Abstract
IL-37 is a new anti-inflammatory cytokine that plays an important role in protecting against tissue injury during infections via limiting immune and inflammatory reactions. This study aimed at determining serum IL-37 concentrations and HBeAg seroconversion in chronic hepatitis B virus (HBV) patients during Telbivudine (LDT) treatment. The serum levels of IL-37 were determined using enzyme-linked immunosorbent assay (ELISA) in 40 chronic hepatitis B virus infection (CHB) patients (HBeAg positive), 30 chronic hepatitis C virus infection (CHC) patients [25 with spontaneously resolved hepatitis C virus (SR-CHC)], and 30 healthy controls (HCs). Anti-inflammatory cytokines such as IL-2 and IL-10 were measured using cytometric bead array, and the concentrations of clinical parameters such as serum hepatitis B surface antigen (HBsAg), hepatitis B nucleocapsid antigen (HBeAg), alanine transaminase (ALT), aspartate transaminase (AST), HBV DNA, and hepatitis C virus (HCV) RNA loads were measured. It was found that the serum levels of IL-37 were higher in chronic HBV patients with high virus loads, but the association was not statistically significant. The serum levels of IL-37 were decreased in HBeAg seroconverted CHB patients after 48 weeks of LDT treatment. The serum levels of IL-37 had no significant difference in CHC patients compared with SR-HCV and HCs. The levels of anti-inflammatory cytokine, IL-2 and IL-10, were lower in CHB and CHC patients than the HC, but IL-2 levels increased after LDT treatment in CHB patients. The concentrations of serum IL-37 in CHB and CHC patients with abnormal levels of serum ALT (>50 U/L) or AST (>40 U/L) were significantly higher than CHB, CHC patients with normal levels of ALT (<50 U/L) or AST (<40 U/L). These results suggest that IL-37 may play a significant role in the immune response of CHB patients with HBeAg seroconversion. The serum levels of IL-37 are associated with liver damage in CHB patients.
Introduction
Both hepatitis B virus (HBV) and hepatitis C virus (HCV) infection are global health problems. Worldwide, 400 million people suffer from chronic HBV infection, and ∼3% of the population worldwide is infected with HCV (Chisari 2005; Rehermann and Nascimbeni 2005). In China, the majority of HBV- as well as HCV-infected individuals become chronic hepatitis patients, which progressively develops into hepatosteatosis, liver fibrosis, liver cirrhosis, and, ultimately, hepatocellular carcinoma (Wang 2003; Zoulim and others 2003). The difference between HBV and HCV infection includes the mechanism of liver injury. Some reports on the pathogenesis of chronic HBV infection have suggested that inflammatory cytokines and lymphocytes participate in the development of liver pathology (Jiang and others 2010). Other reports on chronic HCV infection have showed that the cellular responses triggered by virus infection may participate in the pathogenesis of HCV-associated liver diseases (Ke and Chen 2012). IL-37 acts as an essential inhibitor of inflammation and innate immunity in various diseases (Akdis and others 2011). However, the mechanism of IL-37 in the immune response for the protection against chronic hepatitis B and C virus has not been fully understood.
IL-37 is a new cytokine of the IL-1 family that is expressed in a variety of tissues and cells such as monocytes, Natural killer (NK) cells, and stimulated B cells (Dunn and others 2001; Bufler and others 2002). It includes 5 different splice variants (a–e) (Sharma and others 2008). IL-37 protein can be up-regulated by several pro-inflammatory cytokines and inflammatory stimuli (Nold and others 2010). In addition, expression of IL-37 in macrophages or epithelial cells almost completely inhibited the synthesis of pro-inflammatory cytokines and innate immunocytes (Nold and others 2010; Boraschi and others 2011). Even though the anti-inflammatory mechanism remains unclear, IL-37 is secreted into extracellular space to inhibit the receptor of pro-inflammatory cytokines or locates to the nucleus and interacts intracellularly with Smad3 (Reznikov and others 2002; Boraschi and others 2011). Moreover, it has been reported that IL-37 has a significant protective role in septic shock (Nold and others 2010; Boraschi and others 2011), DSS-induced colitis (McNamee and others 2011), Concanavalin A-Induced Hepatitis (Sakai and others 2012), and Ischemia/Reperfusion-Induced Hepatitis model in-vivo (Bulau and others 2011).
In hepatitis B nucleocapsid antigen (HBeAg)-positive chronic patients, HBV nucleocapsid antigen (HBeAg) likely contributes to the decreased level of core-specific T-cell responses (Bertoletti and Gehring 2006). Furthermore, HBeAg seroconversion is mainly dependent on the host's immune response to HBV (He and others 2012). Thus, this study was conducted to determine serum IL-37 concentrations and HBeAg seroconversion in chronic HBV patients during Telbivudine (LDT) treatment.
