Abstract
Interleukin-8 (IL-8) is a chemokine that has an autocrine and/or paracrine tumor-promoting role and significant potential as a prognostic and/or predictive cancer biomarker. In breast cancer, which is mostly determined by expression of estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2), IL-8 could play a specific role. IL-8 is highly expressed in ER− breast cancers, but it increases invasiveness and metastatic potential of both ER− and ER+ breast cancer cells. It is also highly expressed in HER2+ breast cancers. Because of the complex crosstalk between these receptors and IL-8, its role is mainly determined by delicate balance in their signaling pathways. Therefore, the main point of this review was to analyze the possible influence of IL-8 in breast cancer progression related to its interaction with ER and HER2 and the consequent therapeutic implications of these relations.
Introduction
Cytokines, produced by tumor and endothelial cells, could play an important role in cancer, such as increasing angiogenesis, stimulating tumor progression, enhancing tumor cell migration, and facilitating evasion of immune surveillance, acting either by autocrine or paracrine mechanisms. They can act as growth factors and chemokines (chemotactic cytokines) for both endothelial cells and tumor cells (Keeley and others 2010). The CXC subgroup (defined by the positioning of the conserved cysteines near the amino-terminus), seems to have a crucial role in angiogenesis in both physiologic and pathological settings, but there is evidence that this is not the only role. Moreover, chemokines might promote tumor growth or increase the metastatic potential of cancer cells (Gomperts and Strieter 2006), but the exact mechanisms remain to be elucidated. Analysis of differential cytokine expression levels in various human cancers, including human breast cancer, has shown that cytokine expression is elevated in various types of cancers and that this elevation has a tumor-promoting role. This supports the idea that tumor cells take advantage of the expression of chemokines and chemokine receptors to stimulate the immune response, induce tumor angiogenesis and tumor growth, alter the tumor microenvironment, and facilitate metastasis (Richmond and others 2009). Alterations in chemokine ligand and receptor expression profiles may have different contributions during cancer progression and also practical consequences for cancer diagnosis and therapy (Opdenakker and Van Damme 2004; Vandercappellen and others 2008).
Among chemokines, interleukin (IL)-8 (CXCL8) seems to have significant potential as prognostic and/or predictive cancer biomarker. IL-8 could be a marker for various clinical conditions and IL-8 level increase as a result of many inflammatory conditions. It is also produced by blood cells and many types of tissues. As such, careful interpretation of IL-8 levels is required to determine their correlation with a specific clinical condition's diagnosis or prognosis (Shahzad and others 2010). In addition to being elevated in the sera of cancer patients, IL-8 levels could be elevated in cancer-associated nonvascular extracellular fluids, such as pleural effusion, ascites, cyst fluid, cerebrospinal fluid, urine, saliva, interstitial fluid, and cervicovaginal secretions. Higher IL-8 levels are typically found in high-grade peritumoral fluids rather than low-grade tumors and benign conditions, with the exception of inflammatory processes indicating that local IL-8 production is related to malignant processes (Kotyza 2012). IL-8 is a member of the CXC chemokine family of angiogenesis/inflammation-related chemokines, secreted by stromal (endothelial cells and fibroblasts) and tumor cells. All biological effects of IL-8 are mediated by 2 receptors designated as CXCR-1 (IL-8RA) and CXCR-2 (IL-8RB). IL-8 binds with high specificity to CXCR-1 (Holmes and others 1991) and with less specificity to CXCR-2 (Brat and others 2005) expressed on stromal, endothelial, and tumor cells. The roles of each of these receptors in IL-8-signaling remain controversial. Several signaling pathways could be induced downstream of IL-8 receptors in different cell types, indicating its complex role in normal physiological conditions and in cancer progression (reviewed in Waugh and Wilson 2008). IL-8 signaling activates multiple upstream signaling pathways that affect gene expression via regulation of numerous transcription factor activities, modulate the cellular proteome at the level of translation, and affect the organization of the cell cytoskeleton through post-translational regulation of regulatory proteins (Waugh and Wilson 2008). Due to the diversity of effectors and downstream targets, IL-8 signaling promotes angiogenic responses in endothelial cells, increases the proliferation and survival of endothelial and cancer cells, and potentiates the migration of cancer cells, endothelial cells, and infiltrating neutrophils at the tumor site.
Tumor-Promoting Role of IL-8
IL-8 is overexpressed in various human solid tumors. Ewington and others (2012) found an association between IL-8 and IL-8 receptors and the pathogenesis of endometrial carcinoma. In addition, Nastase and others (2011) were able to use IL-8, to diagnose an early-stage colon cancer and to monitor the progression of colon tumors. Based on current urine-based assays, IL-8 was identified as the most prominent urinary biomarker for the detection of bladder cancer (Urquidi and others 2012). The expression of IL-8 and its receptors in melanoma has demonstrated a positive correlation with disease progression (reviewed in Singh and others 2010). In a large study on association of expression of inflammatory biomarkers with lung cancer risk, there was evidence that only the increase of serum IL-8 is indicative of the development of clinically evident lung cancer. This means that including of IL-8 measurement in diagnostic practice, can lead to improved prediction of lung cancer occurrence (Lagiou and Trichopoulos 2011). The expression level of IL-8 and its receptor CXCR-1 in the tumor tissues and sera of pancreatic cancer patients have also been found to be elevated. In vivo tumor tissues from pancreatic cancer patients with a higher serum IL-8 level grew faster and behaved more aggressively than those with low serum IL-8 levels. Follow-up data showed that patients with high serum IL-8 levels had a relatively lower survival rate than those with low serum IL-8 levels (Chen and others 2012). Survival analyses of nasopharyngeal carcinoma patients revealed that higher IL-8 expression in primary tumor tissue was an independent prognostic factor for overall survival, disease-free survival, and distant metastasis-free survival of the patients (Li and others 2012). In the same study, in vitro experiments also showed that IL-8 increase migration, invasion, and metastasis potential without affecting cellular growth and induces epithelial-mesenchymal transition in various nasopharyngeal carcinoma cell lines. Besides overexpression of the IL-8 ligand, there is evidence of differential expression of its receptors—for example, in benign and malignant breast tissue. In a study by Miller and others (1998), all breast cancer cells expressed the IL-8 receptors CXCR-1 and CXCR-2, whereas only 50% of the benign breast tissue samples expressed either CXCR-1 or CXCR-2. Moreover, recent results suggested that the genetic variation (polymorphism) of IL-8 and CXCR-2 genes in breast cancer, which influences the expression of this chemokine could be the genetic basis of potential tumor progression and invasiveness of breast cancer (Snoussi and others 2010).
