Abstract
Although successful remission has been achieved when cancer is diagnosed and treated during its earliest stages of development, a tumor that has established neovascularization poses a significantly greater risk of mortality. The inability to recapitulate the complexities of a maturing in vivo tumor microenvironment in an in vitro setting has frustrated attempts to identify and test anti-angiogenesis therapies that are effective at permanently halting cancer progression. We have established an in vitro tumor angiogenesis model driven solely by paracrine signaling between MDA-MB-231 breast cancer cells and telomerase-immortalized human microvascular endothelial (TIME) cells co-cultured in a spatially relevant manner. The bilayered bioengineered tumor model consists of TIME cells cultured as an endothelium on the surface of an acellular collagen I hydrogel under which MDA-MB-231 cells are cultured in a separate collagen I hydrogel. Results showed that TIME cells co-cultured with the MDA-MB-231 cells demonstrated a significant increase in cell number, rapidly developed an elongated morphology, and invasively sprouted into the underlying acellular collagen I layer. Comparatively, bioengineered tumors cultured with less aggressive MCF7 breast cancer cells did not elicit an angiogenic response. Angiogenic sprouting was demonstrated by the formation of a complex capillary-like tubule network beneath the surface of a confluent endothelial monolayer with lumen formation and anastomosing branches. In vitro angiogenesis was dependent on vascular endothelial growth factor secretion, matrix concentration, and duration of co-culture. Basic fibroblast growth factor supplemented to the co-cultures augmented angiogenic sprouting. The development of improved preclinical tumor angiogenesis models, such as the one presented here, is critical for accurate evaluation and refinement of anti-angiogenesis therapies.
Introduction
Physiological angiogenesis, or vascular neovessel formation from a pre-existing vessel, is fundamental for tissue maintenance and homeostasis during embryonic development and adult life.1–4 Similar to normal tissue development, an expanding tumor requires oxygen and nutrients to maintain cell function, growth, and survival.4,5 At the early stages of development, solid tumor growth is constrained and remains dormant in the absence of angiogenesis.6 Tumors progress from an avascular to a vascular state in response to microenvironmental changes, specifically the onset of severe hypoxia.7 Tumor angiogenesis is a multifaceted and dynamic progression that is characterized by an imbalance of angiogenic promoters and inhibitors, extracellular matrix remodeling, and endothelial cell migration, proliferation, and differentiation, resulting in neovessel sprouting. This process is initiated by the tumor and stromal cells, which supply an extensive panel of pro-angiogenic growth factors, most prominently vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF).8–10 These ligands interact directly with receptor tyrosine kinases on endothelial cells from the surrounding microvasculature to promote invasive sprouting into the tumor, forming a new vascular network that propagates tumor growth.10
The idea that inhibiting tumor angiogenesis could be an effective strategy for treating solid tumors was proposed by Judah Folkman in 1971.11 Since then, researchers have identified and isolated many angiogenic promoters and inhibitors that represent attractive therapeutic targets. Clinical success has been achieved, and FDA approval suggests that these drugs are capable of significantly halting cancer progression. However, in the absence of validated predictive biomarkers, developed resistance to anti-angiogenic inhibitors frequently occurs through increased redundancy of existing angiogenic factors, activation of alternative angiogenic pathways, and vasculogenic mimicry. Furthermore, anti-angiogenesis therapies often lead to augmented invasiveness and metastasis due to the selection of tumor cells that are capable of surviving in severely hypoxic environments. Hence, anti-angiogenic drugs have only been shown to temporarily extend progression-free survival with merely a small improvement on overall survival.4,12–14 Regardless, targeting the tumor vasculature remains a promising approach for inhibiting tumor growth and merits continued investigation.