Materials and Methods
Patients
A total of 40 patients with CHB, 30 patients with chronic hepatitis C virus infection (CHC) [25 with spontaneously resolved HCV (SR-CHC) infection], and 30 blood donors as healthy controls (HCs) were recruited sequentially at the inpatient service of the Infectious Diseases Division of the Second Part of Jilin University First Hospital. Subjects with chronic HBV infection were confirmed positive for hepatitis B surface antigen (HBsAg), hepatitis B nucleocapsid antigen (HBeAg), and detectable HBV virions for at least 12 months (Yim and Lok 2006). Individuals with chronic HCV infection were confirmed with positive anti-HCV antibodies and serum HCV RNA for at least 6 months (He and others 2012). Individuals with SR-HCV were defined as those subjects who had prior HCV RNA detection (HCV-Ab+) but lacked HCV RNA detection for 12 weeks after enrollment in the absence of drug treatment (Yim and Lok 2006). None of them had been treated with anti-viral and other related drugs, and all patients denied exposure to known hepatotoxin before the treatment. Their demographic and clinical characteristics are shown in Table 1.
Table 1.
The Demographic and Clinical Characteristics of Subjects
| Parameters | Healthy controls | CHB Patients | CHC patients | SR-HCV patients |
|---|---|---|---|---|
| NO. | 30 | 40 | 30 | 25 |
| Age (years) | 35 (21–55) | 38 (24–65) | 41 (23–62) | 39 (21–57) |
| Sex (M/F) | 18/12 | 25/15 | 21/9 | 15/10 |
| HBV DNA (log10 copies/mL) | NA | 7.97a (4.7–9.6) | NA | NA |
| HCV RNA (log10 copies/mL) | NA | NA | 6.27a (3.4–7.3) | 1.61 (1.1–2.6) |
| ALT (U/L) | 14 (5–26) | 180.0a (52.1–479.7) | 45.0a (5–404) | 27 (11–52) |
| AST (U/L) | 13 (8–21) | 151.5a (44.6–448.5) | 35.0a (12–201) | 11 (7–57) |
| HBsAg, pos/neg | Negative | Positive | Negative | Negative |
| HBeAg, pos/neg | Negative | Positive | Negative | Negative |
| Anti-HBe, pos/neg | Negative | Negative | Negative | Negative |
| Anti-HCV | Negative | Negative | Positive | Positive |
Normal values: ALT≤40 IU/L; AST≤40 IU/L; HBV DNA≤3 log10 copies/mL.
P<0.05 versus HC Data were expressed as median and range.
CHB, chronic hepatitis B virus; CHC, chronic hepatitis C virus; HBV, hepatitis B virus; HCV, hepatitis C virus; ALT; AST; NA, not applicable.
The CHB patients were treated orally with 10 mg LDT daily for 24 and 48 weeks. A complete response to LDT treatment was defined as having both HBeAg clearance and serum HBV-DNA level <300 copies/mL. A partial response to LDT treatment was defined as only serum HBV-DNA level <300 copies/mL. Peripheral blood samples were obtained from CHB, CHC, and SR-CHC patients and HC. Sera were prepared and stored at −80°C till needed. Written informed consent was obtained from individual participants, and the study was approved by the First Hospital Ethical Committee of Jilin University.
Measurement of IL-37 by enzyme-linked immunosorbent assay
Serum levels of IL-37 in HC, CHB, CHC, and SR-CHC patients were assayed using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (human IL-37 ELISA; AdipoGen). Briefly, individual sera at 1:1 dilutions in duplicate were subjected to ELISA analysis, and according to the standard curve established using the recombinant IL-37, the concentrations of serum IL-37 in individual samples were calculated. The detection limit of the IL-37 ELISA kit was 0.016–1 ng/mL.
Cytometric bead arrays of serum cytokines
The concentrations of serum IL-2 and IL-10 in HC, CHB, CHC, and SR-CHC patients were determined by cytometric bead array (CBA) (Morgan and others 2004), according to the manufacturer's protocol (BD Biosciences). Briefly, 25 μL of individual sera were used in duplicate for analysis, as previously described (Tárnok and others 2003). The concentrations of serum cytokines were quantified using the CellQuestPro and CBA software (Becton Dickinson) on a FACSCalibur cytometry (BD Biosciences).
Serological and biochemical analysis of hepatitis
The serum HBsAg and HBeAg were detected by a chemiluminescent microparticle immunoassay using an Abbott I 2000 automated chemiluminescence immunoassay analyzer (Abbott Laboratories) (Li and others 2010). The concentrations of serum antibodies against HCV were detected by ELISA II (Abbott Laboratories) (Li and others 2010). The amounts of serum HBV DNA and HCV RNA were measured by quantitative PCR assay using a luciferase quantization detection kit, following the protocols (Roche Amplicor). The detection limit of viral DNA was 300 copies/mL (Wang and others 2012).The levels of serum alanine transaminase (ALT) and aspartate transaminase (AST) were detected using a Biochemistry Automatic Analyzer (Roche Diagnostics).
Statistical analysis
The differences between the groups were analyzed by Mann–Whitney test and Wilcoxon signed rank test using SPSS 18.0 software for unpaired and paired comparisons, respectively. The relationship between variables was evaluated using the Spearman rank correlation test. A 2-side P value<0.05 was considered statistically significant.