It has been suggested that IL-8 has an autocrine and/or paracrine tumor-promoting role in modulating the survival and proliferation of tumor cells. IL-8 biological activity in tumors and the tumor microenvironment might contribute to tumor progression through the regulation of angiogenesis, cancer cell growth and survival, tumor cell motion, leukocyte infiltration, and modification of immune responses (Yuan and others 2005). IL-8 was originally identified as a chemoattractant for neutrophils that release angiogenic growth factors, thus stimulating angiogenesis as a part of cancer progression (enhanced tumor growth and worse survival). IL-8 has a direct role in angiogenesis via enhancement of endothelial cell proliferation and survival and matrix metalloproteinase expression in CXCR-1- and CXCR-2-expressing endothelial cells (Li and others 2003). IL-8 stimulates both endothelial proliferation and capillary tube formation in vitro in a dose-dependent manner, and both of these effects can be blocked by monoclonal antibodies to IL-8. A recent study showed that IL-8 stimulates VEGF expression in endothelial cells, via CXCR-2, and thereby promotes the activation of VEGF receptors in an autocrine fashion (Martin and others 2009). Moreover, IL-8 has been shown to enhance production and secretion of matrix metalloproteinase MMP-2 and MMP-9 by tumor cells (Inoue and others 2000a, 2000b; Li and others 2005) suggesting that it can modulate invasiveness and/or extracellular matrix remodeling in the tumor environment. As cell proliferation, angiogenesis, migration, and invasion are all involved in the metastatic process, IL-8 expression by tumor cells can influence their metastatic capabilities. Indeed, the expression of IL-8 has been shown to correlate with the metastatic potential of human cancer cells. For example, it is well known that highly metastatic melanoma cells express higher levels of IL-8 mRNA than low metastatic cells and this increased mRNA expression directly correlates with IL-8 protein secretion (Singh and others 1994). Increased expression of IL-8 in the tumor microenvironment enhances colon cancer growth and metastasis. Moreover, the absence of its receptor CXCR-2 in the tumor microenvironment could prevent colon cancer cell growth (Lee and others 2012). In a tumor xenograft model, IL-8-expressing cells formed significantly larger tumors than the control cells with increased microvessel density (Ning and others 2011).
Considering the occurrence of both IL-8 receptors in tumor cells, IL-8 could act as an autocrine motility and growth factor providing them with additional growth and progression advantages (Miyamoto and others 1998; Brew and others 2000; De Larco and others 2004; Li and others 2005). IL-8 secreted by tumor cells could potentiate tumor progression by inducing adjacent epithelial tumor cells into an epithelial-to-mesenchymal transition. Epithelial–mesenchymal phenotypic switch of tumor cells during cancer progression is an universal feature of the malignant process, although its contribution to interactions between tumor cells and their microenvironment has not yet been well characterized (Fernando and others 2011). It seems that IL-8 is involved in the phenotypic switch, which induces tumor cell motility and invasiveness and seems to have the essential role for the acquisition and/or maintenance of the mesenchymal and invasive features of tumor cells (Fernando and others 2011). The newest study on this subject revealed that IL-8 is a major cytokine that is highly secreted by human monocyte U937 cells. This secreted IL-8 stimulates the expression of adhesion molecule fibronectin (the hallmark of epithelial mesenchymal transition) in human inflammatory breast carcinoma cells, and the induction of motile phenotype and branch-like structures (Mohamed 2012).
IL-8 signaling plays a critical role in cancer stem cell function (Sharma and Singh 2011). The expression of CXCR-1 is elevated in populations of breast cancer stem cells, such as in breast cancer cell lines with higher frequencies of CD44 +/CD24− cells (Sheridan and others 2006), suggesting that this chemokine should be considered a marker for the identification of breast cancer stem cells. In a study using in vitro assays and mouse models, the addition of IL-8 to breast cancer cell lines was shown to promote the invasion and chemotaxis of cancer stem cells and the blocking of CXCR-1 in breast cancer cell lines. Using a small molecular inhibitor, repertaxin, or CXCR1-specific antibodies reduces the cancer stem cell population and induces apoptosis in vitro and in vivo (Ginestier and others 2010).
The stable and selective overexpression of IL-8 is associated with a drug-resistant phenotype (Duan and others 1999). These findings suggest that the development of therapeutic resistance is accompanied by multiple changes in gene expression, such as stable alterations in selective chemokine and cytokine expression, indicating that cytokines might serve as important survival factors that promote the multidrug-resistant phenotype. In a similar study, Yang and others confirmed this hypothesis. In that study among 120 cytokines screened, marked increases in IL-8 and other 13 proteins were found in a drug resistant MCF-7/R cell line, compared with a sensitive MCF-7/S cell line (Yang and others 2005). Neutralizing antibody IL-8 partially reversed the drug-resistance of MCF-7/R to taxol and doxorubicin. In addition, the inhibition of endogenous IL-8 by siRNA significantly enhanced the drug sensitivity of the MCF-7/R cells. Depleting intracellular IL-8 in androgen-independent prostate cancer increased the cytotoxic activity of multiple chemotherapeutic drugs (Singh and Lokeshwar 2009). In addition, preclinical evaluation of repertaxin effectiveness on animal models showed that mice treated with repertaxin or the combination of repertaxin and chemotherapy, had fewer cancer stem cells than those treated with chemotherapy alone (Ginestier and others 2010). The repertaxin-treated mice developed fewer metastases than the mice treated with chemotherapy alone. The adequate explanation would be that dying cells in tumors exposed to chemotherapy, produce IL-8, which stimulates cancer stem cells to replicate. Adding repertaxin to chemotherapy specifically targets breast cancer stem cells by blocking CXCR-1, thereby increasing the effect of chemotherapy.
