Abstract
Interferon-alpha (IFN-α) shows potent immunomodulatory properties, which underlies its use for low-dose oral treatments of diverse viral infections and immunopathological conditions. The studies on oral administration have been hampered by the lack of recognized in vitro models, reproducing the in vivo control action of IFN-α over inflammatory cytokine responses. Owing to these reasons, the aim of our study was to validate IPEC-J2 (a continuous cell line of porcine intestinal epithelial cells) as a reporter system of the properties of IFN-α. Three different experimental conditions (oxidative stress, inflammatory response, and amplification of lymphoid cell signals) were selected to evaluate the effects of porcine recombinant IFN-α1 (rIFN-α) and 2 natural porcine IFN-α preparations (nIFN-α). The IFNs under study showed significantly different control actions in IPEC-J2 cells. In particular, rIFN-α was shown to down-regulate interleukin (IL)-8, IL-1β, tumor necrosis factor (TNF)-α, and β-defensin 1 genes either directly, or indirectly through second messengers released by IFN-α-treated lymphoid cells. With regard to IL-6, only second messengers from IFN-α-treated lymphoid cells could regulate the expression of this cytokine. Our results suggest that IPEC-J2 cells can be a useful tool for investigating the regulatory actions of type I IFNs and the second messengers thereof. The results provided by this model could be conveniently exploited in studies on enteric diseases sustained by infectious or noninfectious stressors.
Introduction
Interferons (IFNs) are proteins named after their capacity to interfere with viral infections of animal cells and are also endowed with immunomodulatory, anti-proliferative, and anti-inflammatory activities (Amadori 2008; Wang and Fish 2012). Three distinct classes of IFN molecules are known to date: Type I, Type II, and Type III IFNs. Type I IFNs are a heterogeneous group including several distinct families: IFN-α, IFN-β, IFN-ɛ, IFN-κ, IFN-ω, IFN-δ, and IFN-τ. These may be associated with distinct profiles of antiviral and anti-tumor activities, as well as of regulation of the T-helper 1/T-helper 2 ratio (Garcia-Sastre 2011; Gajewski 2012). The IFN system plays a pivotal role in the innate immune system as well as in the regulation of the adaptive immune response (Gonzalez-Navajas and others 2012). In addition, recent evidence accumulated in humans, mice, and farm animals points at type I IFN as a crucial homeostatic system that is aimed at avoiding unnecessary tissue damage and waste of food energy due to a dysregulated inflammatory response (Amadori 2007; Trevisi and others 2011).
Among farm animal species, pigs show interesting properties of their Type I IFN system in their response to environmental stressors. In particular, the constitutive expression of several IFN-α subtypes was shown to be modulated in a model of early weaning stress on which IFN-α can exert a regulatory role (Razzuoli and others 2010). Such a regulation is badly needed, as the stress associated to weaning leads to mast cell activation and low feed intake, both of which play a pivotal role in the loss of barrier function of gut (Wijtten and others 2011). In this scenario, a low-dose IFN-α treatment at weaning was shown to be effective, the results being probably due to an anti-inflammatory control action of this cytokine (Amadori and others 2009). Understanding the direct and indirect control actions of oral IFN-α in pigs is difficult, because there are no recognized in vitro models that evaluate the biological effects mentioned earlier after oral administration (Peters and others 2011). For this reason, the objective of this study was to demonstrate the suitability of IPEC-J2 (a continuous line of porcine intestinal epithelial cells) as an in vitro reporter system of the anti-inflammatory control action of IFN-α at different concentrations.
Materials and Methods
Cells and IFNs
IPEC-J2 cells (porcine intestinal epithelial cells, IZSLER Cell Bank code BS CL 205) were grown in Minimum Essential Medium (MEM) enriched with Fetal Calf Serum (FCS) (10% v/v), 2 mM glutamine, and antibiotics (50 μg/mL penicillin, 50 μg/mL streptomycin, and 10 μg/mL neomycin). These cells show a spontaneous secretion of interleukin (IL)-8 and were previously employed in studies on the inflammatory response (Sargeant and others 2011). They have a typical epithelial morphology and are permissive for commensal and pathogenic bacteria; their profile of cytokine and chemokine expression makes them suited for studies on innate immunity. Cells were seeded into 12-well tissue culture plates (2 mL per well, 2×105 cells/mL) and incubated at 37°C in 5% CO2 until confluence (about 24 h).
Porcine recombinant IFN-α1 (rIFN-α) was purchased from PBL Biomedical Laboratories (cat. 17100-1). Its concentration is expressed in terms of U/mL with regard to the international reference standard for human leukocyte IFN (Ga-902-530) provided by National Institutes of Health (Bethesda, MD). Natural porcine IFN-α (nIFN-α) was obtained from Paramyxovirus-stimulated peripheral blood mononuclear cells (PBMC) of 2 different pigs as described in our previous paper (Razzuoli and others 2011a). The concentration of the 3 IFNs under study was measured by ELISA (Razzuoli and others 2011a) and a bioassay on MDBK cells, calibrated with rIFN-α (Meager 1987).
Flow cytometry
Staining of cells for IL-1β was carried out according to established procedures (Schuerwegh and others 2001; Walravens and others 2002), with minor modifications. Samples were analyzed by the A40 Apogee Flow System (Enterprise House, Hertfordshire, United Kingdom). The percentage of positive cells beyond the threshold FL2 fluorescence channel was assessed in each sample on 20,000 events and compared between mAb-treated and control cells using Fischer exact test (threshold for significance set at P<0.05).
Gene expression
The expression of porcine IFN-β, IL-8, IL-6, bD1, bD2, IL-1β, and tumor necrosis factor (TNF)-α was investigated using primer sets described in previous studies (Amadori and others 2009; Veldhuizen and others 2009; Collado-Romero and others 2010; Razzuoli and others 2011b). Porcine β2-microglobulin (B2M) was used as a housekeeping control gene (Table 1). EVA Green Real-time PCR amplification was performed in a CFX96™ Real-time System (Bio-Rad, Milan, Italy) after the reverse transcription step as previously described (Razzuoli and others 2011b). In each sample of IPEC-J2 cells, the relative expression of the selected genes was calculated using the formula ΔCt=Ct (target gene)–Ct (housekeeping), where Ct (cycle of threshold) values are the mean of 3 test replicates±1 standard deviation. Negative samples were given a Ct 40 fictitious value for further statistical examination. The average intensity of expression (mean ΔCt sample-ΔCt negative control) of the genes under study was compared by one-way analysis of variance (ANOVA). The threshold for significance was set at P<0.05.
