isolated from blood using Ficoll-paque gradient centrifugation, stimulated with phytohemagglutinin for 2 days, and then grown in the presence of IL-2 (20 U/ml, Roche) for a further 3 days before use.
HIV-1(WT) and HIV-1(del Vpu) versions of the HIV-1 molecular clone NL4-3 have been previously described (Neil et al., 2006). Envelopedefective derivatives, as well as derivatives carrying YFP in the stalk region of matrix (HIV-1/MA-YFP), were constructed using standard molecular biology techniques or have been described previously (Jouvenet et al., 2006). Virus stocks were generated by transient transfection of semiconfluent 10 cm plates of 293T cells with 8 µg of plasmid complexed with polyethylenimine. To generate VSV-G-pseudotyped or mixed-pseudotyped virus stocks, 1 µg of pCMV-VSV-G was included in the transfection. Virus-containing supernatants were harvested 48 hr posttransfection and filtered, and infectious titers were determined as β-galactosidase+ colony-forming units using HeLa-TZM indicator cells.
Virus Release Assays
For virus release assays involving adherent or suspension cells, 1 × 105 cells were plated per well of a 12-well plate. The next day the cells were infected with VSV-G-pseudotyped virus stocks at an moi of 0.2, and the inoculum was removed 6 hr later. Eighteen hours later, cells were treated with or without recombinant human IFNα (Sigma), and the cells were cultured for a further 24 hr. Supernatants were then harvested and filtered, and particles were pelleted through a 20% sucrose layer at 30,000 g. Particle pellets and cell lysates were analyzed by SDS-PAGE and western blot assays using α-p24 and α-mouse-peroxidase antibodies, as previously described. To measure infectious virus release from human cells, virus containing supernatants were serially diluted and used to inoculate HeLa-TZM indicator cells. After 48 hr, the HeLa-TZM cells were lysed, and β-galactosidase activity was determined using a chemiluminescent assay.
Alternatively, 293T or HeLa cells were transfected with HIV-1 proviral, Ebola VP40 (Martin-Serrano et al., 2004), and/or HIV-1 Vpu expression plasmids. IFNα was added to transfected cells at 24 hr after transfection. Cells and extracellular particles were harvested 24 hr after IFNα addition and analyzed as described above.
Virus Replication Assays
Jurkat cells or PBMCs (1 × 105) in 1 ml of medium were infected with HIV-1(WT) or HIV-1(del Vpu) at an moi of 0.005. At 24 hr postinfection, recombinant human IFNα (100 U/ml) was added to the cultures. Samples of supernatants were harvested at 48 hr intervals thereafter for the ensuing 13–15 days and stored at −80°C. Infectious virus titers in the collected supernatant samples were determined using HeLa-TZM indicator cells, as described above.
Fluorescent Microscopy
Cells were infected, as described above, with VSV-G-pseudotyped HIV-1/MA-YFP derivatives at an moi of 0.2 and either were left untreated or were treated with 1000 U/ml IFNα. Microscopy was done as described previously (Jouvenet et al., 2006; Neil et al., 2006). Mouse monoclonal EEA1 (BD Biosciences) and CD63 (Chemicon) were used to stain early and late endosomal compartments, respectively, followed by an α-mouse Alexa Fluor 595 conjugate (Invitrogen).
Electron Microscopy
COS-7 cells were transfected with HIV-1 plasmids (1.2 µg/well of a 6-well plate) and, in some cases, cotransfected with the expression construct for Rab5a(S34N) (0.3 µg/well) using FuGENE6 (Roche), according to the manufacturer’s instructions. Control experiments confirmed the efficacy of the Rab5a(S34N) in inhibiting endocytosis of the EGF receptor (data not shown) Fifteen hours posttransfection, the media were changed and supplemented with 500 or 2500 U/ml IFNα. Thirty-two hours later, the cell supernatants and cells were harvested for electron microscopy, western blot, and infectivity analyses, as described previously (von Schwedler et al., 2003). Cells were fixed with 2.5% glutaraldehyde/1% paraformaldehyde in cacodylate buffer (0.1 M sodium cacodylate [pH 7.4], 35 mM sucrose, 4 mM CaCl2). After staining in 2% OsO4 and in 4% uranyl acetate, the cells were dehydrated in a graded ethanol series (50%–100%) and embedded in epoxy resin Embed-812 (Electron Microscopy Sciences). Thin sections (60–90 nm) were stained with saturated uranyl acetate, rinsed with water, and then stained with Reynolds’ lead citrate. Electron micrographs were taken on a Hitachi H-7100 transmission electron microscope at an accelerating voltage of 75 kV. Note that only data from the 500 U/ml treatments are shown in Figure 4, but very similar phenotypes were observed upon treatment with 2500 U/ml.