IL-8 in Breast Cancer: Basic Assumptions
There is evidence of a strong correlation between the metastatic potential of breast carcinoma cell lines and ectopic expression of IL-8. It has been shown that undifferentiated, highly metastatic cell lines produce much more IL-8 than their differentiated lower metastatic counterparts. This could be a consequence of possible epigenetic modifications, such as, the aberrant methylation pattern in the IL-8 gene, which might be responsible for the differences in IL-8 release between the high and low metastatic cell lines (De Larco and others 2003). There are few clinical studies regarding the role of IL-8 in breast cancer progression. Recent results have shown that the polymorphisms in IL-8 and CXCR-2 genes are associated with increased breast cancer risk and disease progression (Snoussi and others 2010). According to one clinical study, IL-8 has independent prognostic significance for post-relapse survival (Benoy and others 2004). In that study, serum IL-8 levels were higher in patients with breast cancer compared with healthy volunteers, especially in patients with more advanced disease. An elevated serum IL-8 level is related to the worse outcomes, a higher tumor load, and the presence of liver or lymph node involvement. Although no correlation was observed between the expression of IL-8 mRNA and clinicopathological variables such as tumor grade, patient age, or nodal status, a much higher expression was measured in neoplastic tissues compared with normal tissues. IL-8 stimulates osteoclastogenesis and bone resorption, and higher IL-8 levels predict early metastatic spread of breast cancer. In vivo testing using a nude mouse model to measure IL-8 production from breast cancer cells that had metastasized to bone showed that estrogen-receptor negative cell line MDA-231-BSC (metastatic cells isolated from bone metastases) produce much higher levels of IL-8 compared with the parental MDA-231 cells (Singh and others 2006). A recent immunohistochemical study showed an inverse correlation between IL-8 expression and metastasis and/or local recurrence (Zuccari and others 2012). In the patient group, which received post-surgery chemotherapy and radiotherapy, a lower IL-8 expression was found in those women who showed local recurrence. Elevated expression of IL-8 in breast cancer cells is associated with breast cancer invasiveness and angiogenesis (Lin and others 2004).
IL-8 and Estrogen Receptor/Human Epidermal Growth Factor Receptor 2 Relation in Breast Cancer
Breast cancer is a heterogeneous disease, determined mostly by expression of estrogen receptor/progesteron receptor (ER/PR) and human epidermal growth factor receptor 2 (HER2), according to recent microarray-based expression profiling studies (Sandhu and others 2010; Colombo and others 2011). Clinical and pathologic responses to different types of therapy vary, depending on this novel molecular subclasification of breast cancer. In addition to the worst prognosis of triple negative subtype ER-PR-HER2− (mostly because there is no targeted therapy for this particular phenotype), ER-PR-HER2+ is also the phenotype with poor outcome (Aedayo and others 2009; Parise and others 2009; Early Breast Cancer Trialists' Collaborative Group 2011). Regardless, steroid receptor negative phenotypes had the worst prognoses (Osborne 1998). Although ER positivity is a pre-requisite for antiestrogen treatment (tamoxifen), a number of patients will not respond (intrinsic resistance) and others will in time fail to respond (acquired resistance). Tamoxifen resistance is an old and unsolved issue. There are many possible causes, one of which could be hidden in the main mechanism of its action (ie, blocking the ER function). By blocking ER function, tamoxifen could also influence on expression of ER-regulated growth factors and cytokines (ER+ cancer expression profile).
In addition to the relationship of IL-8 to increased invasiveness of cancer cells, it seems that the most important association in breast cancer is with ER and/or HER2 status. However, there are only few studies regarding potential significance of IL-8 related to ER/HER2 status. In a recent study that compared triple negative (ER-PR-HER2-) breast cancer patients with or without recurrence, IL-8 was identified as the top-ranked gene associated with poor prognosis (Rody and others 2011). A subgroup of triple negative breast cancer patients with a low IL-8 metagene expression is characterized by relatively low risk of recurrence, even in the absence of systemic therapy, and has a recurrence-free survival rate of 84% at 5-years. An in vitro study showed that the basal level of IL-8 expression is substantially higher in the metastatic- estrogen-independent cell lines when compared with the nonmetastatic estrogen-dependent lines (De Larco and others 2001). Comparing the expression of several cytokines in normal and malignant breast tissues, by multiplexed flow cytometry technology (Chavey and others 2007) revealed overexpression of IL-8 in high-grade ER− breast carcinoma, in addition to increased expression of several cytokines, especially IL-8 in breast carcinoma compared with normal breast. In addition, IL-8 was more abundant in HER2+ tumors than in HER2- tumors. Human cytokine antibody arrays confirmed that IL-8 expression is inversely associated with ER status, but positively associated with the metastatic potential of ER− breast cancer cells (Freund and others 2004). Moreover, IL-8 has the potential to increase the invasiveness of ER+ breast cancer cells. Transfection assays showed that ER significantly downregulate IL-8 promoter activity, but this regulation was independent on estrogen. In agreement with this, a transient transfection test showed that IL-8 expression in the MDA-MB-231 cells transfected with ER was lower than that of ER−MDA-MB-231 cells (Yao and others 2007). Knockdown of the IL-8 expression, with RNA interference technology in ER− breast cancer cell line MDA-MB-231, induce decreased expression levels of IL-8. This decrease was associated with reduced cell invasion in vitro (no effect on cell proliferation), inhibited neutrophils infiltration, and decreased metastatic potential in vivo (Lin and others 2005). In contrast, in another study (Bendrik and Dabrosin 2009) a significant positive correlation was found between IL-8 and estradiol in normal breast tissue and hormone-dependent breast cancer tissue in vivo. Estradiol exposure increased IL-8 secretion of normal whole breast tissue in culture and in experimental breast cancer, whereas the anti-estrogen tamoxifen inhibited the secretion of IL-8 both in vitro and extracellularly in vivo in tumors in nude mice. In vitro experiments also have shown that epidermal growth factor (EGF) potently upregulated IL-8 secretion by breast tumor cells, and its effect was promoted by a consecutive treatment of the cells by estrogen (Azenshtein and others 2005). These findings suggest a complex regulation pattern for IL-8.