Table 1.
Oligonucleotide Primer Sequences for Evagreen Quantitative Reverse Transcription Real-Time Polymerase Chain Reaction Amplification of Porcine Genes
| Gene | Protein | Primers | GeneBank gi-number |
|---|---|---|---|
| IL-8 | porcine IL-8 | F: 5′- CTGTACAACCTTCTGCACCCA-3′ | M86923 |
| R: 5′-TTCGATGCCAGTGCATAAATA-3′ | |||
| IL-6 | porcine IL-6 | F: 5′-CAGAGATTTTGCCGAGGATG-3′ | NM_214399 |
| R: 5′-TGGCTACTGCCTTCCCTACC-‘3 | |||
| IL-1β | porcine IL-1β | F: 5′-AATTCGAGTCTGCCCTGTACCC-3′ | NM_001005149 |
| R: 5′-GCCAAGATATAACCGACTTCACCA | |||
| TNF-α | porcine TNF-α | F: 5′-TGCCTACTGCACTTCGAGGTTATC-3′ | NM_214022 |
| R: 5′CAGATAAGCCCGTCGCCCAC-3′ | |||
| βD1 | porcine defensin-1β | F: 5′-TGCCACAGGTGCTCT-3′ | NM_213838 |
| R: 5′CTGTTAGCTGCTTAAGGAATAAAGGC-3′ | |||
| βD2 | porcine defensin-2β | F: 5′-CCAGAGGTCCGACCACTA-3′ | NM_214442 |
| R: 5′-GGTCCCTTCAATCCTGTT-3 | |||
| B2M | Sus scrofa, beta-2-microglobulin | F: 5′CGCCCCAGATTGAAATTGATTTGC 3′ | 397033 |
| R: 5′GCTATACTGATCCACAGCGTTAGG 3′ | |||
| IFNB | porcine IFN-β | F: 5-AGTTGCCTGGGACTCCTCAA-3 | NM_21455 |
| R: 5-CCTCAGGGACCTCAAAGTTCAT-3 |
F, forward primer; R, reverse primer; IFN, interferon.
Assays for cytokine concentrations
Swine IL-8 and IL-1β were measured by commercial ELISA kits as suggested by the manufacturer (R&D system, DUOset cat. DY535 and cat. DY681). Plates were read spectrophotometrically at 492 nm. Cytokine concentrations were calculated from a standard curve that had been created using seven 3-fold dilutions of porcine recombinant IL-8 and IL-1β. Data were analyzed by software Prism 2.01, (Graph Pad Software; Avenida de la Playa, La Jolla, CA); the LOQ (limits of quantification) corresponded to 10 and 5 pg/mL for swine IL-8 and IL-1β, respectively.
TNF-α and IL-6 were measured by 3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyl tetrazolium bromide (MTT)-based biological assays, as previously described (Grenett and others 1991; Asai and others 1993). Cytokine concentrations were calculated from a standard curve that had been created with reference preparations of porcine recombinant TNF-α and IL-6 (Pierce Endogen, Rockford, IL). Depending on the number of cell passages in culture, the LOQ in both tests varied between 5 and 50 pg/mL.
Treatments of IPEC-J2 cells
Each of the following experiments was performed thrice (see Table 2):
Table 2.
Treatments of IPEC-J2 Cells
| Name | Scope | Treatment |
|---|---|---|
| Experiment 1 | Evaluation of IFN-α effects on oxidative stress. | Pretreatment of IPEC-J2 cells with different concentrations of IFN-α. After 2 h of incubation, wash, and induction of oxidative stress. |
| Experiment 2 | Evaluation of IFN-α effects on inflammatory responses. | Treatment of IPEC-J2 cells with different concentrations of LPS and/or IFN-α at 1 or 100 U/mL. |
| Experiment 3 | Evaluation of the ability of IFN-α to amplify lymphoid cell signals. | Treatment of IPEC-J2 cells with supernatants obtained after tonsil cell treatment with IFN-α at 1 or 100 U/mL. |
Oxidative stress
IPEC-J2 cells were seeded and incubated in 12-well tissue culture plates until confluence as described earlier. Six wells were used as an unstimulated control. The others were pretreated with rIFN-α and 2 nIFN-α at 100, 25, 6, and 1 U/mL. After 2 h at 37°C in 5% CO2, cells were washed once with MEM medium and stimulated with 1 μM H2O2 and 2 ng/mL of porcine Tumor Necrosis Factor-α (TNF-α, R&D system, cat. 690-PT) for 18 h. Three out of 6 control wells were treated with MEM only (K−), whereas the 3 wells left (K+) were stimulated with H2O2 and TNF-α as described earlier. After this phase, cells were used for a colorimetric apoptosis test (Titer Tacs, R&D system, cat. TA600) and supernatants for a measurement of IL-1β and IL-8 release.
Inflammatory response
IPEC-J2 cells grown in 96- or 12-well plates were stimulated with lipopolysaccharide (LPS) only (from Escherichia coli O111:B4, Sigma–Aldrich, cat. L4391) at 1, 2, and 4 μg/mL, or LPS (at the same concentrations)+rIFN-α at 1 and 100 U/mL. Untreated cells were used as a negative control. After 18 h of incubation at 37°C in 5% CO2, supernatants were harvested and stored at −80°C for ELISA analyses (IL-1β, IL-8) and bioassays (TNF-α, IL-6). RNA from cells grown in 12-well plates was extracted to evaluate cytokine gene expression. Cells from 96-well plates (treated with LPS only at 0, 1, 2, and 4 μg/mL) were tested for caspase-1 activity (Colorimetric Assay Kit; BIOVISION, San Francisco, CA, cat. K111-100).
Amplification of lymphoid cell signals
Pig tonsils were collected at the slaughterhouse from 5 healthy, 9- to 10-month-old, Landrace x Large White pigs, and processed as previously described (Razzuoli and others 2012). Viable cells were resuspended at 3 million mL–1 and cultivated in RPMI 1640 medium+2×10−5 M 2-mercaptoethanol (2-ME)+10% FCS. Tonsil cells were treated with rIFN-α at 0, 1, and 100 U/mL, and then incubated at 37°C in 5% CO2. Supernatants were harvested 24 h later and stored under aseptic conditions in aliquots at–80°C. IPEC-J2 cells at confluence were washed once with MEM and treated with 1:4 diluted tonsil supernatants for 18 h at 37°C in 5% CO2.
IL-1β and IL-8 were measured in supernatants of IPEC-J2 cells; their total RNA was extracted to evaluate the expression of β2 and β1-defensins (bD1 and bD2), IL-1β, TNF-α, IL-8, and IL-6 genes.