Results
Demographic and clinical characteristics of study subjects
The study was conducted on 40 chronic HBV patients (range 24–65 years of age), 30 chronic HCV patients (range 23–62 years of age), 25 SR-HCV patients (range 21–57 years of age), and 30 healthy blood donors (range 21–55 years of age). The concentrations of serum HBsAg, HBeAg, ALT, AST, and HBV DNA in CHB patients were significantly higher than the HC at baseline. The levels of serum HCV RNA loads in CHC patients were higher than the SR-HCV and HCs. Both CHC and SR-HCV patients have a detectable anti-HCV antibody. The data are expressed as median and range unless specified (Table 1).
The serum levels of IL-37 in chronic HBV patients with HBeAg seroconversion
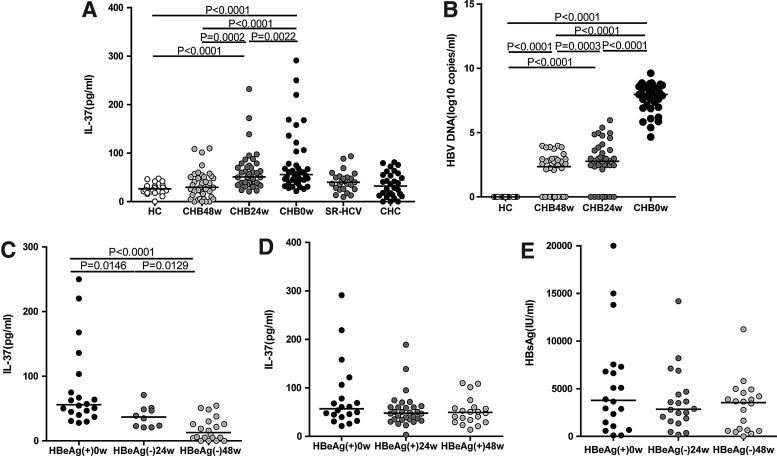
It is known that IL-37 has an anti-inflammatory role and inhibits the immune response in various diseases (Akdis and others 2011). However, the effects of HBV and HCV infection on IL-37 are still unknown. This study, therefore, assessed the levels of serum IL-37 among chronic HBV-infected, long-term LDT-treated patients, CHC and SR-HCV patients, and HCs. The serum levels of IL-37 and HBV DNA in chronic HBV patients were higher than the HCs at baseline, and there were a significant decrease in IL-37 and HBV DNA concentration after 48 weeks of treatment (Fig 1A, P<0.0001; 1B, P<0.0001, respectively). However, the serum levels of IL-37 had no significant difference in CHC and SR-HCV patients compared with the HCs (Fig. 1A). There were no statistically significant correlations between virus loads and serum IL-37 levels. (data not shown). We supposed that other factors may also affect serum IL-37 levels. Based on the LDT therapy outcome on HBeAg clearance or not, the 40 HBeAg-positive CHB patients were divided into 2 groups. 10 out of 40 HBeAg-positive CHB patients had HBeAg seroconversion after 24 weeks of LDT treatment (Fig. 1C). After 48 weeks, 20 out of 40 HBeAg-positive CHB patients had HBeAg clearance. The data revealed that the serum levels of IL-37 were significantly lower in CHB patients with complete HBeAg clearance (Fig. 1C, P<0.0001), whereas it had no significant difference in CHB patients without HBeAg clearance under LDT therapy compared with CHB patients at the baseline (Fig. 1D). The serum levels of HBsAg in HBeAg-seroconverted CHB patients were still higher after LDT treatment (Fig. 1E). From the findings provided earlier, it is possible to confirm that HBeAg was a critical factor that influenced the serum levels of IL-37 in chronic HBV infection during LDT treatment.
FIG. 1.
The levels of serum IL-37 in chronic HBV and HCV infection. (A) The levels of serum IL-37 in HC and total CHB patients at baseline, 24 and 48 weeks after LDT treatment, SR-HCV and CHC patients. (B) The levels of HBV DNA loads in correspondence to (A). (C) The levels of serum IL-37 in HBeAg-seroconverted CHB patients group under LDT therapy. (D) The levels of serum IL-37 in CHB patients without HBeAg seroconversion under LDT therapy. (E) The concentrations of HBsAg in HBeAg-seroconverted CHB patients group. The horizontal lines indicate the median values of different groups. CHB, chronic hepatitis B virus; CHC, chronic hepatitis C virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HC, healthy control; LDT, telbivudine.