The main controversy regarding ER and IL-8 relation is that IL-8 expression is inversely correlated with ER status (ER downregulate IL-8 expression), but positively correlated with estradiol (estradiol seems to increase IL-8 expression). This is the same paradox as with ER and estrogen itself: estrogen is known as a potent tumor-promoting factor in breast cancer, but on the other hand breast cancer patients who lack ER expression have worse prognoses. However, IL-8 increases invasiveness and metastatic potential of both ER− and ER+ breast cancer cells. The question is how does this apply to the breast cancer patient's outcome and treatment? It would be interesting to know if this relationship influences the effectiveness of hormonal therapies, such as tamoxifen, primary given to ER+ patients. For ER− patients who have higher IL-8 expression, IL-8 could contribute to a worse patient outcome by increasing the invasiveness of cancer cells. In addition, these patients are not candidates for antiestrogen therapy. In ER+ patients, if ER downregulate IL-8 expression (independent of estrogen action) and tamoxifen as estrogen antagonist blocks ER action, then it would be reasonable to postulate that this treatment could result in increased IL-8 production, regulated via different mechanisms. The risk of recurrence of breast cancer in patients taking antiestrogen therapy decreases, although that is dependent on the potency of still-existing tumor cells to secrete growth factors and chemokines. These growth factors and chemokines can stimulate development of an adverse and resistant cellular phenotype that exhibits altered adhesive interactions and enhanced migratory and invasive behavior (Hayes and others 2011). If that is true, IL-8 could be a cause and indicator of tamoxifen resistance, as a part of the IL-8-mediated breast cancer progression. There are only a few studies related to this issue. A study on ovarian cancer cells showed that autocrine production IL-8 in epithelial ovarian cancer cell lines is inversely associated with cell response to tamoxifen, indicating that higher IL-8 expression implies less responsiveness by tumor cells (Wang and others 2010). Tamoxifen, an ER antagonist, completely blocks estrogen-stimulated cell growth and the expression of IL-6 and IL-8 (Wang and others 2005; Yang and others 2009). As postulated, chronic exposure of breast cancer cells to endocrine agents such as tamoxifen induces the acquisition of a resistant and aggressive phenotype. It has been shown that tamoxifen-resistant derivative cells (generated from culturing MCF7 cells in the presence of tamoxifen until the resistant growth was seen) among other characteristics, exhibit increased IL-8 expression. Only one study on breast cancer patients revealed that serum cytokine levels (including IL-8), which were elevated in untreated breast cancer patients, were significantly reduced after tamoxifen therapy for more than 1 year (Premkumar and others 2007).
In the context of breast cancer patient prognoses and predictions, interactions between ER and HER2 are often considered as the main molecular mechanisms in resistance to different types of therapy. The crosstalk between ER and the growth factor signaling such as the HER2 pathway is complex and bidirectional. Because these 2 receptors have the potential to coactivate each other in ER+HER2+ breast cancers, the expected net effect of their mutual action would be increased survival and proliferation of tumor cells. In this context, the tumor-promoting role of IL-8 could be important in HER2-induced antiestrogen-resistant breast cancer phenotype. Measuring cytokine expression in breast cancer samples, by multiplexed flow cytometry technology revealed that among other cytokines, IL-8 was not only related to the ER-negative phenotype, but there was a strong positive correlation between HER2 expression and IL-8 (Freund and others 2003). A similar study (Vazquez-Martin and others 2007) involving cytokine antibody arrays revealed a strong similarity between the HER2 “cytokine signature” observed in serum samples and that obtained in media conditioned by breast cancer-derived cell lines. In addition, IL-8 serum levels were significantly higher in HER2+ breast cancer patients than in HER2- patients. These experiments identified IL-8 as the most prominent chemokine with at least a 10-fold increased expression in HER2-overexpressing transfectants when compared with matched control. Moreover, treatment with tyrosine kinase inhibitor gefitinib decreased the expression level of IL-8. On the other hand, in vitro studies using the mammosphere colony assay showed that IL-8 promotes cancer stem cell activity in HER2-overexpressing breast cancer and that a combined treatment involving an IL-8 inhibitor and lapatinib (an HER2 inhibitor) is optimal (Singh and others 2011). Re-expression of HER2 in breast cancer cells that endogenously express low levels of HER2 (MCF-7 and T-47D) results in higher expression of IL-8 (and VEGF). Inhibition of HER2 with a humanized anti-HER2 antibody (trastuzumab) or a retrovirus-mediated, small, interfering RNA against HER2 (siHER2) decreased IL-8 expression via the phosphatidylinositol 3′-kinase (PI3K)-AKT pathway (Wen and others 2006). In another study, the evaluation of angiogenic plasma markers in patients with metastatic breast cancer treated with docetaxel HER2-ECD and IL-8 plasma levels seem to be of prognostic value for survival (Papaxoinis and others 2010). The experiments investigating the effects of HER2/HER3 overexpression and activation on gene expression in breast cancer revealed that among 80 upregulated transcripts, IL-8 was highly expressed (11-fold upregulation) (Aceto and others 2012). IL-8 was especially highly expressed in HER2-enriched and basal-like primary breast tumors, characterized by a particularly poor prognosis and correlated with a high tumor grade and ER-negative status. Moreover, treatment with IL-8-neutralizing antibodies prevented invasion of MCF10A-HER2/HER3 and BT474 cells in 3D cultures, highlighting the importance of IL-8 autocrine signaling upon HER2/HER3 activation.
IL-8 in Breast Cancer: Summary
IL-8 is a marker of ER-negative and/or HER2-positive breast cancer.
Expression of IL-8 dependent on ER and HER2 crosstalk could influence breast cancer prognosis and prediction.
Although negatively linked to ER status, IL-8 expression could increase the invasiveness of ER-positive breast cancer cells.
In the context of antiestrogen therapy (tamoxifen) given presumably to ER-positive breast cancer patients, involvement of HER2 and multidirectional interactions between these biomarkers (ER, HER2, and IL-8) in breast cancer could influence the efficiency of the treatment.