Statistical analysis
Differences in terms of protein release and gene expression after the treatments with different IFN-α preparations were evaluated by one-way ANOVA for repeated measures. The threshold for significance was set at P<0.05.
Results
Flow cytometry
IPEC-J2 cells were shown to produce IL-1β. Under our test conditions, there were 42.5%±6.5% positive cells (difference between mAb-treated and control cell) for intracellular IL-1β (data not shown).
Effects of IFN-α in an oxidative stress model
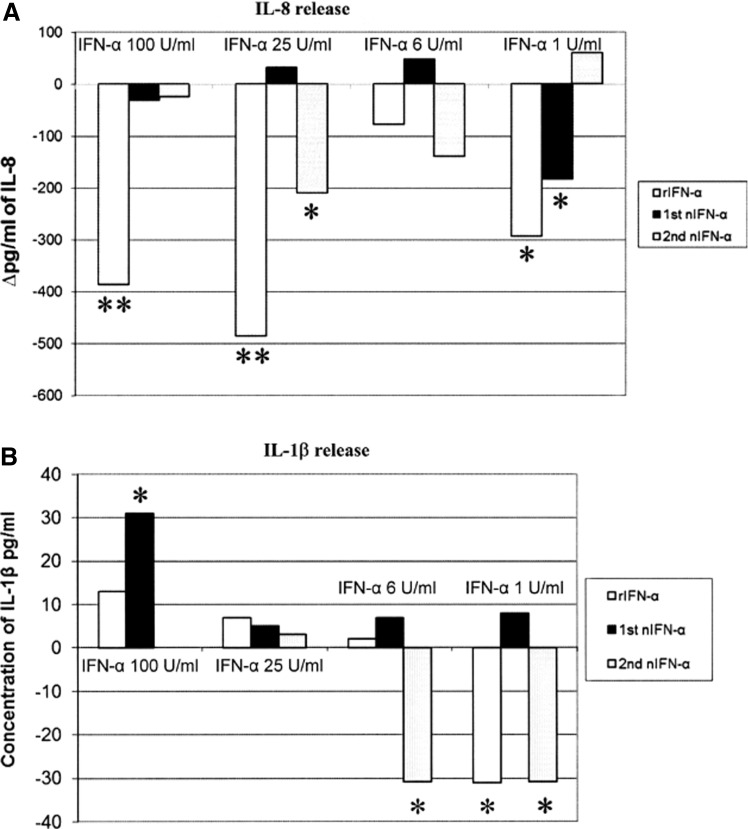
The treatment of IPEC-J2 cells with H2O2 and TNF-α caused a significant (P<0.05) increase (39±2.8 mOD in our colorimetric test) in cell apoptosis with regard to untreated wells. The cells spontaneously released 405±64 pg/mL of IL-8; after treatment with H2O2 and TNF-α, IL-8 release increased to 825±124 pg/mL (+420 pg/mL with regard to the spontaneous release, P<0.05). The IFN-α treatments partly restored the control condition (Fig. 1). Differences in terms of regulatory action on IL-8 release were also shown among different types of IFNs; in particular, 25 U/mL of rIFN-α completely reversed the agonist effect of the applied oxidative stress on the secretion of IL-8 (Fig. 1).
FIG. 1.
Effect of Interferon (IFN)-α treatments on IPEC-J2 cells stimulated with H2O2 and tumor necrosis factor (TNF)-α. IPEC-J2 cells were treated with recombinant (r), or natural (n) IFN-α (2 preparations) at different concentrations (100, 25, 6, and 1 U/mL) or kept as untreated control. Then, they were submitted to oxidative stress by adding TNF-α and H2O2 (2 ng/mL and 1 mM, respectively); supernatants were harvested 18 h later. Results are expressed as a difference between release of IL-8 by IFN α-treated and control wells (A), and a concentration of IL-1β (B). *indicates a significant difference between control and IFN-α treatment (one-way ANOVA for repeated measures, P<0.05) **P<0.01.
Stimulation with TNF-α and H2O2 did not cause any increase of IL-1β release; this occurred after treatment with one of the 2 nIFN-α under study at 100 U/mL, whereas 1 U/mL of rIFN-α and of the second nIFN-α significantly decreased IL-1β release (both −31 pg/mL, Fig. 1).
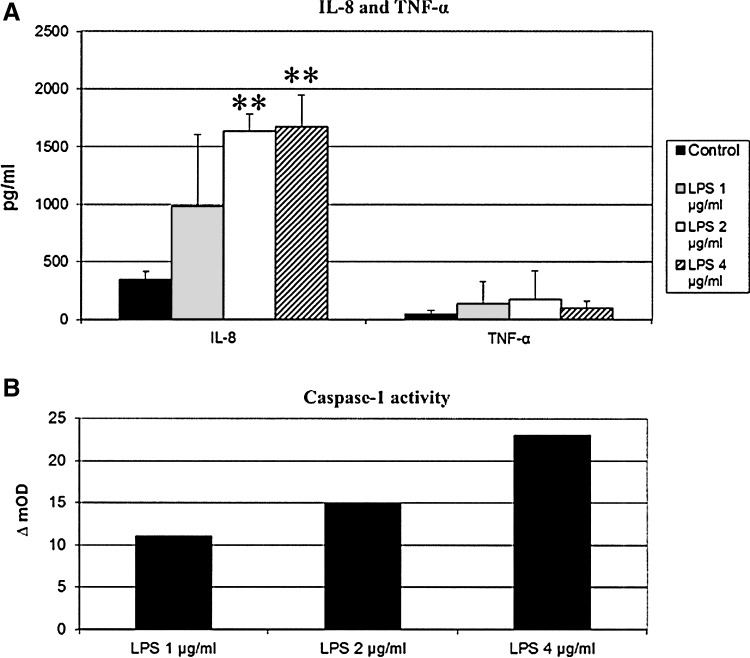
Effect of IFN-α on the LPS-driven inflammatory response
LPS treatments (2 and 4 μg/mL) gave rise to significant increases (P<0.01) of IL-8 release by IPEC-J2 cells (+1,290 and 1,330 pg/mL respectively, see Fig. 2). TNF-α release was also increased but not significantly (Fig. 2), nor were there effects in terms of IL-6 and IL-1β secretion (data not shown). There was instead an increase, albeit not significant, of caspase-1 activity, as shown by our colorimetric test. In particular 1, 2, and 4 μg/mL of LPS caused increases of 11, 15, and 23 mOD in our caspase-1 colorimetric test (nonsignificant differences, see Fig. 2).