Conventional in vitro angiogenesis models have been designed to characterize the angiogenic activity of endothelial cells cultured on a compliant matrix, such as a collagen or fibrin hydrogel, in response to known pro-angiogenic factors.1,15–21 While these assays provide the foundation for contemporary angiogenesis research, a comprehensive understanding of the mechanism and interplay of angiogenic growth factors in relation to dynamic cellular and environmental interactions during tumor development is lacking. The emergence of tumor engineering, defined as the development of complex three-dimensional (3D) in vitro tumor models that reproduce the phenotypes and physiological responses of the in vivo tumor microenvironment,22 is facilitating the establishment of new platforms for identifying and testing more efficacious anti-angiogenic drugs. These systems are beginning to successfully incorporate multiple cell types (endothelial, tumor and/or stromal) into controlled 3D in vitro environments, demonstrating reproducible angiogenic sprouting23–28 as well as inhibition in response to known angiogenic blockers.25,26,28 However, many of these models are unable to recapitulate co-culture induced angiogenic sprouting from a confluent monolayer,24,26,28 which is important for maintaining proper endothelial cell polarity. Furthermore, most co-culture angiogenesis models have been unsuccessful in achieving invasive sprouting without the contribution of exogenous angiogenic growth factors26,27 or fibroblasts.23–25,28
To address this deficiency, we introduce an in vitro angiogenesis model based on microvascular endothelial cells cultured as a confluent monolayer on the surface of previously characterized pro-angiogenic bioengineered tumors29 in the absence of exogenous angiogenic growth factors. These in vitro tumors, composed of MDA-MB-231 breast cancer cells cultured within a bulk collagen I hydrogel, have been shown to exhibit phenotypic characteristics that are ideal for encouraging an in vivo-like angiogenic response, including the development of a necrotic core, intracellular levels of hypoxia, and subsequent up-regulation of VEGFA gene expression. In the current study, paracrine signaling between telomerase-immortalized human microvascular endothelial (TIME) cells cultured as an endothelium on the surface of an acellular collagen I hydrogel and MDA-MB-231 cells cultured beneath in a separate collagen I hydrogel led to augmented angiogenic activity; specifically a significant increase in TIME cell number, the development of an elongated and aligned TIME morphology, and invasive angiogenic sprouting. This system allowed for the decoupling of important cellular and environmental factors that influence co-culture-induced in vitro angiogenesis; particularly cancer cell aggressiveness, seeding density (tumor and endothelial), matrix concentration, growth factor involvement (VEGF and bFGF), and duration of co-culture. The in vitro tumor angiogenesis platform presented here can be used to gain a clearer understanding of specific mechanisms involved in tumor angiogenesis as well as potentially provide a more realistic and reproducible response to anti-angiogenesis therapies.
Materials and Methods
Cell culture
MDA-MB-231 human breast cancer cells (American Type Culture Collection [ATCC], Manassas, VA) were cultured in DMEM/F12 (1:1) +L-Glutamine, +15 mM HEPES (Invitrogen, Carlsbad, CA) and supplemented with 10% fetal bovine serum (FBS; Sigma Aldrich, St. Louis, MO) and 1% Penicillin/Streptomycin (P/S; Invitrogen). MCF7 human breast cancer cells were cultured in EMEM with L-Glutamine (ATCC) and supplemented with 10% FBS, 1% P/S, and 0.01 mg/mL insulin (Sigma Aldrich). TIME cells stably transduced with an mKate lentivirus (generously provided by the Wake Forest Institute of Regenerative Medicine, Winston-Salem, NC) were cultured in EBM-2 (Lonza Biomedical, Walkersville, MD) and supplemented with an EGM-2 SingleQuots® Kit (Lonza Biomedical), which contains 2% FBS, hydrocortisone, VEGF (2 ng/mL), hFGF-B (4 ng/mL), R3-insulin growth factor, ascorbic acid, human epidermal growth factor, GA-1000, and heparin. Fluorescently labeled endothelial cells enabled convenient monitoring of angiogenic sprouting before immunofluorescence staining. Cell cultures were incubated in a humidified atmosphere of 95% air and 5% CO2 at a constant temperature of 37°C.