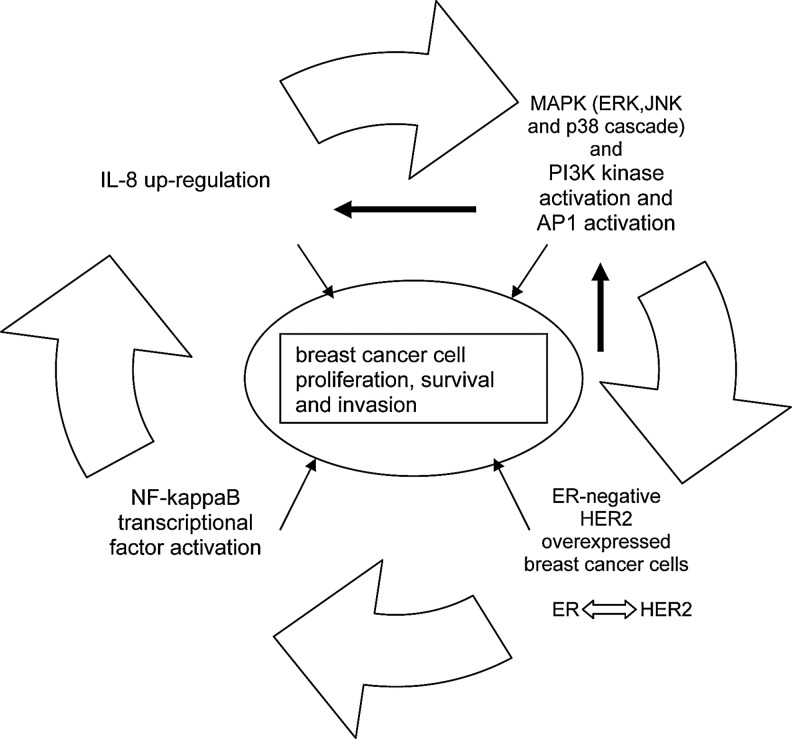
The signaling pathways by which HER2 modulates IL-8 expression are still not defined. It is known that PI3K is involved in the regulation of IL-8. Activated HER2 induce activation of signaling pathways such as the PI3K/Akt and ERK1/2 mitogen-activated protein kinase (MAPK) pathways. Moreover, most of these kinases can activate ER signaling (ER-dependent or ER-independent gene expression) by phosphorylation of specific sites (ligand-independent activation) or indirectly by phosphorylation of a variety of coregulatory proteins. Conversely, ER could downregulate HER2 expression (Russell and Hung 1992). Growth factor and ER might form a structured complex for signal transduction to MAPK and/or PI3K, which are involved in the regulation of IL-8 expression. IL-8 signaling also regulates the activity of MAPK signaling cascade, with downstream phosphorylation of ERK1/2 in both neutrophils and cancer cells (Knall and others 1996; Venkatakrishnan and others 2000). In vitro experiments have revealed that the influence of these pathways varies in different cell lines and that inhibition of the dominantly active pathway can downregulate IL-8 expression (Chelouche-Lev and others 2004). IL-8 signaling indeed transactivates growth factor receptors and activation of MAPK signaling is consistent with cell proliferation and cell survival-promoting effects of IL-8 in neutrophils, endothelial, and cancer cell lines (Waugh and Wilson 2008). Further, nuclear factor (NF)-kappaB, transcription factor with pleiotropic effects and downstream mediator of growth signaling, which controls the expression of genes that promote cell growth and survival, seems to be the main regulator controlling IL-8 gene activity (Freund and others 2004). The inhibition of NF-kappaB activity results in downregulation of IL-8 expression levels and inhibition of cell proliferation and metastasis (Patel and others 2002). NF-kappaB is predominately activated in ER−, and particularly, HER2 receptor-positive cancer (Singh and others 2007). Moreover, the exogenous expression of ER leads to ligand-independent decreased activity of IL-8 promoter, and this inhibition is likely to occur through decreased NF-kappaB activity (Freund and others 2003). On the other hand, overexpression of HER2 in Ba/F3 cells have been shown to lead to constitutive PI3- and AKT kinase activities and the induction of NF-kappaB (Pianetti and others 2001). Overall, these findings showed that NF-kappaB could be a point of convergence between ER and HER2 signaling, related to the effect on IL-8 expression. Accordingly, the net effect of IL-8 action in breast cancer is the result of a delicate balance between ER inhibition and HER2 induction of NF-kappaB activity, and consequently, IL-8. The proposed model of IL-8 regulation/expression in breast cancer is presented in Fig. 1.
FIG. 1.
Functional feedback between estrogen receptor (ER), human epidermal growth factor receptor 2 (HER2), and Interleukin (IL)-8 in breast cancer. In ER negative, HER2-overexpressed breast cancer tumors IL-8 could be upregulated by nuclear factor-kappaB signaling cascade or via mitogen-activated protein kinase (MAPK). In contrast, MAPK cascade as a part of IL-8 signaling could influence ER and growth factor function. This points to the role of IL-8 as estrogen “agonist,” in a context of ER negative, HER2-overexpressed breast cancer cells.
A recent study (Haim and others 2011) indicated that EGF (a HER2 ligand) and estrogen promoted in an additive manner the transcription and the release of IL-8 by breast tumor cells. They postulated that, EGF and estrogen probably do not act through intracellular crosstalk, but that they act in independent transcriptional pathways that complement each other, in the upregulation of IL-8. In the EGF and estrogen stimulatory setup, the effects of estrogen were channeled to transcription-related activities, mediated by ER. Specifically, after joint stimulation by EGF and estrogen, estrogen upregulated IL-8 by activating the transcriptional activity of ER, whereas EGF induced IL-8 expression through the activation of AP-1. In contrast, when concomitant stimulation by EGF and estrogen was applied, estrogen was able to partly or completely downregulate the ability of EGF to promote IL-8 expression through intracellular signaling pathways. This study confirms the existence of a complex interaction between ER and growth factor signaling that has consequences in the regulation of cytokines expression, thereby providing reasonable hypothesis that puts IL-8 at the center of HER2 and ER crosstalk.
Conclusion and Future Directions
ER/HER2 crosstalk could be of crucial significance in the understanding of ER+HER2+ breast cancer progression and, consequently, antiestrogen resistance. Because in ER+HER2+ breast cancers this crosstalk is bidirectional the expected net effect of their mutual action is increased survival and proliferation of tumor cells. Involvement of other molecules in this crosstalk could only lead to a better understanding of possible mechanisms of cancer progression. In this context, the tumor-promoting role of IL-8 could be important in the HER2-induced, antiestrogen-resistant, breast cancer phenotype. First, it is well known that there is an inverse correlation between ER and HER2 expression, and between ER and IL-8. On the other hand, it seems that HER2 increases IL-8 expression (Wen and others 2006). Consequently, although ER decreases IL-8 expression, a complex, interactive and bidirectional signaling network in ER+HER2+ tumors could be expected, leading to increased survival and proliferation of breast cancer cells (Prat and Baselga 2008). In such tumors, increased IL-8 expression could be the cause of cancer progression and therapeutic resistance.
Because the majority of clinical studies confirm overexpression of IL-8 in the most advanced stages of cancer, suppressing the effects of IL-8 may have important implications (ie, a favorable clinical outcome by preventing the switch of tumor cells from an epithelial to a mesenchymal metastatic phenotype). The therapeutic relevance of targeting IL-8 signaling is indisputable, as decreasing chemokine signaling might influence disease progression and modulate the therapy response. Neutralizing antibodies to IL-8 receptors, a humanized antibody against IL-8, and a small-molecule IL-8 receptor inhibitor, repertaxin, have all been used in several preclinical studies showing their ability to inhibit angiogenesis, tumor growth, and metastasis of various xenograft tumor models (Ginestier and others 2010). In context of breast cancer, targeting IL-8 and/or IL-8 receptors seem necessary for the treatment of tumors that express ER and overexpress HER2.
Acknowledgment
This work was supported by Grant No. 175068 “Molecular biomarkers of breast cancer and changes of their significance depending on the follow-up of the disease” from the Ministry of Science and Environment Protection of Republic of Serbia.