FIG. 2.
Inflammatory response of IPEC-J2 cells to different lipopolysaccharide (LPS) concentrations. IPEC-J2 cells were treated with LPS at 1, 2, and 4 μg/mL. After 18 h of incubation, supernatants were harvested and stored at −80°C for ELISA analyses (IL-8, TNF-α, A) and a colorimetric caspase-1 assay (B). Data are expressed as pg/mL of secreted cytokines (A) and OD difference between LPS-stimulated and control wells (ΔmOD, B). **P<0.01.
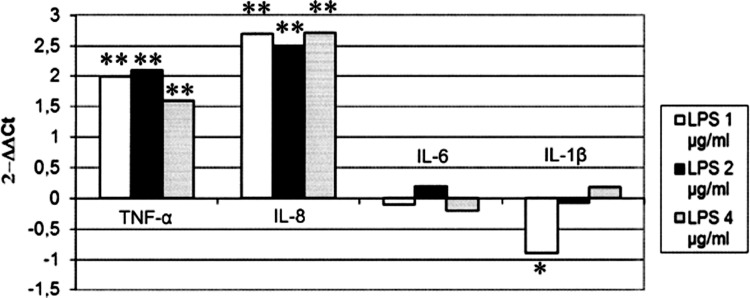
As for gene expression analyses, the LPS treatments significantly increased IL-8 and TNF-α expression (P<0.01) at all concentrations used (Fig. 3). 1 μg/mL of LPS resulted in a significant (P<0.05) decrease of IL-1β expression (Fig. 3), while the other concentrations of LPS under study showed no effects. IL-6 expression was not modulated by any concentration of LPS (Fig. 3), whereas IFN-β gene expression (data not shown) slightly increased after treatment with 2 and 4 μg/mL of LPS (nonsignificant).
FIG. 3.
Effects of LPS on the expression of cytokine genes in IPEC-J2 cells. IPEC-J2 cells were treated with LPS at 1, 2, and 4 μg/mL. Untreated cell cultures were used as negative controls. After 18 h of incubation, RNA was extracted to evaluate cytokine gene expression by RT Real-time PCR. Results are expressed as 2−ΔΔCt (ΔCt cell control - ΔCt treatment). *indicates a significant difference between control and LPS treatment (one-way ANOVA for repeated measures). The threshold for significance was set at P<0.05. *P<0.05 **P<0.01.
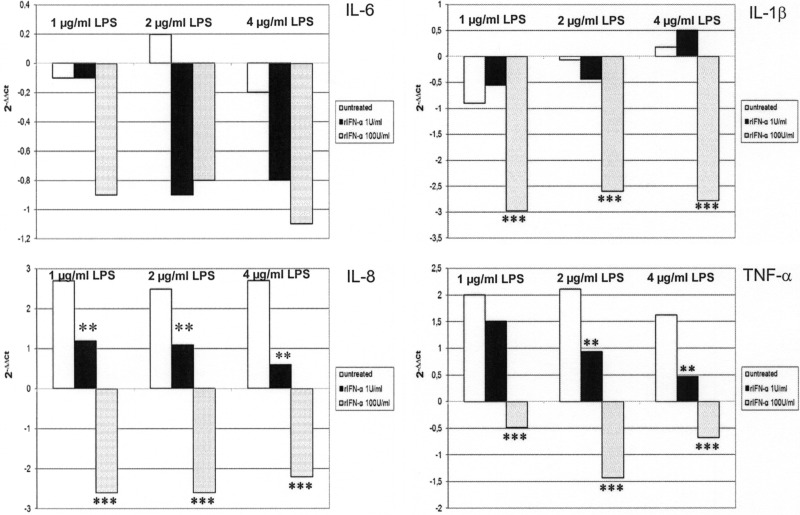
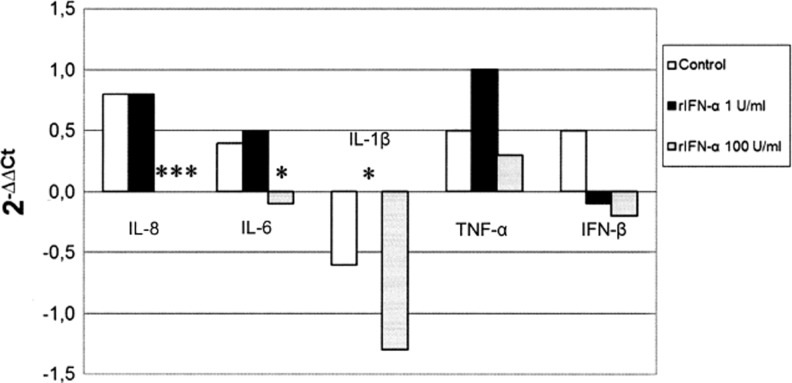
With the exception of IL-6, treatment with 100 U/mL of rIFN-α significantly decreased (Fig. 4) pro-inflammatory cytokine gene expression at all tested LPS concentrations (IL-1β P<0.001, IL-8 P<0.001, and TNF-α P<0.001). TNF-α and IL-8 expression was significantly up-regulated (P<0.01) after IFN-α treatment at a much lower concentration (1 U/mL). In addition, IFN-β was significantly (P<0.05) modulated (−1.24 ΔΔCt) in cells treated with 4 μg/mL of LPS and 1 U/mL of IFN-α but not at other LPS/IFN combinations (data not shown).
FIG. 4.
IFN-α effects on cytokine gene expression in IPEC-J2 cells. IPEC-J2 cells were treated with LPS only at 1, 2, and 4 μg/mL or LPS (at the same concentrations)+rIFN-α at 1 and 100 U/mL. Untreated cells were used as a negative control. After 18 h of incubation, RNA from cells was extracted to evaluate cytokine gene expression. Data are expressed as 2−ΔΔCt, where ΔΔCt=(ΔCt of untreated cells)- (ΔCt of treated cells). The asterisks indicate significant differences between control and IFN-α treatments (one way ANOVA). **P<0.01 ***P<0.001.
IPEC-J2 as reporter system of signals generated by lymphoid cells
In this experiment, IPEC-J2 cells did not release IL-1β. Treatment of IPEC-J2 with tonsil cell supernatants obtained after 24 h in culture resulted in a significant increase of IL-8 release (P<0.05). The same effect was not observed using supernatants of tonsil lymphocytes treated with rIFN-α (Table 3).
Table 3.