Bilayered, collagen I hydrogel bioengineered tumors
Bioengineered tumors were fabricated in a similar manner to our previous study.29 MDA-MB-231 and MCF7 cells were cultured separately in an 8 mg/mL collagen solution at a seeding density of 5×106 cells/mL. The tumor cell-collagen suspension was pipetted into 12-well costar transwell inserts with 0.4 μm pore polyester membranes (Corning Incorporates, Corning, NY). Two-thirds of the final volume (226 μL to achieve 2 mm thickness) was allowed to polymerize for 5 min at 37°C before the final one-third of the suspension was added to fill in the naturally occurring meniscus of the collagen solution, thereby creating a flat top surface of the hydrogel. After polymerization of the tumor cell-collagen layer, a thin acellular layer of 8 mg/mL collagen hydrogel (113 μL to achieve 1 mm thickness) was similarly added in two separate and equal volumes. Respective cell culture mediums were added to the well and insert, and medium was replenished every 24 h.
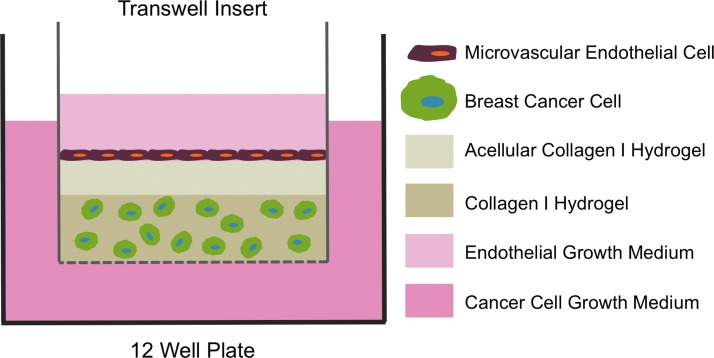
The tumor cells were cultured for 24 h, after which the TIME cells were seeded at a density of 3×104 cells/cm2 on the surface of the acellular layer of the bilayered bioengineered tumors. The use of transwell inserts provided two separate medium compartments for the different cell types, which protected the endothelial cells from the acidic growth conditions generated by the tumor cells cultured at a high density. Respective cell culture mediums were added to the well and insert, and medium was replenished every 24 h. Figure 1 illustrates the co-culture experimental setup. The co-culture experimental setup maintains the appropriate spatial relationship between the tumor and endothelial cells as well as correct endothelial cell polarity. The model was designed to focus on the cellular interactions between the tumor and endothelial cells during angiogenic sprouting without the influence of vascular flow. An acellular collagen layer was added as a barrier between the two cell types to preserve endothelial health. Previous studies have reported that direct contact between breast cancer cells and endothelial cells in vivo and in vitro leads to the induction of endothelial cell apoptosis.30 During preliminary experiments, similar results were observed. TIME cells cultured in direct contact with the breast cancer cells cultured within the collagen hydrogels (without an acellular collagen layer) were only able to grow for a few days before there was a noticeable decrease in TIME cell number (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tec).
FIG. 1.
Schematic illustration of the bilayered bioengineered tumor model cultured in a transwell insert. Breast cancer cells were cultured in the bulk collagen I hydrogel, and microvascular endothelial cells were cultured on the surface of an acellular layer of collagen I hydrogel that separated the two cell types. Color images available online at www.liebertpub.com/tec
Angiogenic growth factor expression
VEGF protein secretion from the bioengineered tumors (breast cancer cell monocultures only) was measured by an enzyme-linked immunosorbent assay (ELISA) using Quantikine Human Immunoassay kits (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol. Conditioned medium samples from the inserts were collected on days 1, 3, 5, and 7. In order to monitor temporal changes in protein secretion, fresh cell culture medium was replaced 24 h before each time point.