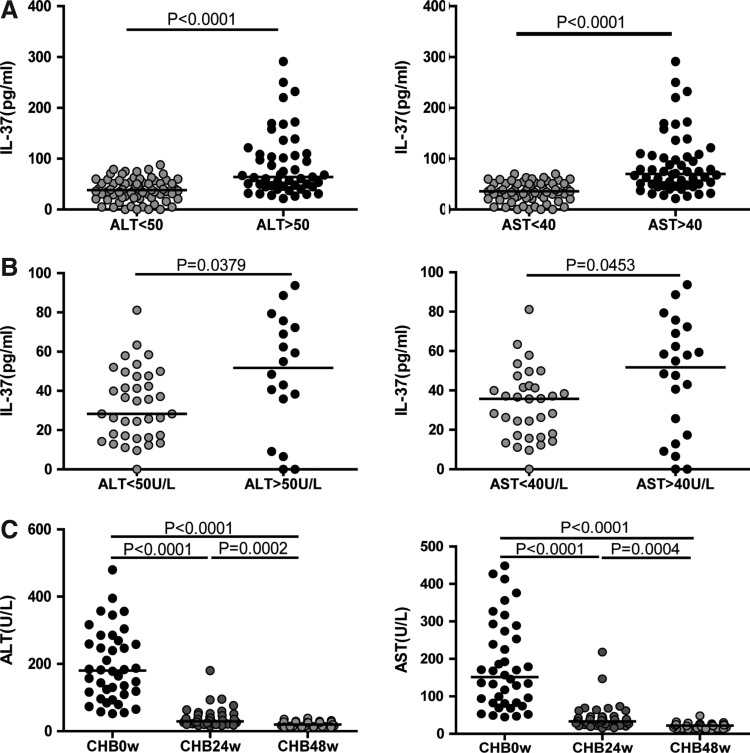
The serum levels of IL-37 in CHB and CHC patients with abnormal levels of serum ALT and AST
Studies have revealed that IL-37 has a protective effect in tissue injury; however, the effect of IL-37 on the liver in CHB and CHC patients is still unknown. In this study, the serum levels of IL-37 in CHB and CHC patients with abnormal serum ALT and AST were compared with those with normal ALT and AST. We found that the concentrations of IL-37 in CHB and CHC patients with abnormal ALT (>50 U/L) or AST (>40 U/L) were significantly higher than those with normal ALT (<50 U/L) or AST (<40 U/L) (Fig. 2A, P<0.0001 and Fig. 2B, P=0.0379, P=0.0453, respectively). In addition, the serum levels of ALT and AST in CHB patients were decreased progressively after LDT treatment (Fig. 2C), but the decrease in serum levels of ALT and AST were less progressive in SR-HCV patients (data not shown).
FIG. 2.
The levels of serum IL-37 influenced by ALT and AST. The concentrations of serum IL-37 in the HBV infection group (A) and HCV infection group (B) with normal and abnormal ALT/AST. (C) The levels of ALT and AST in CHB patients at baseline, 24 and 48 weeks after LDT treatment. The horizontal lines show the median. ALT, alanine transaminase; AST, aspartate transaminase.