Effects of Tonsil Cell Supernatants on Spontaneous Interleukin-8 Release by IPEC J2 Cells
| Treatment | IL-8 (pg/mL±1 SD) |
|---|---|
| IPEC-J2 untreated cells | 565±207 |
| IPEC-J2 cells+tonsil cell supernatant | 1055±338* |
| IPEC-J2 cells+tonsil cell supernatant+rIFN-α at 100 U/mL | 820±244 |
| IPEC-J2 cells+tonsil cell supernatant+rIFN-α at 1 U/mL | 820±348 |
Pig tonsils were collected at the slaughterhouse. Viable tonsil mononuclear leukocytes of 5 healthy pigs were resuspended at 3 million mL–1 and cultivated in RPMI 1640 medium+2-mercaptoethanol (5×10−4 M)+10% fetal calf serum. Tonsil cells were treated with rIFN-α at 0, 1, and 100 U/mL, and then incubated at 37°C in 5% CO2. Supernatants were harvested 24 h later and stored under aseptic conditions in aliquots at −80°C. IPEC-J2 cells at confluence were washed once with MEM and treated with 1:4 diluted tonsil cell supernatants for 18 h at 37°C in 5% CO2. Supernatants were harvested 18 h later and stored under aseptic conditions in aliquots at −80°C for an IL-8 ELISA assay.
Results are shown in terms of pg/mL of IL-8±1 standard deviation in 5 tests. The asterisk indicates a significant difference with regard to untreated control cells (one-way ANOVA for repeated measures, P<0.05).
IL, interleukin.
P<0.05.
No significant effect was shown in terms of TNF-α and IFN-β gene expression after treatment with control tonsil cell supernatant (Fig. 5). With regard to IL-8 and IL-6, stimulation with control tonsil cell supernatants significantly increased cytokine gene expression (P<0.01 and P<0.05 respectively) with regard to untreated IPEC-J2 cells. The treatment with supernatant of tonsil cells stimulated with 100 U/mL of rIFN-α restored the initial expression levels of IL-8 and IL-6 in IPEC-J2 cells, whereas no effect was observed with supernatants of tonsil cells treated with 1 U/mL of rIFN-α. With regard to IL-1β, treatment with control tonsil supernatant nonsignificantly decreased the expression of this cytokine; the value of untreated IPEC-J2 cells was observed after stimulation with tonsil supernatant treated with 1 U/mL of rIFN-α (P<0.05, Fig. 5); no significant effect was instead observed with supernatant of tonsil cells treated with 100 U/mL of rIFN-α.
FIG. 5.
Indirect effects of IFN-α on cytokine gene expression in IPEC-J2 cells. Pig tonsil cells were treated with rIFN-α at 0, 1, and 100 U/mL, at 37°C in 5% CO2. Supernatants were harvested 18 h later and stored under aseptic conditions in aliquots at–80°C. IPEC-J2 cells at confluence were washed once with MEM medium and treated with 1:4 diluted tonsil supernatants for 18 h at 37°C in 5% CO2. RNA of IPEC-J2 cells was extracted to evaluate the expression of IL-1β, TNF-α, IL-8, IL-6, and IFN-β genes. Data are expressed as 2−ΔΔCt where ΔΔCt=(ΔCt of untreated cells)- (ΔCt of treated cells). The asterisks indicate significant differences between IPEC-J2 cells exposed to supernatants of control and IFN α-treated tonsil cells, respectively (one-way ANOVA). *P<0.05 ***P<0.001.
Antimicrobial peptide expression: direct and indirect modulation
With regard to β1 and 2 defensin gene expressions, there were no significant effects of LPS on IPEC-J2 cells (data not shown). After IFN-α stimulation, direct or indirect effects on β2-defensin gene expression were not shown (Table 4). On the contrary, the β1-defensin gene was significantly down-regulated with regard to control IPEC-J2 cells by direct treatment with 100 or 1 U/mL of rIFN-α, and also by supernatants of tonsil cells treated with 100 U/mL of rIFN-α (Table 4).
Table 4.
Direct and Indirect Effects of Interferon-Alpha on β-Defensin Gene Expression
| Control | β1-Defensin (ΔCt±1 SD) | β2-Defensin (ΔCt±1 SD) |
|---|---|---|
| IPEC-J2 untreated cells | 6.3±0.3 | 13.9±1.5 |
| Treatment/indirect effects | ||
| IPEC-J2+tonsil supernatant | 6.4±0.15 | 13.5±1.2 |
| IPEC-J2+tonsil supernatant+rIFN-α 100 UI/mL | 7.5±0.05*** | 13.8±1.3 |
| IPEC-J2+tonsil supernatant+rIFN-α 1 UI/mL | 6.6±0.2 | 14.1±1.5 |
| Treatment/direct effects | ||
| IPEC-J2+rIFN-α 100 UI/mL+LPS 1 μg/mL | 10.5±0.4*** | 15.3±1.9 |
| IPEC-J2+rIFN-α 1 UI/mL+LPS 1 μg/mL | 9.1±0.5** | 15.2±0.6 |
| IPEC-J2+rIFN-α 100 UI/mL+LPS 2 μg/mL | 11±0.2*** | 16.4±1.7 |
| IPEC-J2+rIFN-α 1 UI/mL+LPS 2 μg/mL | 8.6±0.07* | 16±0.2 |
| IPEC-J2+rIFN-α 100 UI/mL+LPS 4 μg/mL | 10.8±0.01*** | 16.3±1.2 |
| IPEC-J2+rIFN-α 1 UI/mL+LPS 4 μg/mL | 8.7±0.07* | 15.5±0.7 |
IPEC-J2 cells were submitted to the indicated treatments for 18 h at 37°C. Total RNA was extracted to evaluate the expression of β2 and β1-defensin genes by reverse-transcription Real-time PCR. Results are shown in terms of ΔCt±1 standard deviation. Asterisks indicate significant differences with regard to untreated cells (one-way ANOVA for repeated measures).
P<0.001.
P<0.01.
P<0.05.