Analysis of angiogenic activity
The capacity of the breast cancer cells cultured in the bioengineered tumors to induce angiogenic activity by the TIME cells cultured as an endothelium was characterized by monitoring endothelial cell number, morphological changes, and angiogenic sprouting. VEGF and bFGF were removed from the endothelial growth medium to determine whether the tumor cells used in the model could provide the necessary growth factors that would generate an angiogenic response without the aid of exogenous stimuli. For the proliferation and morphology experiments, the tumors cells were seeded at a density of 5×106 cells/mL, the TIME cells were seeded at a density of 3×104 cells/cm2, and the acellular collagen layer concentration was 8 mg/mL. The culturing parameters were varied for the sprouting experiments, as described next. The control group was a TIME cell monoculture on an acellular collagen hydrogel cultured with complete endothelial growth medium.
Quantification of endothelial cell number
Quantification of endothelial cell number was conducted on days 1, 3, and 5 (following TIME cell seeding on bioengineered tumors). Samples were fixed with 10% formalin and permeabilized with 0.5% Triton X-100 (Sigma Aldrich). NucBlue™ Fixed Cell Stain with DAPI (Invitrogen) was used to stain nuclei. A set of 12 images were taken from each sample using a Leica DMI 6000 fluorescent microscope, and Image J (NIH, Bethesda, MA) was used to count nuclei. For these experiments, all experimental and control groups were cultured simultaneously to reduce variability due to cell passage and health, collagen batch, quality of serum, and other conditions of growth.
Analysis of morphological changes
Endothelial cell morphology was analyzed on days 1, 3, 5, and 7 as previously described.31 Briefly, cells were stained with Oregon Green® 488 phalloidin (Invitrogen), a high-affinity probe for F-actin, and NucBlue™ Fixed Cell Stain with DAPI (Invitrogen). Imaging was performed with a Zeiss LSM 510 laser scanning confocal microscope (Carl Zeiss, Thornwood, NY). Representative images are shown for all groups.
Characterization of angiogenic sprouting
For the sprouting experiments, parameters were varied to characterize the cellular and environmental factors that influence co-culture-induced in vitro angiogenesis. In addition to using two breast cancer cell lines with differing degrees of aggressiveness (more aggressive MDA-MB-231 and less aggressive MCF7), the effect of growth conditions such as tumor cell seeding density (1×106 cells/mL and 5×106 cells/mL), endothelial cell seeding density (3×104 cells/cm2 and 1×105 cells/cm2), acellular collagen I hydrogel concentration (2, 4, and 8 mg/mL), exogenous growth factor supplementation (complete EGM-2 with 2 ng/mL VEGF and 4 ng/mL bFGF and EGM-2 containing 10 ng/mL bFGF), and time delay of co-culture (seeding TIME cells 1 and 5 days after initial culture of the bioengineered tumors) were investigated. Confirmation of sprouting was visualized with the fluorescent mKate lentivirus before immunofluorescence staining with Oregon Green 488 phalloidin, as described earlier. Imaging was performed with a Zeiss LSM 510 laser scanning confocal microscope. Representative images are shown for all groups. While the trends between experimental groups remained constant throughout the study, minor variability in angiogenic sprouting within the same group was attributed to differences in cell passage and health, collagen batch, quality of serum, and other conditions of growth.
Statistical analysis
A Student's t-test was used to determine statistical significance of the ELISA and endothelial cell number data. p<0.05 was considered significant. p<0.01 and p<0.001 were also noted.