Author Disclosure Statement
The authors declare that there is no conflict of interest.
References
- Aceto N. Duss S. Macdonald G. Meyer DS. Roloff TC. Hynes NE, et al. Co-expression of HER2 and HER3 receptor tyrosine kinases enhances invasion of breast cells via stimulation of interleukin-8 autocrine secretion. Breast Cancer Res. 2012;14:R131. doi: 10.1186/bcr3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azenshtein E. Meshel T. Shina S. Barak N. Keydar I. Ben-Baruch A. The angiogenic factors CXCL8 and VEGF in breast cancer: regulation by an array of pro-malignancy factors. Cancer Lett. 2005;217:73–86. doi: 10.1016/j.canlet.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Bendrik C. Dabrosin C. Estradiol increases IL-8 secretion of normal human breast tissue and breast cancer in vivo. J Immunol. 2009;182:371–378. doi: 10.4049/jimmunol.182.1.371. [DOI] [PubMed] [Google Scholar]
- Benoy IH. Salgado R. Van Dam P. Eboers K. Van Marck E. Scharpé S, et al. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res. 2004;10:7157–7162. doi: 10.1158/1078-0432.CCR-04-0812. [DOI] [PubMed] [Google Scholar]
- Brat DJ. Bellail AC. Van Meir EG. The role of Interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neurooncology. 2005;7:122–133. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew R. Erikson JS. West DC, et al. Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro. Cytokine. 2000;12:78–85. doi: 10.1006/cyto.1999.0518. [DOI] [PubMed] [Google Scholar]
- Chavey C. Bibeau F. Gourgou-Bourgade S. Burlinchon S. Boissière F. Laune D, et al. Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res. 2007;9:R15. doi: 10.1186/bcr1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelouche-Lev D. Miller CP. Tellez C. Ruiz M. Bar-Eli M. Price JE. Different signalling pathways regulate VEGF and IL-8 expression in breast cancer: implications for therapy. Eur J Cancer. 2004;40:2509–2518. doi: 10.1016/j.ejca.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Chen Y. Shi M. Yu G-Z. Qin X-R. Jin G. Chen P, et al. Interleukin-8, a promising predictor for prognosis of pancreatic cancer. World J Gastroenterol. 2012;18:1123–1129. doi: 10.3748/wjg.v18.i10.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo PE. Milanezi F. Weigelt B. Reis-Filho JS. Microarrays in the 2010s: the contribution of microarray-based gene expression profiling to breast cancer classification, prognostication and prediction. Breast Cancer Res. 2011;13:212. doi: 10.1186/bcr2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Larco JE. Wuertz BR. Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res. 2004;10:4895–4900. doi: 10.1158/1078-0432.CCR-03-0760. [DOI] [PubMed] [Google Scholar]
- De Larco JE. Wuertz BR. Rosner KA. Erickson SA. Gamache DE. Manivel JC. Furcht LT. A potential role for interleukin-8 in the metastatic phenotype of breast carcinoma cells. Am J Pathol. 2001;158:639–646. doi: 10.1016/S0002-9440(10)64005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Larco JE. Wuertz BR. Yee D. Rickert BL. Furcht LT. Atypical methylation of the interleukin-8 gene correlates strongly with the metastatic potential of breast carcinoma cells. Proc Natl Acad Sci U S. 2003;A100:13988–13993. doi: 10.1073/pnas.2335921100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z. Feller AJ. Penson RT. Chabner BA. Seiden MV. Discovery of differentially expressed genes associated with paclitaxel resistance using cDNA array technology: analysis of interleukin (IL) 6, IL-8, and monocyte chemotactic protein 1 in the paclitaxel-resistant phenotype. Clin Cancer Res. 1999;5:3445–3453. [PubMed] [Google Scholar]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewington L. Taylor A. Sriraksa R. Horimoto Y. Lam EW. El-Bahrawy MA. The expression of interleukin-8 and interleukin-8 receptors in endometrial carcinoma. Cytokine. 2012;59:417–422. doi: 10.1016/j.cyto.2012.04.036. [DOI] [PubMed] [Google Scholar]
- Fernando RI. Castillo MD. Litzinger M. Hamilton DH. Palena C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res. 2011;71:5296–5306. doi: 10.1158/0008-5472.CAN-11-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A. Chauveau C. Brouillet JP. Lucas A. Lacroix M. Licznar A, et al. IL-8 expression and its possible relationship with estrogen-receptor-negative status of breast cancer cells. Oncogene. 2003;22:256–265. doi: 10.1038/sj.onc.1206113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A. Jolivel V. Durand S. Kersual N. Chalbos D. Chavey C, et al. Mechanisms underlying differential expression of interleukin-8 in breast cancer cells. Oncogene. 2004;23:6105–6114. doi: 10.1038/sj.onc.1207815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestier C. Liu S. Diebel ME. Korkaya H. Luo M. Brown M, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomperts BN. Strieter RM. Chemokine-directed metastasis. Contrib Microbiol. 2006;13:170–190. doi: 10.1159/000092972. [DOI] [PubMed] [Google Scholar]
- Haim K. Weitzenfeld P. Meshel T. Ben-Baruch A. Epidermal growth factor and estrogen act by independent pathways to additively promote the release of the angiogenic chemokine CXCL8 by breast tumor cells. Neoplasia. 2011;13:230–243. doi: 10.1593/neo.101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes E. Nicholson RI. Hiscox S. Acquired endocrine resistance in breast cancer: implications for tumour metastasis. Front Biosci. 2011;16:838–848. doi: 10.2741/3723. [DOI] [PubMed] [Google Scholar]
- Holmes WE. Lee J. Kuang WJ. Rice GC. Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278–1280. doi: 10.1126/science.1840701. [DOI] [PubMed] [Google Scholar]
- Inoue K. Slaton JW. Eve BY. Kim SJ. Perrotte P. Balbay MD. Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clin Cancer Res. 2000a;6:2104–2119. [PubMed] [Google Scholar]
- Inoue K. Slaton JW. Kim SJ. Perrotte P. Eve BY. Bar-Eli M. Radinsky R. Dinney CP. Interleukin 8 expression regulates tumorigenicity and metastasis in human bladder cancer. Cancer Res. 2000b;60:2290–2299. [PubMed] [Google Scholar]