Discussion
Type I IFNs represent a crucial link between innate and adaptive immune responses, which has stimulated many investigations into their role in established disease models (Gonzalez-Navajas and others 2012). These cytokines cause concentration-dependent effects: high doses of IFN-α (≥100 U/mL) usually show anti-proliferative, antiviral, and pro-inflammatory activities; whereas low doses (<10 U/mL) show preferential immuno-modulatory and anti-inflammatory activities (Amadori 2007). In this scenario, Type I IFNs act as important homeostatic agents in the control of environmental, noninfectious stressors such as early weaning in pigs, which causes transient anorexia and up-regulation of inflammatory cytokine genes in both proximal and distant tracts of the small intestine (McCracken and others 1999; Pie and others 2004). In this model, the control circuits of the inflammatory response can be adequately targeted by Type I IFNs to prevent its excessive amplification at weaning and the related disease losses (Amadori and others 2012). These findings prompted us to develop an in vitro model depicting the critical interaction between type I IFNs and intestinal epithelial cells and the relevant regulation of the inflammatory response. The IPEC-J2 line was developed from the jejunal epithelial cells of a newborn, nonfed piglet, and it represents a good model to investigate the pathogenesis of microbial intestinal infections (Schierack and others 2006; Liu and others 2010). In a comprehensive study, the complete morphological and functional characterization of these cells was presented, and their suitability for microbiological studies was demonstrated (Brosnahan and Brown 2012); in addition, the expression of mRNAs for pro-inflammatory cytokines and chemokines such as IL-6, IL-8, and TNF-α was confirmed (Mariani and others 2009). This profile was further characterized in our study, which demonstrated the ability of this cell line to produce IL-1β and to express both IL-1β and IFN-β genes. These features, along with the production of other pro-inflammatory cytokines, characterize IPEC-J2 cells as a good model for studies on gut inflammation and enteric disease (Zhou and others 2012).
In our work, this cell line was used to investigate IFN-α in a model based on LPS and oxidative stress-driven changes of both expression and secretion of inflammatory cytokines and chemokines (IL-8); the choice of an oxidative stress approach is relevant to the present lean-type pig phenotypes, characterized by high plasma concentrations of Reactive Oxygen Metabolites under resting conditions, as a result of a major imbalance between cardio-circulatory system and development of the muscular mass (Brambilla and others 2002). In this regard, previous studies had shown that IPEC-J2 cells secrete cytokines and chemokines as a response to oxidative stress (Paszti-Gere and others 2012). These results were confirmed in our study, whereby an oxidative stress caused a significant increase of IL-8 release. Interestingly, pretreatment of IPEC-J2 cells with different types of IFN-α could significantly decrease such a response.
With regard to natural, nonpurified IFNs, the different results obtained in terms of inflammatory response could be related to the presence of contaminating cytokines or to different ratios among IFN-α subtypes. This assumption is in line with our previous study, where we demonstrated in vitro the expression of different porcine IFN-α subtypes after PBMC stimulation with Newcastle Disease Virus (NDV) LaSota strain (Razzuoli and others 2011a). These subtypes can show wide differences in terms of antiviral activities (Sang and others 2010), but at the moment, there is no precise information about their different anti-inflammatory potentials. For this reason, we decided to use only rIFN-α for the subsequent experiments.
Treatment with different concentrations of LPS caused an inflammatory response in terms of IL-8 gene expression and protein secretion. Interestingly, no significant effect was detected in terms of TNF-α, IL-1β, and IL-6 protein release. In addition, caspase-1 activity was not significantly increased by LPS stimulation. It should be stressed that inflammatory cytokines are regulated by both gene expression and mRNA stability in the cytoplasm; an increase of IL-1β and TNF-α also causes a compensatory release of IL-6 (Myers and Murtaugh 1995). Accordingly, in our study, LPS stimulation caused a significant increase of TNF-α gene expression but not of cytokine release, nor was LPS stimulation associated to an increased expression of IL-6 and IL-1β genes. These results differ from the data obtained in human macrophages and monocytes, where LPS causes an increase of all the cytokines mentioned earlier through TLR4/NF-KB signaling; remarkably, these latter components are expressed in IPEC-J2 cells as well (Myers and Murtaugh 1995; Mariani and others 2009). The absence of IL-1β and IL-6 responses to LPS in IPEC-J2 cells could represent a form of endotoxin tolerance, that is, a physiological condition of intestinal epithelial cells that are refractory to inflammatory signals of commensal bacteria under healthy gut conditions (Lotz and others 2006).
On the basis of the findings cited earlier, we investigated the activity of IFN-α in the same experimental model. Our results highlight the ability of a moderate concentration of IFN-α (100 U/mL) to regulate the LPS-driven inflammatory response in terms of pro-inflammatory cytokine gene expression. 1 U/mL of rIFN-α showed an opposite regulation in terms of IL-8 and TNF-α gene expression, whereas no control action was exerted on IL-1β. In a global view, the lack of a control action on IL-1β at low IFN−α concentrations would not be of concern because of further biological check points; among these, the availability of Caspase-1 through the inflammosome reaction is likely to play a major role (Rathinam and others 2012).
The observed control action of IFN-α on inflammatory cytokines could be performed through different, nonmutually exclusive dose-dependent pathways: mRNA stability control by tristetraprolin induction (Anderson and others 2004), TAM receptor-mediated activation of SOCS proteins through IFNAR I signaling (Lemke and Rothlin 2008), and down-regulation of CD14 expression (Begni and others 2005).
With regard to the indirect effects of IFN-α, supernatants of untreated tonsil cells caused a moderate inflammatory response in IPEC-J2 cells, as opposed to supernatants of tonsil cells treated with rIFN-α. In particular, untreated tonsil cell supernatants up-regulated IL-8 and IL-6 gene expression. This might be related to IL-6 inducing cytokines such as TNF-α or IL-1β released by tonsil cells (Murtaugh 1994). IFN-α could inhibit their expression, or induce anti-inflammatory components such as IL-10 (Ouyang and others 2011). Interestingly, supernatants of tonsil cells treated with 1 U/mL of IFN-α kept IL-1β expression at the levels observed in untreated IPEC-J2 cells (Fig. 5).
β-defensins were included in our study because of their involvement in the regulation of the inflammatory response (Yang and others 2002). In addition, a previous study (Mariani and others 2009) showed that IPEC-J2 cells constitutively express porcine bD1 and (to a lesser extent) bD2; this latter defensin is also expressed in the small intestine (Sang and others 2006), which justifies the use of IPEC-J2 cells as a useful investigation model of β-defensin expression. Treatment of IPEC-J2 cells with LPS caused no effects in terms of β-defensin expression; this finding is in agreement with the results by Zhang and co-workers (Zhang and others 1999), who demonstrated that porcine bD1 activity was not inducible by an inflammatory stimulation (LPS, TNF-α, IL-1); this is probably due to the lack of consensus binding sites in the defensin promoter region for both NF-kB and NF-IL6 (Yang and others 2002). We also demonstrated the ability of IFN-α to down-regulate bD1 expression (Table 3); our findings are compatible with both a direct regulation on the bD1 gene and an indirect action of IFN-α through second messengers secreted by tonsil cells, IPEC-J2 cells, or both (experiments B and C, see Materials and Methods).