Protease Stripping Experiments
Cells were infected with VSV-G-pseudotyped HIV-1(WT) or HIV-1(del Vpu) and treated with IFNα as described above. Constitutively released particles were harvested from culture supernatants, and cells in parallel wells were then either directly harvested or, alternatively, incubated for 15 min at 37°C in 100 µl of Tris/HCl (pH8.0), 150 mM NaCl, and 5 mM CaCl2 with or without the addition of subtilisin (1 mg/ml). The reaction was then stopped with 0.5 ml DMEM/FCS containing 5 mM PMSF. Cells were pelleted and the supernatants were filtered (0.2 µm) prior to harvesting released particles, by pelleting through sucrose, as described above.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Bieniasz laboratory for helpful discussions. This work was supported by grants from the NIH to P.D.B. (R01AI50111) and W.I.S. (R01 AI051174) and a grant from the American Foundation for AIDS research (to V.S.). P.D.B. is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation.
Footnotes
Supplemental Data
The Supplemental Data include two supplemental figures and can be found with this article online at http://www.cellhostandmicrobe.com/cgi/content/full/2/3/193/DC1/.
REFERENCES
- Abada P, Noble B, Cannon PM. Functional domains within the human immunodeficiency virus type 2 envelope protein required to enhance virus production. J. Virol. 2005;79:3627–3638. doi: 10.1128/JVI.79.6.3627-3638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaoka K, Ikeda K, Hishinuma T, Horie-Inoue K, Takeda S, Inoue S. A retrovirus restriction factor TRIM5alpha is transcriptionally regulated by interferons. Biochem. Biophys. Res. Commun. 2005;338:1950–1956. doi: 10.1016/j.bbrc.2005.10.173. [DOI] [PubMed] [Google Scholar]
- Bieniasz PD. Intrinsic immunity: A front-line defense against viral attack. Nat. Immunol. 2004;5:1109–1115. doi: 10.1038/ni1125. [DOI] [PubMed] [Google Scholar]
- Bilello JA, Wivel NA, Pitha PM. Effect of interferon on the replication of mink cell focus-inducing virus in murine cells: Synthesis, processing, assembly, and release of viral proteins. J. Virol. 1982;43:213–222. doi: 10.1128/jvi.43.1.213-222.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas P, Poli G, Kinter AL, Justement JS, Stanley SK, Maury WJ, Bressler P, Orenstein JM, Fauci AS. Interferon gamma induces the expression of human immunodeficiency virus in persistently infected promonocytic cells (U1) and redirects the production of virions to intracytoplasmic vacuoles in phorbol myristate acetate-differentiated U1 cells. J. Exp. Med. 1992;176:739–750. doi: 10.1084/jem.176.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Ho-flack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Hunter E. Recombinant human interferons inhibit replication of Mason-Pfizer monkey virus in primate cells. Virology. 1987;157:548–551. doi: 10.1016/0042-6822(87)90299-6. [DOI] [PubMed] [Google Scholar]
- Christeff N, Melchior JC, de Truchis P, Perronne C, Gou-geon ML. Increased serum interferon alpha in HIV-1 associated lipodystrophy syndrome. Eur. J. Clin. Invest. 2002;32:43–50. doi: 10.1046/j.0014-2972.2001.00940.x. [DOI] [PubMed] [Google Scholar]