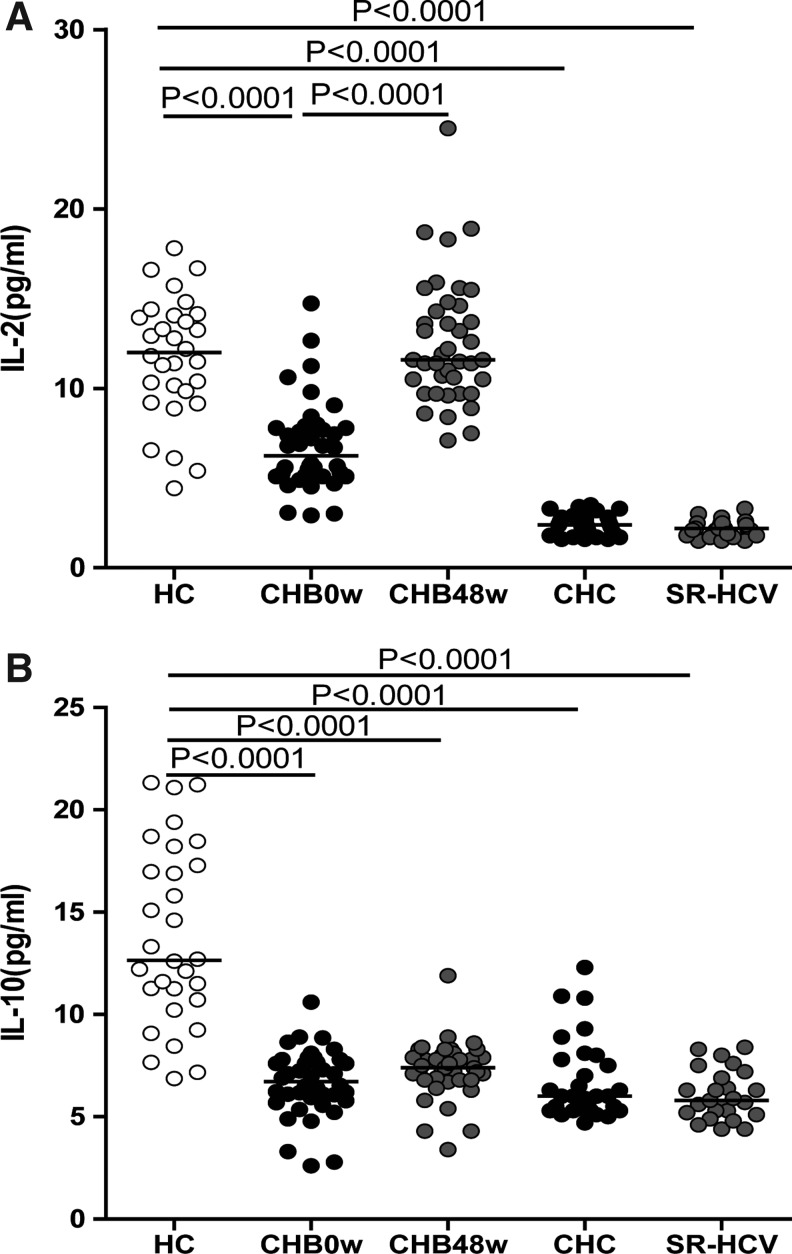
Changes in IL-2 and IL-10 during chronic HBV and HCV infection
Although IL-2, IL-10, and IL-37 have anti-inflammatory characteristics, their roles in chronic HBV and HCV infection are still unknown. The study's result showed that the levels of anti-inflammatory cytokines, IL-2 and IL-10, were lower in CHB and CHC patients compared with the HCs (Fig. 3A, P<0.0001 and Fig. 3B, P<0.0001), and only IL-2 level was increased in CHB patients within LDT treatment compared with CHB patients at baseline (Fig. 3A, P<0.0001).
FIG. 3.
The changes of IL-2 and IL-10 in chronic HBV and HCV infection. The concentrations of anti-inflammatory cytokines IL-2 (A) and IL-10 (B) in the HBV infection group and HCV infection group. The horizontal lines show the median.
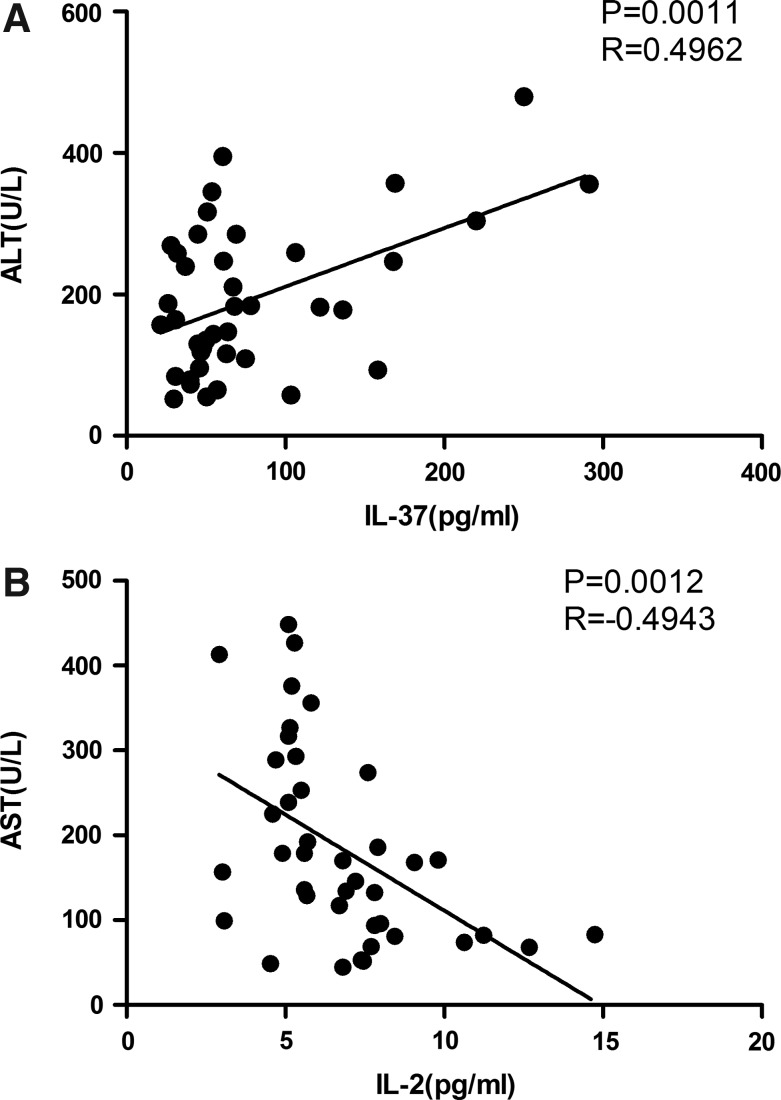
IL-37 and serum ALT in chronic HBV infection
To further determine the roles of IL-37 on the liver injury in CHB patients, the relationship between serum IL-37 and ALT in CHB patients was analyzed. The study found that the levels of serum IL-37 were positively correlated with serum ALT (Fig. 4A, P=0.0011 R=0.4962), and the levels of IL-2 were negatively correlated with serum AST in CHB patients (Fig. 4B, P=0.0012 R=−0.4943).