- Keeley EC. Mehrad B. Strieter RM. CXC chemokines in cancer angiogenesis and metastases. Adv Cancer Res. 2010;106:91–111. doi: 10.1016/S0065-230X(10)06003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knall C. Young S. Nick JA. Buhl AM. Worthen GS. Johnson GL. Interleukin-8 regulation of the Ras/Raf/mitogen-activated protein kinase pathway in human neutrophils. J Biol Chem. 1996;271:2832–2838. doi: 10.1074/jbc.271.5.2832. [DOI] [PubMed] [Google Scholar]
- Kotyza J. Interleukin-8 (CXCL8) in tumor associated non-vascular extracellular fluids: its diagnostic and prognostic values. A review. Int J Biol Markers. 2012;27:e169–e178. doi: 10.5301/JBM.2012.9261. [DOI] [PubMed] [Google Scholar]
- Lagiou P. Trichopoulos D. Inflammatory biomarkers and risk of lung cancer Pagona lagiou and dimitrios trichopoulos. J Natl Cancer Inst. 2011;103:1073–1075. doi: 10.1093/jnci/djr220. [DOI] [PubMed] [Google Scholar]
- Lee YS. Choi I. Ning Y. Kim NY. Khatchadourian V. Yang D, et al. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br J Cancer. 2012;106:1833–1841. doi: 10.1038/bjc.2012.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A. Dubey S. Varney ML. Dave BJ. Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- Li A. Varney ML. Valasek J. Godfrey M. Dave BJ. Singh RK. Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis. Angiogenesis. 2005;8:63–71. doi: 10.1007/s10456-005-5208-4. [DOI] [PubMed] [Google Scholar]
- Li XJ. Peng LX. Shao JY. Lu WH. Zhang JX. Chen S, et al. As an independent unfavorable prognostic factor, IL-8 promotes metastasis of nasopharyngeal carcinoma through induction of epithelial-mesenchymal transition and activation of AKT signaling. Carcinogenesis. 2012;33:1302–1309. doi: 10.1093/carcin/bgs181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. Huang R. Chen L. Li S. Shi Q. Jordan C, et al. Identification of interleukin-8 as estrogen receptor-regulated factor involved in breast cancer invasion and angiogenesis by protein arrays. Int J Cancer. 2004;109:507–515. doi: 10.1002/ijc.11724. [DOI] [PubMed] [Google Scholar]
- Lin Y. Wang SM. Huang RP. Angiogenic effect of interleukin-8 in breast cancer and its association with estrogen receptor. Zhonghua Yi Xue Za Zhi. 2005;85:1419–1423. [PubMed] [Google Scholar]
- Martin D. Galisteo R. Gutkind JS. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1complex. J Biol Chem. 2009;284:6038–6042. doi: 10.1074/jbc.C800207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LJ. Kurtzman SH. Wang Y, et al. Expression of interleukin-8 receptors on tumor cells and vascular endothelial cells in human breast cancer tissue. Anticancer Res. 1998;18:77–81. [PubMed] [Google Scholar]
- Miyamoto M. Shimizu Y. Okada K, et al. Effect of interleukin-8 on production of tumor-associated substances and autocrine growth of human liver and pancreatic cancer cells. Cancer Immunol Immunother. 1998;47:47–57. doi: 10.1007/s002620050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed MM. Monocytes conditioned media stimulate fibronectin expression and spreading of inflammatory breast cancer cells in three-dimensional culture: a mechanism mediated by IL-8 signaling pathway. Cell Commun Signal. 2012;10:3. doi: 10.1186/1478-811X-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastase A. Pâslaru L. Niculescu AM. Ionescu M. Dumitraşcu T. Herlea V, et al. Prognostic and predictive potential molecular biomarkers in colon cancer. Chirurgia (Bucur) 2011;106:177–185. [PubMed] [Google Scholar]
- Ning Y. Manegold PC. Hong YK. Zhang W. Pohl A. Lurje G, et al. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer. 2011;128:2038–2049. doi: 10.1002/ijc.25562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdenakker G. Van Damme J. The countercurrent principle in invasion and metastasis of cancer cells. Recent insights on the roles of chemokines. Int J Dev Biol. 2004;48:519–527. doi: 10.1387/ijdb.041796go. [DOI] [PubMed] [Google Scholar]
- Osborne CK. Steroid hormone receptors in breast cancer management. Breast Cancer Res Treat. 1998;51:227–238. doi: 10.1023/a:1006132427948. [DOI] [PubMed] [Google Scholar]
- Papaxoinis G. Pectasides DG. Korantzis I. Koutras A. Kosmidis PA. Linardou H, et al. Plasma angiogenic markers in patients with metastatic breast cancer treated with weekly docetaxel. J Clin Oncol. 2010;28:e21004. [Google Scholar]
- Parise CA. Bauer KR. Brown MM. Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999–2004. Breast J. 2009;15:593–602. doi: 10.1111/j.1524-4741.2009.00822.x. [DOI] [PubMed] [Google Scholar]
- Patel PS. Varney ML. Dave BJ. Singh RK. Regulation of constitutive and induced NF-kappaB activation in malignant melanoma cells by capsaicin modulates interleukin-8 production and cell proliferation. J Interferon Cytokine Res. 2002;22:427–435. doi: 10.1089/10799900252952217. [DOI] [PubMed] [Google Scholar]
- Pianetti S. Arsura M. Romieu-Mourez R. Coffey RJ. Sonenshein GE. Her-2/neu overexpression induces NF-kappaB via a PI3-kinase/Akt pathway involving calpain-mediated degradation of IkappaB-alpha that can be inhibited by the tumor suppressor PTEN. Oncogene. 2001;20:1287–99. doi: 10.1038/sj.onc.1204257. [DOI] [PubMed] [Google Scholar]
- Prat A. Baselga J. The role of hormonal therapy in the management of hormonal-receptor-positive breast cancer with co-expression of HER2. Nat Clin Pract Oncol. 2008;5:531–542. doi: 10.1038/ncponc1179. [DOI] [PubMed] [Google Scholar]
- Premkumar VG. Yuvaraj S. Vijayasarathy K. Gangadaran SG. Sachdanandam P. Serum cytokine levels of interleukin-1beta, −6, −8, tumour necrosis factor-alpha and vascular endothelial growth factor in breast cancer patients treated with tamoxifen and supplemented with co-enzyme Q(10), riboflavin and niacin. Basic Clin Pharmacol Toxicol. 2007;100:387–391. doi: 10.1111/j.1742-7843.2007.00065.x. [DOI] [PubMed] [Google Scholar]