- Demirov DG, Ono A, Orenstein JM, Freed EO. Over-expressionof the N-terminal domain of TSG101 inhibits HIV-1 budding by blocking late domain function. Proc. Natl. Acad. Sci. USA. 2002;99:955–960. doi: 10.1073/pnas.032511899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrus JE, von Schwedler UK, Pornillos OW, Morham SG, Za-vitz KH, Wang HE, Wettstein DA, Stray KM, Cote M, Rich RL, et al. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 2001;107:55–65. doi: 10.1016/s0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Goff SP. Retrovirus restriction factors. Mol. Cell. 2004;16:849–859. doi: 10.1016/j.molcel.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Gottlinger HG, Dorfman T, Sodroski JG, Haseltine WA. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc. Natl. Acad. Sci. USA. 1991;88:3195–3199. doi: 10.1073/pnas.88.8.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlinger HG, Dorfman T, Cohen EA, Haseltine WA. Vpu protein of human immunodeficiency virus type 1 enhances the release of capsids produced by gag gene constructs of widely divergent retroviruses. Proc. Natl. Acad. Sci. USA. 1993;90:7381–7385. doi: 10.1073/pnas.90.15.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gummuluru S, Kinsey CM, Emerman M. An in vitro rapid-turnover assay for human immunodeficiency virus type 1 replication selects for cell-to-cell spread of virus. J. Virol. 2000;74:10882–10891. doi: 10.1128/jvi.74.23.10882-10891.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O, Kochs G. Interferon-induced mx proteins: Dy-namin-like GTPases with antiviral activity. Traffic. 2002;3:710–717. doi: 10.1034/j.1600-0854.2002.31003.x. [DOI] [PubMed] [Google Scholar]
- Harila K, Prior I, Sjoberg M, Salminen A, Hinkula J, Suoma-lainen M. Vpu and Tsg101 regulate intracellular targeting of the human immunodeficiency virus type 1 core protein precursor Pr55gag. J. Virol. 2006;80:3765–3772. doi: 10.1128/JVI.80.8.3765-3772.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hout DR, Mulcahy ER, Pacyniak E, Gomez LM, Gomez ML, Stephens EB. Vpu: A multifunctional protein that enhances the pathogenesis of human immunodeficiency virus type 1. Curr. HIV Res. 2004;2:255–270. doi: 10.2174/1570162043351246. [DOI] [PubMed] [Google Scholar]
- Jouvenet N, Neil SJ, Bess C, Johnson M, Virgen C, Simon S, Bieniasz P. Plasma membrane is the site of productive HIV-1 particle assembly. PLoS Biol. 2006;4:435. doi: 10.1371/journal.pbio.0040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimkait T, Strebel K, Hoggan MD, Martin MA, Orenstein JM. The humanimmunodeficiency virustype 1-specific protein vpu is required for efficient virus maturation and release. J. Virol. 1990;64:621–629. doi: 10.1128/jvi.64.2.621-629.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth RS, Oh PS, Munis JR, Cleveland PH, Richman DD. Interferons and bacterial lipopolysaccharide protect macrophages from productive infection by human immunodeficiency virus in vitro. J. Exp. Med. 1989;169:1137–1151. doi: 10.1084/jem.169.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lama J, Mangasarian A, Trono D. Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr. Biol. 1999;9:622–631. doi: 10.1016/s0960-9822(99)80284-x. [DOI] [PubMed] [Google Scholar]
- Levy JA, Scott I, Mackewicz C. Protection from HIV/ AIDS: The importance of innate immunity. Clin. Immunol. 2003;108:167–174. doi: 10.1016/s1521-6616(03)00178-5. [DOI] [PubMed] [Google Scholar]
- Li G, Stahl PD. Structure-function relationship of the small GTPase rab5. J. Biol. Chem. 1993;268:24475–24480. [PubMed] [Google Scholar]
- Martin-Serrano J, Zang T, Bieniasz PD. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat. Med. 2001;7:1313–1319. doi: 10.1038/nm1201-1313. [DOI] [PubMed] [Google Scholar]