FIG. 4.
The relationship between serum IL-37 and ALT in CHB patients. (A) The levels of serum IL-37 were positively associated with serum ALT. (B) The levels of cytokine IL-2 were negatively associated with serum AST. The horizontal lines show the median.
Discussion
Even though both HBV and HCV infections contribute to the liver injury, their pathogenesis is completely different. The immunocytes and pro-inflammatory cytokines are involved in the pathogenesis of HBV infection (Jiang and others 2010), whereas the hepatocellular stress response is involved in the pathogenesis of HCV infection (Ke and Chen 2012). Many studies have revealed that the expression of IL-37 was apparently associated with immune cells and inflammation (Nold and others 2008; Boraschi and others 2011; Moschen and others 2011), yet the roles of IL-37 in chronic HBV and HCV infection are not clear. In this work, we examined the levels of serum IL-37 in HBV and HCV infections and in HC. A virus load-dependent increase in the concentrations of IL-37 in HBV infection was observed, but there was no obvious difference in HCV infection. Nold and others have reported that diseased synovial lining in rheumatoid arthritis contained larger amounts of IL-37 compared with HC; supporting the notion that cytokine stimulation induces the production of IL-37, and then, endogenous IL-37 inhibits over-expression inflammation initiated by innate pathways (Nold and others 2010). In HBeAg-positive CHB patients, HBeAg seroconversion and HBV DNA level are the tools that are currently used to follow and predict the response to treatment (He and others 2012). The decreased IL-37 levels only happened in CHB patients with HBeAg clearance. Therefore, IL-37 could be considered a crucial cytokine in HBV infection with HBeAg seroconversion during LDT treatment.
Studies from the mouse model have demonstrated that the expression of IL-37 has protective effects in tissue injury in inflammatory diseases. These effects were related to the roles of IL-37, which acts as an inhibitor of innate immunity and inflammation (Reznikov and others 2002; Nold and others 2010; Boraschi and others 2011). For example, IL-37 expression protected mice from DSS-induced colitis via reducing the recruitment of leukocyte populations (Dentritic cells, Macrophage, Neutrophils, CD4+ T cells, CD8+ T cells, and CD19+ B cells) to the colonic lamina propria and the production of pro-inflammatory cytokines IL-1β, TNF-α but increasing the synthesis of anti-inflammatory cytokines IL-10 (Reznikov and others 2002). In addition, IL-37 also protects mice from LPS-induced shock via reducing the number of activated DCs, Macrophages, NK cells, and CD4+ T cells and the levels of pro-inflammatory cytokines IL-6, IL-1β, IL-17, and IFN-γ in LPS-induced mice (Nold and others 2010). Therefore, IL-37 acts in a feedback mechanism to suppress an exacerbated immune reaction (Boraschi and others 2011). In this study, the concentrations of serum IL-37 in CHB and CHC patients with abnormal levels of serum ALT (>50 U/L) or AST (>40 U/L) were higher than those with normal levels of ALT (<50 U/L) or AST (<40 U/L). Accordingly, we presumed that the increased IL-37 levels were accompanied by higher levels of serum ALT, to inhibit the excessive inflammatory damage.
Both IL-2 and IL-10 have anti-inflammatory characteristics, but it is not known whether their expressions on HBV and HCV are similar to IL-37. IL-2 is mainly produced by Th1 cells, IL-10 is produced by Th2 cells, and other lymphocyte populations can prevent the uncontrolled expansion of immune responses and limit overall inflammation by increasing the number of Treg cells and decreasing the number of TH17 and TFH cells (Banchereau and others 2012). Patients with chronic virus infection show immune tolerance with a strong reduction in CD4+ Th and CD8+ cytotoxic T-lymphocyte responses (Chen and others 2011; Piao and others 2012). Our study also found that there was a significant decrease in the serum levels of IL-2 and IL-10 in CHB and CHC patients than the HCs. Although IL-2 and IL-10 are anti-inflammatory cytokines, the expression of IL-2 and IL-10 in hepatitis B and C infection is not the same as anti-inflammatory cytokine IL-37. After drug treatment, HBeAg-seroconverted patients had a gradually recovered immune system that displayed increased amount of immune cells and cytokines (Kang and others 2006). In this study, only serum levels of IL-2 were increased in HBeAg-seroconverted CHB patients compared with the pretreated patients. A previous study has reported that the balance of Th1/Th2 type cytokines played an important role in the outcome of patients with hepatitis B e antigen-positive chronic hepatitis B treated with LDT (Zhang and others 2009). Therefore, we found that IL-2 secreted by Th1 cells, but not IL-10 produced by Th2 cells, was increased in CHB patients whose pathogenic condition was improved under LDT therapy. Moreover, anti-inflammatory cytokines IL-2 and IL-10 were still lower in SR-HCV patients than the HCs but had no significant differences compared with CHC patients at the baseline; this is because the immune system in SR-HCV patients is not fully back to normal. These results indicated that IL-37, but not IL-2 and IL-10, activated by HBeAg or other factors may play an important role in the immune tolerance stage of chronic HBV infection.