Results
VEGF expression from the bioengineered tumors
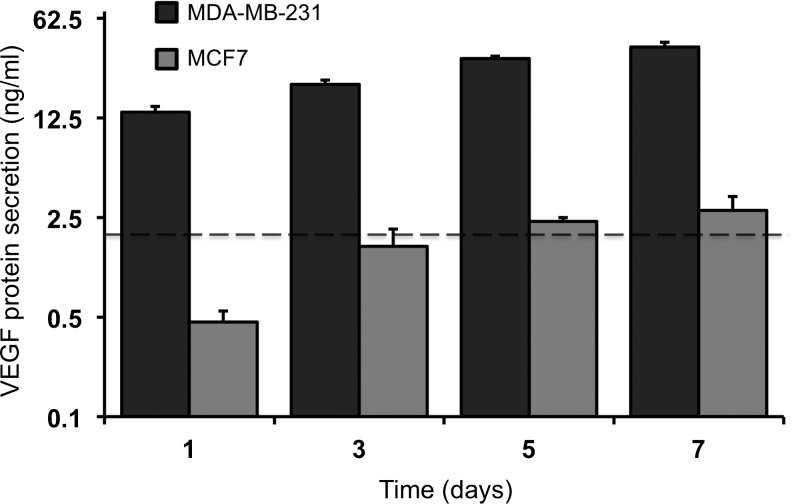
Two human breast cancer cell lines of differing degrees of aggressiveness were cultured individually within bulk collagen I hydrogels cast in 12-well transwell inserts. The more aggressive MDA-MB-231 cells were shown to proliferate and interact with the surrounding collagen matrix to a greater extent than the less aggressive MCF7 cells over 7 days in culture (Supplementary Fig. S2). In addition, the MDA-MB-231 bioengineered tumors secreted a significantly greater (p<0.001) concentration of VEGF than the MCF7 bioengineered tumors at each time point over 7 days in culture (Fig. 2). There was a significant increase in protein secretion by the MDA-MB-231 bioengineered tumors at each time point (day 3, 5: p<0.001; day 7: p<0.01), compared with the previous time point, which correlated with the observed increase in MDA-MB-231 cell number. The MCF7 bioengineered tumors exhibited a significant increase (p<0.05) in protein secretion only from days 1–3, with no statistical difference observed after day 3.
FIG. 2.
VEGF protein secretion from bioengineered tumors seeded with MDA-MB-231 or MCF7 cells. There was a significant difference (p<0.001) in protein secretion between the two groups at each time point. There was a significant increase in protein secretion by the MDA-MB-231 bioengineered tumors at each time point (day 3, 5: p<0.001; day 7: p<0.01), as compared with the previous time point. The MCF7 bioengineered tumors exhibited a significant increase (p<0.05) in protein secretion only on day 3, as compared with on day 1. The dotted line represents the concentration of VEGF supplemented in the complete endothelial growth medium (2 ng/mL). VEGF, vascular endothelial growth factor.
The dotted line in Figure 2 represents the concentration of VEGF supplemented in the complete endothelial growth medium (2 ng/mL). This is important to note, as the control group for subsequent co-culture experiments was a TIME cell monoculture on an acellular collagen hydrogel cultured with complete endothelial growth medium. While the MCF7 bioengineered tumors secreted a similar concentration of VEGF compared with the complete medium, the MDA-MB-231 bioengineered tumors secreted a considerably greater concentration of VEGF at each time point. An ELISA was unable to detect bFGF in the bioengineered tumor conditioned medium (data not shown).