In conclusion, our results indicate that IPEC-J2 cells can be employed as a model for in vitro studies on the regulation of the intestinal inflammatory response, the onset and the course of enteric disease. Moreover, our results show different control actions of nIFN-α and rIFN-α, as well as different effects of nIFN-α depending on the PBMC cultures employed for IFN induction. In addition, our results show that a substantial regulation of the inflammatory response is exerted at moderate concentrations of IFN-α (100 U/mL), which may be found at the initial stages of a microbial infection. In a global view, this might imply that pro and anti-inflammatory control actions of Type I IFNs take place at the same time on different cellular targets. Among these, intestinal epithelial cells need a very stringent control of their response to enteric infections and/or dysbiosis of intestinal microbiota; this can be accounted for by the state of controlled gut inflammation in the presence of commensal bacteria.
Acknowledgments
The authors want to thank C. Mantovani and G. Ferrari for their skillful technical assistance; their work is gratefully acknowledged.
This study was partly supported by the Italian Ministry of Health, grant PRF2010006, and a EU EPIZONE Grant.
Author Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- Amadori M. The role of IFN-alpha as homeostatic agent in the inflammatory response: a balance between danger and response? J Interferon Cytokine Res. 2007;27(3):181–189. doi: 10.1089/jir.2006.0110. [DOI] [PubMed] [Google Scholar]
- Amadori M. Physiological response and constitutive expression of interferons. Role and functions. In: Durand M, editor; Morel C, editor. New research on innate immunity. New York: Nova Science Publisher; 2008. pp. 1–11. [Google Scholar]
- Amadori M. Farinacci M. Begni B. Faita R. Podavini D. Colitti M. Effects of interferon-alpha on the inflammatory response of swine peripheral blood mononuclear cells. J Interferon Cytokine Res. 2009;29(4):241–247. doi: 10.1089/jir.2008.0074. [DOI] [PubMed] [Google Scholar]
- Amadori M. Razzuoli E. Nassuato C. Issues and possible intervention strategies relating to early weaning of piglets. CAB Rev. 2012;7(046):1–15. [Google Scholar]
- Anderson P. Phillips K. Stoecklin G. Kedersha N. Post-transcriptional regulation of proinflammatory proteins. J Leukoc Biol. 2004;76(1):42–47. doi: 10.1189/jlb.1103536. [DOI] [PubMed] [Google Scholar]
- Asai T. Okada M. Ono M. Irisawa T. Mori Y. Yokomizo Y. Sato S. Increased levels of tumor necrosis factor and interleukin 1 in bronchoalveolar lavage fluids from pigs infected with mycoplasma hyopneumoniae. Vet Immunol Immunopathol. 1993;38(3–4):253–260. doi: 10.1016/0165-2427(93)90085-i. [DOI] [PubMed] [Google Scholar]
- Begni B. Amadori M. Ritelli M. Podavini D. Effects of IFN-alpha on the inflammatory response of swine leukocytes to bacterial endotoxin. J Interferon Cytokine Res. 2005;25(4):202–208. doi: 10.1089/jir.2005.25.202. [DOI] [PubMed] [Google Scholar]
- Brambilla G. Civitareale C. Ballerini A. Fiori M. Amadori M. Archetti LI. Regini M. Betti M. Response to oxidative stress as a welfare parameter in swine. Redox Rep. 2002;7(3):159–163. doi: 10.1179/135100002125000406. [DOI] [PubMed] [Google Scholar]
- Brosnahan AJ. Brown DR. Porcine IPEC-J2 intestinal epithelial cells in microbiological investigations. Vet Microbiol. 2012;156(3–4):229–237. doi: 10.1016/j.vetmic.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado-Romero M. Arce C. Ramirez-Boo M. Carvajal A. Garrido JJ. Quantitative analysis of the immune response upon salmonella typhimurium infection along the porcine intestinal gut. Vet Res. 2010;41(2):23. doi: 10.1051/vetres/2009072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski TF. Cancer immunotherapy. Mol Oncol. 2012;6(2):242–250. doi: 10.1016/j.molonc.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sastre A. Induction and evasion of type I interferon responses by influenza viruses. Virus Res. 2011;162(1–2):12–18. doi: 10.1016/j.virusres.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Navajas JM. Lee J. David M. Raz E. Immunomodulatory functions of type I interferons. Nat Rev Immunol. 2012;12(2):125–135. doi: 10.1038/nri3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenett HE. Danley DE. Strick CA. James LC. Otterness IG. Fuentes N. Nesbitt JE. Fuller GM. Isolation and characterization of biologically active murine interleukin-6 produced in escherichia coli. Gene. 1991;101(2):267–271. doi: 10.1016/0378-1119(91)90422-8. [DOI] [PubMed] [Google Scholar]
- Lemke G. Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8(5):327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. Li G. Wen K. Bui T. Cao D. Zhang Y. Yuan L. Porcine small intestinal epithelial cell line (IPEC-J2) of rotavirus infection as a new model for the study of innate immune responses to rotaviruses and probiotics. Viral Immunol. 2010;23(2):135–149. doi: 10.1089/vim.2009.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M. Gutle D. Walther S. Menard S. Bogdan C. Hornef MW. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med. 2006;203(4):973–984. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani V. Palermo S. Fiorentini S. Lanubile A. Giuffra E. Gene expression study of two widely used pig intestinal epithelial cell lines: IPEC-J2 and IPI-2I. Vet Immunol Immunopathol. 2009;131(3–4):278–284. doi: 10.1016/j.vetimm.2009.04.006. [DOI] [PubMed] [Google Scholar]
- McCracken BA. Spurlock ME. Roos MA. Zuckermann FA. Gaskins HR. Weaning anorexia may contribute to local inflammation in the piglet small intestine. J Nutr. 1999;129(3):613–619. doi: 10.1093/jn/129.3.613. [DOI] [PubMed] [Google Scholar]
- Meager A. Quantification of interferons by anti-viral assays and their standardization. In: Clemens MJ, editor; Morris AG, editor; Gearing AJH, editor. Lymphokines and interferons, a practical approach. Oxford: IRL Press; 1987. pp. 129–147. [Google Scholar]