The data collected from CHB patients showed that IL-37 in the pretreatment phase was positively correlated with ALT, but IL-2 was negatively correlated with AST. From this, it is possible to suggest that the serum levels of IL-37 and IL-2 are associated with liver damage in hepatitis B infection.
In conclusion, IL-37 is highly expressed in the serum of HBeAg-positive CHB patients with high virus load and decreased significantly in patients with HBeAg conversion under LDT therapy. HBV virus load, HBe antigen, or other unknown factors may affect the expression of IL-37 in HBV infection. Moreover, HBeAg seroconversion is mainly dependent on the host's immune response to HBV (Kang and others 2006). Therefore, we presumed that IL-37 may play a significant role in the immune response of CHB patients with HBeAg seroconversion. The serum levels of IL-37 were associated with liver damage in CHB patients. Although more detailed studies are necessary to determine the role and the mechanism of IL-37 in regulating the pathogenic process in CHB, our novel findings may provide new insights into understanding the pathogenesis of CHB.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No. 30972610 and 81273240), Jilin Province Science and Technology Agency (No.20110716), the Health Department Research Projects in Jilin Province (2009Z054), and the Cutting-edge Science and Interdisciplinary Innovation Projects of Jilin University.
Author Disclosure Statement
No competing financial interests exist.
References
- Akdis M. Burgler S. Crameri R. Eiwegger T. Fujita H. Gomez E. Klunker S. Meyer N. O'Mahony L. Palomares O. Rhyner C. Ouaked N. Schaffartzik A. Van De Veen W. Zeller S. Zimmermann M. Akdis CA. Interleukins, 1 to 37, and interferon-γ: Receptors, functions, and roles in disease. J Allergy Clin Immunol. 2011;127(3):701–721. doi: 10.1016/j.jaci.2010.11.050. [DOI] [PubMed] [Google Scholar]
- Banchereau J. Pascual V. O'Garra A. From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat Immunol. 2012;13(10):925–931. doi: 10.1038/ni.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoletti A. Gehring AJ. The immune response during hepatitis B virus infection. J Gen Virol. 2006;87(pt6):1439–1449. doi: 10.1099/vir.0.81920-0. [DOI] [PubMed] [Google Scholar]
- Boraschi D. Lucchesi D. Hainzl S. Leitner M. Maier E. Mangelberger D. Oostingh GJ. Pfaller T. Pixner C. Posselt G. Italiani P. Nold MF. Nold-Petry CA. Bufler P. Dinarello CA. IL-37: a new anti-inflammatory cytokine of the IL-1 family. Eur Cytokine Netw. 2011;22(3):127–147. doi: 10.1684/ecn.2011.0288. [DOI] [PubMed] [Google Scholar]
- Bufler P. Azam T. Gamboni-Robertson F. Reznikov LL. Kumar S. Dinarello CA. Kim SH. A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proc Natl Acad Sci U S A. 2002;99(21):13723–13728. doi: 10.1073/pnas.212519099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulau AM. Fink M. Maucksch C. Kappler R. Mayr D. Wagner K. Bufler P. In vivo expression of interleukin-37 reduces local and systemic inflammation in concanavalin a-induced hepatitis. Sci World J. 2011;11:2480–2490. doi: 10.1100/2011/968479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. Li X. Ye B. Yang X. Wu W. Chen B. Pan X. Cao H. Li L. Effect of telbivudine therapy on the cellular immune response in chronic hepatitis B. Antiviral Res. 2011;91(1):23–31. doi: 10.1016/j.antiviral.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Chisari FV. Unscrambling hepatitis C virus-host interactions. Nature. 2005;436(7053):930–932. doi: 10.1038/nature04076. [DOI] [PubMed] [Google Scholar]
- Dunn E. Sims JE. Nicklin MJ. O'Neill LA. Annotating genes with potential roles in the immune system: six new mumbers of the IL-1 family. Trends Immunol. 2001;22(10):533–536. doi: 10.1016/s1471-4906(01)02034-8. [DOI] [PubMed] [Google Scholar]
- He D. Yan G. Wang Y. Serum levels of interleukin-12 in various clinical states with hepatitis B virus infection. Cell Immunol. 2012;272(2):162–165. doi: 10.1016/j.cellimm.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Jiang Y. Ma Z. Xin G. Yan H. Li W. Xu H. Hao C. Niu J. Zhao P. Th1 and Th2 immune response in chronic hepatitis B patients during a long-term treatment with adefovir dipivoxil. Mediat Inflamm. 2010;2010:143026. doi: 10.1155/2010/143026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang EH. Kown TY. Oh GT. Park WF. Park S-I. Park SK. Lee YI. The flavonoid ellagic acid from a medicinal herb inhibits host immune tolerance induced by the hepatitis B virus-e antigen. Antiviral Res. 2006;72:100–106. doi: 10.1016/j.antiviral.