- Martin-Serrano J, Perez-Caballero D, Bieniasz PD. Context-dependent effects of L domains and ubiquitination on viral budding. J. Virol. 2004;78:5554–5563. doi: 10.1128/JVI.78.11.5554-5563.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil SJ, Eastman SW, Jouvenet N, Bieniasz PD. HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane. PLoS Pathog. 2006;2:39. doi: 10.1371/journal.ppat.0020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura A, Lu G, Pitha-Rowe I, Pitha PM. Innate antiviral response targets HIV-1 release by the induction of ubiquitin-like protein ISG15. Proc. Natl. Acad. Sci. USA. 2006;103:1440–1445. doi: 10.1073/pnas.0510518103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G, Lei KJ, Jin W, Greenwell-Wild T, Wahl SM. Induction of APOBEC3 family proteins, a defensive maneuver underlying interferon-induced anti-HIV-1 activity. J. Exp. Med. 2006;203:41–46. doi: 10.1084/jem.20051512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G, Orenstein JM, Kinter A, Folks TM, Fauci AS. Interferon-alpha but not AZT suppresses HIV expression in chronically infected cell lines. Science. 1989;244:575–577. doi: 10.1126/science.2470148. [DOI] [PubMed] [Google Scholar]
- Ross TM, Oran AE, Cullen BR. Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral Nef protein. Curr. Biol. 1999;9:613–621. doi: 10.1016/s0960-9822(99)80283-8. [DOI] [PubMed] [Google Scholar]
- Samuel CE. Antiviral actions of interferons. Clin. Microbiol. Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi Y, Pitha PM. Alpha interferon inhibits early stages of the human immunodeficiency virus type 1 replication cycle. J. Virol. 1992;66:1321–1328. doi: 10.1128/jvi.66.3.1321-1328.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi Y, Pitha PM. Interferon alpha-mediated inhibition of human immunodeficiency virus type 1 provirus synthesis in T-cells. Virology. 1993;193:303–312. doi: 10.1006/viro.1993.1126. [DOI] [PubMed] [Google Scholar]
- Siegal FP, Fitzgerald-Bocarsly P, Holland BK, Shodell M. Interferon-alpha generation and immune reconstitution during antiretroviral therapy for human immunodeficiency virus infection. AIDS. 2001;15:1603–1612. doi: 10.1097/00002030-200109070-00002. [DOI] [PubMed] [Google Scholar]
- Smith MS, Thresher RJ, Pagano JS. Inhibition of human immunodeficiency virus type 1 morphogenesis in T cells by alpha interferon. Antimicrob. Agents Chemother. 1991;35:62–67. doi: 10.1128/aac.35.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruen-berg J, Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373–381. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Strebel K, Klimkait T, Martin MA. A novel gene of HIV-1, vpu, and its 16-kilodalton product. Science. 1988;241:1221–1223. doi: 10.1126/science.3261888. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Marusawa H, Seno H, Matsumoto Y, Ueda Y, Ko-dama Y, Endo Y, Yamauchi J, Matsumoto T, Takaori-Kondo A, et al. Anti-viral protein APOBEC3G is induced by interferon-alpha stimulation in human hepatocytes. Biochem. Biophys. Res. Commun. 2006;341:314–319. doi: 10.1016/j.bbrc.2005.12.192. [DOI] [PubMed] [Google Scholar]
- van Boxel-Dezaire AH, Rani MR, Stark GR. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity. 2006;25:361–372. doi: 10.1016/j.immuni.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Varthakavi V, Smith RM, Bour SP, Strebel K, Spearman P. Viral protein U counteracts a human host cell restriction that inhibits HIV-1 particle production. Proc. Natl. Acad. Sci. USA. 2003;100:15154–15159. doi: 10.1073/pnas.2433165100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schwedler UK, Stray KM, Garrus JE, Sundquist WI. Functional surfaces of the human immunodeficiency virus type 1 capsid protein. J. Virol. 2003;77:5439–5450. doi: 10.1128/JVI.77.9.5439-5450.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R, Matrosovich M, Klenk HD. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev. Med. Virol. 2002;12:159–166. doi: 10.1002/rmv.352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.