- Murtaugh MP. Porcine cytokines. Vet Immunol Immunopathol. 1994;43(1–3):37–44. doi: 10.1016/0165-2427(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Myers MJ. Murtaugh MP. Biology of tumor necrosis factor. In: Myers MJ, editor; Murtaugh MP, editor. Cytokines in animal health and disease. New York: Marcel Dekker, Inc; 1995. pp. 121–151. [Google Scholar]
- Ouyang W. Rutz S. Crellin NK. Valdez PA. Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- Paszti-Gere E. Csibrik-Nemeth E. Szeker K. Csizinszky R. Jakab C. Galfi P. Acute oxidative stress affects IL-8 and TNF-alpha expression in IPEC-J2 porcine epithelial cells. Inflammation. 2012;35(3):994–1004. doi: 10.1007/s10753-011-9403-8. [DOI] [PubMed] [Google Scholar]
- Peters SM. Yancy H. Bremer E. Monroe J. Paul D. Stubbs JT., 3rd Myers MJ. In vitro identification and verification of inflammatory biomarkers in swine. Vet Immunol Immunopathol. 2011;139(1):67–72. doi: 10.1016/j.vetimm.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Pie S. Lalles JP. Blazy F. Laffitte J. Seve B. Oswald IP. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J Nutr. 2004;134(3):641–647. doi: 10.1093/jn/134.3.641. [DOI] [PubMed] [Google Scholar]
- Rathinam VA. Vanaja SK. Fitzgerald KA. Regulation of inflammasome signaling. Nat Immunol. 2012;13(4):333–332. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzuoli E. Villa R. Sossi E. Amadori M. Characterization of the interferon-alpha response of pigs to the weaning stress. J Interferon Cytokine Res. 2011a;31(2):237–247. doi: 10.1089/jir.2010.0041. [DOI] [PubMed] [Google Scholar]
- Razzuoli E. Villa R. Sossi E. Amadori M. Reverse transcription real-time PCR for detection of porcine interferon alpha and beta genes. Scand J Immunol. 2011b;74(4):412–418. doi: 10.1111/j.1365-3083.2011.02586.x. [DOI] [PubMed] [Google Scholar]
- Razzuoli E. Dotti S. Archetti IL. Amadori M. Clinical chemistry parameters of piglets at weaning are modulated by an oral, low-dose interferon-alpha treatment. Vet Res Commun. 2010;34(Suppl 1):S189–S192. doi: 10.1007/s11259-010-9402-5. [DOI] [PubMed] [Google Scholar]
- Razzuoli E. Faggionato E. Dotti S. Villa R. Lombardo T. Boizza L. Ferrari M. Amadori M. Isolation and culture of pig tonsil lymphocytes. Vet Immunol Immunopathol. 2012;148(3–4):320–325. doi: 10.1016/j.vetimm.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Sang Y. Rowland RR. Hesse RA. Blecha F. Differential expression and activity of the porcine type I interferon family. Physiol Genomics. 2010;42(2):248–258. doi: 10.1152/physiolgenomics.00198.2009. [DOI] [PubMed] [Google Scholar]
- Sang Y. Patil AA. Zhang G. Ross CR. Blecha F. Bioinformatic and expression analysis of novel porcine beta-defensins. Mamm Genome. 2006;17(4):332–339. doi: 10.1007/s00335-005-0158-0. [DOI] [PubMed] [Google Scholar]
- Sargeant HR. Miller HM. Shaw MA. Inflammatory response of porcine epithelial IPEC J2 cells to enterotoxigenic E. coli infection is modulated by zinc supplementation. Mol Immunol. 2011;48(15–16):2113–2121. doi: 10.1016/j.molimm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Schierack P. Nordhoff M. Pollmann M. Weyrauch KD. Amasheh S. Lodemann U. Jores J. Tachu B. Kleta S. Blikslager A, et al. Characterization of a porcine intestinal epithelial cell line for in vitro studies of microbial pathogenesis in swine. Histochem Cell Biol. 2006;125(3):293–305. doi: 10.1007/s00418-005-0067-z. [DOI] [PubMed] [Google Scholar]
- Schuerwegh AJ. Stevens WJ. Bridts CH. De Clerck LS. Evaluation of monensin and brefeldin A for flow cytometric determination of interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha in monocytes. Cytometry. 2001;46(3):172–176. doi: 10.1002/cyto.1102. [DOI] [PubMed] [Google Scholar]
- Trevisi E. Amadori M. Archetti I. Lacetera N. Bertoni G. Inflammatory response and acute phase proteins in the transition period of high-yielding dairy cows. Acute phase proteins as early non-specific biomarkers of human and veterinary disease. In: Veas F, editor. Rijeka: InTech Publisher; 2011. pp. 355–379. [Google Scholar]
- Veldhuizen EJ. Koomen I. Ultee T. van Dijk A. Haagsman HP. Salmonella serovar specific upregulation of porcine defensins 1 and 2 in a jejunal epithelial cell line. Vet Microbiol. 2009;136(1–2):69–75. doi: 10.1016/j.vetmic.2008.09.072. [DOI] [PubMed] [Google Scholar]
- Walravens K. Wellemans V. Weynants V. Boelaert F. deBergeyck V. Letesson JJ. Huygen K. Godfroid J. Analysis of the antigen-specific IFN-gamma producing T-cell subsets in cattle experimentally infected with mycobacterium bovis. Vet Immunol Immunopathol. 2002;84(1–2):29–41. doi: 10.1016/s0165-2427(01)00398-1. [DOI] [PubMed] [Google Scholar]
- Wang BX. Fish EN. The yin and yang of viruses and interferons. Trends Immunol. 2012;33(4):190–197. doi: 10.1016/j.it.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijtten PJ. van der Meulen J. Verstegen MW. Intestinal barrier function and absorption in pigs after weaning: A review. Br J Nutr. 2011;105(7):967–981. doi: 10.1017/S0007114510005660. [DOI] [PubMed] [Google Scholar]
- Yang D. Biragyn A. Kwak LW. Oppenheim JJ. Mammalian defensins in immunity: More than just microbicidal. Trends Immunol. 2002;23(6):291–296. doi: 10.1016/s1471-4906(02)02246-9. [DOI] [PubMed] [Google Scholar]
- Zhang G. Hiraiwa H. Yasue H. Wu H. Ross CR. Troyer D. Blecha F. Cloning and characterization of the gene for a new epithelial beta-defensin. genomic structure, chromosomal localization, and evidence for its constitutive expression. J Biol Chem. 1999;274(34):24031–24037. doi: 10.1074/jbc.274.34.24031. [DOI] [PubMed] [Google Scholar]
- Zhou C. Liu Z. Jiang J. Yu Y. Zhang Q. Differential gene expression profiling of porcine epithelial cells infected with three enterotoxigenic escherichia coli strains. BMC Genomics. 2012;13(1):330. doi: 10.1186/1471-2164-13-330. [DOI] [PMC free article] [PubMed] [Google Scholar]