Tumor-endothelial cell co-culture regulates endothelial cell proliferation
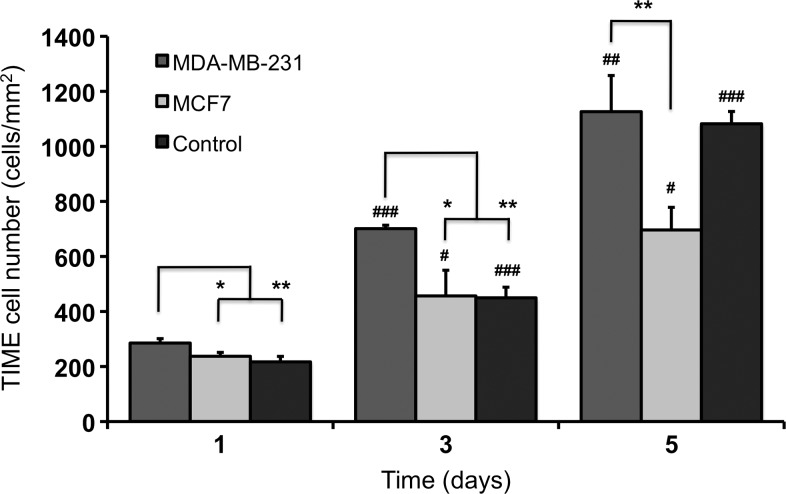
Tumor-endothelial cell co-culture led to significant changes in TIME cell number compared with the control group, with the MDA-MB-231 bioengineered tumors stimulating endothelial proliferation and the MCF7 bioengineered tumors reducing endothelial proliferation (Supplementary Fig. S3). TIME cell number in the MDA-MB-231 bioengineered tumors was significantly greater (day 1, 3: p<0.05; day 5: p<0.01) at each time point compared with the MCF7 bioengineered tumors (Fig. 3). This difference was most likely related to the significant increase in VEGF protein secretion by the MDA-MB-231 bioengineered tumors, as determined by an ELISA (Fig. 2). Compared with the control group, TIME cell number in the MDA-MB-231 bioengineered tumors was significantly greater (p<0.01) on days 1 and 3. By day 5, TIME cells cultured in the MDA-MB-231 bioengineered tumors and the control group had reached confluence; therefore, there was no statistical difference in cell number. TIME cell number was statistically less (p<0.01) in the MCF7 bioengineered tumors than in the control group only on day 5. This difference was likely due to a combination of low VEGF protein secretion levels from the MCF7 bioengineered tumors and decreased availability of growth supplements in the medium (such as FBS), as there was a greater total number of cells competing for the same volume of medium compared with the control group. It is also possible that MCF7 cells secrete anti-angiogenic factors that suppress endothelial cell growth, similar to pancreatic cancer cells,32 which may allow them to maintain their less aggressive phenotype in vivo. TIME cell number increased significantly from day 1 to day 3 and from day 3 to day 5 in all groups.
FIG. 3.
TIME cell number when cultured on the surface of the bioengineered tumors. TIME cells cultured on the surface of MDA-MB-231 bioengineered tumors proliferated at a faster rate than TIME cells cultured on MCF7 bioengineered tumors with TIME cell number significantly greater at each time point. TIME cell number in the MDA-MB-231 bioengineered tumors was significantly greater on days 1 and 3 compared with the control group. By day 5, TIME cells cultured in the MDA-MB-231 and control groups reached confluence; therefore, there was no statistical difference in cell number. TIME cell number in each group was significantly greater than the previous time point on days 3 and 5. * denotes a significant difference between groups, and # denotes a significant difference within the same group compared with the previous time point. */#, **/##, and ### indicate p<0.05, 0.01, and 0.001, respectively. TIME, telomerase-immortalized human microvascular endothelial.
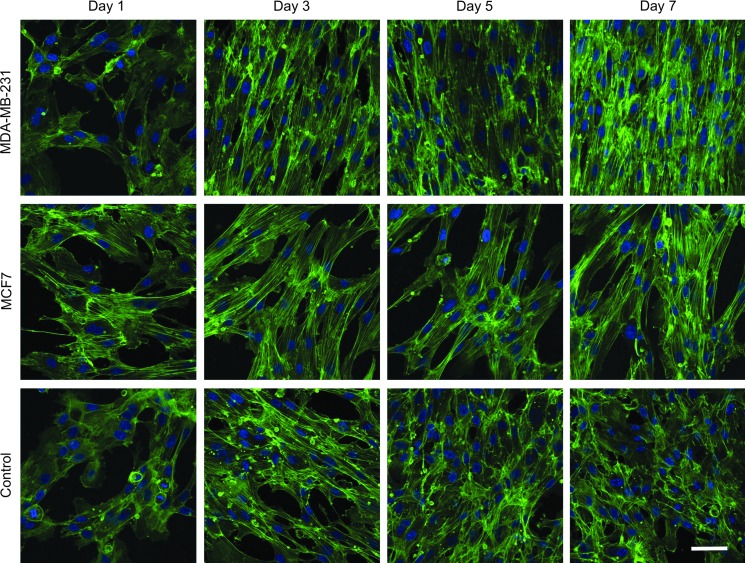
Tumor-endothelial cell co-culture elicits an elongated endothelial cell morphology