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Ke PY. Chen SS. Hepatitis C virus and cellular stress response: implications to molecular pathogenesis of liver diseases. Viruses. 2012;4(10):2251–2290. doi: 10.3390/v4102251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A. Yuan Q. Huang Z. Fan J. Guo R. Lou B. Zheng Q. Ge S. Chen Y. Su Z. Yeo AE. Chen Y. Zhang J. Xia N. Novel double-antigen sandwich immunoassay for human hepatitis B core antibody. Clin Vaccine Immunol. 2010;17(3):464–469. doi: 10.1128/CVI.00457-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamee EN. Masterson JC. Jedlicka P. McManus M. Grenz A. Collins CB. Nold MF. Nold-Petry C. Bufler P. Dinarello CA. Rivera-Nieves J. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci U S A. 2011;108(40):16711–16716. doi: 10.1073/pnas.1111982108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan E. Varro R. Sepulveda H. Ember JA. Apgar J. Wilson J. Lowe L. Chen R. Shivraj L. Agadir A. Campos R. Ernst D. Gaur A. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin Immunol. 2004;110(3):252–266. doi: 10.1016/j.clim.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Moschen AR. Molnar C. Enrich B. Geiger S. Ebenbichler CF. Tilg H. Adipose and liver expression of interleukin(IL)-1 family members in morbid obesity and effects of weight loss. Mol Med. 2011;17(7–8):840–845. doi: 10.2119/molmed.2010.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nold MF. Nold-Petry CA. Zepp JA. Bufler P. Dinarello CA. The IL-1 family member IL-1F7 reduces innate immunity by inhibiting Toll-like receptor and IL-1 signaling pathways. Cytokine. 2008;43(3):264. [Google Scholar]
- Nold MF. Nold-Petry CA. Zepp JA. Palmer BE. Bufler P. Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11(11):1014–1022. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao RL. Liu YY. Tian D. Ma ZH. Zhang M. Zhao C. Niu JQ. Adefovir dipivoxil modulates cytokine expression in Th1/Th2 cells in patients with chronic hepatitis B. Mol Med Rep. 2012;5(1):184–189. doi: 10.3892/mmr.2011.627. [DOI] [PubMed] [Google Scholar]
- Rehermann B. Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5(3):215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- Reznikov LL. Kim SH. Zhou L. Bufler P. Goncharov I. Tsang M. Dinarello CA. The combination of soluble IL-18Rα and IL-18Rβ chains inhibits IL-18-induced IFN-γ. J Interferon Cytokine Res. 2002;22(5):593–601. doi: 10.1089/10799900252982070. [DOI] [PubMed] [Google Scholar]
- Sakai N. Van Sweringen HL. Belizaire RM. Quillin RC. Schuster R. Blanchard J. Burns JM. Tevar AD. Edwards MJ. Lentsch AB. IL-37 reduces liver inflammatory injury via effects on hepatocytes and non-parenchymal cells. J Gastroenterol Hepatol. 2012;27(10):1609–1616. doi: 10.1111/j.1440-1746.2012.07187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. Kulk N. Nold MF. Gräf R. Kim SH. Reinhardt D. Dinarello CA. Bufler P. The IL-1 family member 7b translocates to the nucleus and down-regulates proinflammatory cytokines. J Immunology. 2008;8(180):5477–5482. doi: 10.4049/jimmunol.180.8.5477. [DOI] [PubMed] [Google Scholar]
- Tárnok A. Hambsch J. Chen R. Varro R. Cytometric bead array to measure six cytokines in twenty-five microliters of serum. Clin Chem. 2003;49(6):1000–1002. doi: 10.1373/49.6.1000. [DOI] [PubMed] [Google Scholar]
- Wang FS. Current status and prospects of studies on human genetic alleles associated with hepatitis B virus infection. World J Gastroenterol. 2003;9(4):641–644. doi: 10.3748/wjg.v9.i4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Cai Y. Ji H. Feng J. Ayana DA. Niu J. Jiang Y. Serum IL-33 levels are associated with liver damage in patients with chronic hepatitis B. J Interferon Cytokine Res. 2012;32(6):248–253. doi: 10.1089/jir.2011.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim HJ. Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology. 2006;43(2 Suppl 1):S173–S181. doi: 10.1002/hep.20956. [DOI] [PubMed] [Google Scholar]
- Zhang L. Zhang DZ. Chen M. He H. Guo SH. Dynamic changes of Th1/Th2 type cytokines in peripheral blood of patients with hepatitis B e antigen-positive chronic hepatitis B treated with telbivudine. Zhonghua Gan Zang Bing Za Zhi. 2009;17(3):175–179. [PubMed] [Google Scholar]
- Zoulim F. Chevallier M. Maynard M. Trepo C. Clinical consequences of hepatitis C virus infection. Rev Med Virol. 2003;13(1):57–68. doi: 10.1002/rmv.371. [DOI] [PubMed] [Google Scholar]