- Richmond A. Yang J. Su Y. The good and the bad of chemokines/chemokine receptors in melanoma. Pigment Cell Melanoma Res. 2009;22:175–186. doi: 10.1111/j.1755-148X.2009.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rody A. Karn T. Liedtke C. Pusztai L. Ruckhaeberle E. Hanker L, et al. A clinically relevant gene signature in triple negative and basal-like breast cancer. Breast Cancer Res. 2011;13:R97. doi: 10.1186/bcr3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell K. Hung M-C. Transcriptional repression of the neu protooncogene by estrogen stimulated estrogen receptor. Cancer Res. 1992;52:6624–6629. [PubMed] [Google Scholar]
- Sandhu R. Parker JS. Jones WD. Livasy CA. Coleman WB. Microarray-based gene expression profiling for molecular classification of breast cancer and identification of new targets for therapy. LabMedicine. 2010;41:364–372. [Google Scholar]
- Shahzad A. Knapp M. Lang I. Köhler G. Interleukin 8 (IL-8)—a universal biomarker? Int Arch Med. 2010;3:11. doi: 10.1186/1755-7682-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma B. Singh RK. Emerging candidates in breast cancer stem cell maintenance, therapy resistance and relapse. J Carcinogen. 2011;10:36. doi: 10.4103/1477-3163.91119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan C. Kishimoto H. Fuchs RK. Mehrotra S. Bhat-Nakshatri P. Turner CH, et al. CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006;8:R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B. Berry JA. Shoher A. Lucci A. COX-2 induces IL-11 production in human breast cancer cells. J Surg Res. 2006;131:267–275. doi: 10.1016/j.jss.2005.11.582. [DOI] [PubMed] [Google Scholar]
- Singh JK. Farnie G. Bundred NJ. Simoes BM. Shergill A. Landberg G. Howell SJ. Clarke RB. Targetting CXCR½ significantly reduces breast cancer stem cell activity and increases the efficacy of inhibiting HER2 via HER2-dependent and -independent mechanisms. Clin Cancer Res. 2013;19:643–656. doi: 10.1158/1078-0432.CCR-12-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RK. Gutman M. Radinsky R. Bucana CD. Fidler IJ. Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res. 1994;54:3242–3247. [PubMed] [Google Scholar]
- Singh RK. Lokeshwar BL. Depletion of intrinsic expression of interleukin-8 in prostate cancer cells causes cell cycle arrest, spontaneous apoptosis and increases the efficacy of chemotherapeutic drugs. Mol Cancer. 2009;8:57. doi: 10.1186/1476-4598-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. Shi Q. Bailey ST. Palczewski MJ. Pardee AB. Iglehart JD, et al. Nuclear factor-kappaB activation: a molecular therapeutic target for estrogen receptor-negative and epidermal growth factor receptor family receptor-positive human breast cancer. Mol Cancer Ther. 2007;6:1973–1982. doi: 10.1158/1535-7163.MCT-07-0063. [DOI] [PubMed] [Google Scholar]
- Singh S. Singh AP. Sharma B. Owen LB. Singh RK. CXCL8 and its cognate receptors in melanoma progression and metastasis. Future Oncol. 2010;6:111–116. doi: 10.2217/fon.09.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoussi K. Mahfoudh W. Bouaouina N. Fekih M. Khairi H. Helal AN, et al. Combined effects of IL-8 and CXCR2 gene polymorphisms on breast cancer susceptibility and aggressiveness. BMC Cancer. 2010;10:283. doi: 10.1186/1471-2407-10-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquidi V. Chang M. Dai Y. Kim J. Wolfson ED. Goodison S, et al. IL-8 as a urinary biomarker for the detection of bladder cancer. BMC Urol. 2012;12:12. doi: 10.1186/1471-2490-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandercappellen J. Van Damme J. Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–244. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- Vazquez-Martin A. Colomer R. Menendez JA. Protein array technology to detect HER2 (erbB-2)-induced ‘cytokine signature’ in breast cancer. Eur J Cancer. 2007;43:1117–1124. doi: 10.1016/j.ejca.2007.01.037. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan G. Salgia R. Groopman JE. Chemokine receptors CXCR-1/2 activate mitogen-activated protein kinase via the epidermal growth factor receptor in ovarian cancer cells. J Biol Chem. 2000;275:6868–6875. doi: 10.1074/jbc.275.10.6868. [DOI] [PubMed] [Google Scholar]
- Wang Y. Guo XQ. Niu XL. Wu J. Zhu YQ. Mao LQ. Relationship of IL-6 and IL-8 secretion in epithelial ovarian cancer cell lines with their sensitivity to tamoxifen as well as MAPK, Akt and estrogen receptor phosphorylation. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2010;1:21–24. [PubMed] [Google Scholar]
- Wang Y. Yang J. Gao Y. Du Y. Bao L. Niu W, et al. Regulatory effect of e2, IL-6 and IL-8 on the growth of epithelial ovarian cancer cells. Cell Mol Immunol. 2005;2:365–272. [PubMed] [Google Scholar]
- Waugh DJJ. Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- Wen XF. Yang G. Mao W. Thornton A. Liu J. Bast RC., Jr Le XF. HER2 signaling modulates the equilibrium between pro- and antiangiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene. 2006;25:6986–6996. doi: 10.1038/sj.onc.1209685. [DOI] [PubMed] [Google Scholar]
- Yang J. Wang Y. Gao Y. Shao J. Zhang XJ. Yao Z. Reciprocal regulation of 17beta-estradiol, interleukin-6 and interleukin-8 during growth and progression of epithelial ovarian cancer. Cytokine. 2009;46:382–391. doi: 10.1016/j.cyto.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Yang W. Li-Pai C. Huang R. Huang R-P. Inhibition of IL-6 and IL-8 enhances chemosensitization in multidrug resistant human breast cancer cells. Proc Amer Assoc Cancer Res. 2005;46:5079. doi: 10.1007/s10549-012-2196-0. Abstract No. [DOI] [PubMed] [Google Scholar]
- Yao C. Lin Y. Ye CS. Bi J. Zhu YF. Wang SM. Role of interleukin-8 in the progression of estrogen receptor-negative breast cancer. Chin Med J (Engl) 2007;120:1766–1772. [PubMed] [Google Scholar]
- Yuan A. Chen JJ. Yao PL. Yang PC. The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci. 2005;10:853–865. doi: 10.2741/1579. [DOI] [PubMed] [Google Scholar]
- Zuccari DA. Leonel C. Castro R. Gelaleti GB. Jardim BV. Moscheta MG, et al. An immunohistochemical study of interleukin-8 (IL-8) in breast cancer. Acta Histochem. 2012;114:571–576. doi: 10.1016/j.acthis.2011.10.007. [DOI] [PubMed] [Google Scholar]