TIME cells cultured on the surface of the MDA-MB-231 bioengineered tumors developed elongated and tightly aligned morphologies over a 7-day period, with morphological changes becoming evident by day 3 (Fig. 4). Although TIME cells cultured on the MCF7 bioengineered tumors did not reach confluence after 7 days of co-culture, an elongated morphology was observed. When complete endothelial growth medium was provided to the TIME cells cultured on the MCF7 bioengineered tumors, the cells became confluent and displayed similar elongated and tightly aligned morphologies to the TIME cells cultured on the MDA-MB-231 bioengineered tumors (Supplementary Fig. S4). Conversely, TIME cells in the control group reached confluence by day 5 but did not undergo any morphological changes. The development of an elongated morphology was not a consequence of over confluence, as endothelial cell number on the MDA-MB-231 bioengineered tumors and the control group were similar on day 5 (Fig. 3). Furthermore, since the supplemented VEGF and bFGF were removed from the co-culture groups, these results suggest that paracrine growth factors from both types of breast cancer cells, other than VEGF and bFGF, are responsible for encouraging an in vivo-like elongated and aligned endothelial morphology in the absence of vascular flow. Similar morphological changes were observed when the TIME cells were cultured on lower concentrations of the acellular collagen hydrogel layer (2 and 4 mg/mL) (data not shown).
FIG. 4.
TIME cell morphology when cultured on the surface of the bioengineered tumors. TIME cells became elongated and tightly aligned when co-cultured on MDA-MB-231 bioengineered tumors. Although TIME cells did not reach confluence on the MCF7 bioengineered tumors, an elongated morphology was observed. Conversely, TIME cells in the control group reached confluence but did not undergo morphological changes. Green, F-actin; blue, nuclei. Scale bar represents 50 μm. Color images available online at www.liebertpub.com/tec
Tumor-endothelial cell co-culture induces invasive angiogenic sprouting
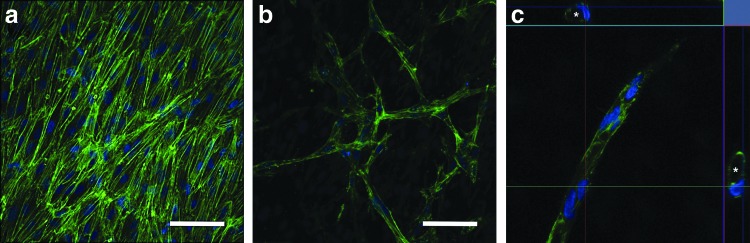
Invasive angiogenic sprouting was observed when TIME cells were cultured on the surface of the more aggressive MDA-MB-231 bioengineered tumors (Fig. 5) but not with the less aggressive MCF7 bioengineered tumors. In order to facilitate sprouting, the concentration of the acellular collagen layer was reduced, as sprouting could not be attained when the TIME cells were cultured on an 8 mg/mL acellular collagen hydrogel. A capillary-like tubule network was shown to develop beneath the surface of a confluent monolayer of TIME cells (2 mg/mL acellular collagen layer) after 7 days of co-culture (Fig. 5a, b). Select tubules contained patent lumens, which were typically surrounded by a single endothelial cell (4 mg/mL acellular collagen layer) (Fig. 5c). These results validate that paracrine signaling between the tumor and endothelial cells cultured in the bilayered bioengineered tumor in vitro angiogenesis model is sufficient to induce angiogenic sprouting with complex capillary-like tubule formation without the addition of exogenous pro-angiogenic growth factors (VEGF and bFGF removed from endothelial growth medium). Angiogenic sprouting was also observed in the control group when TIME cells were cultured on 2 mg/mL acellular collagen hydrogels with complete endothelial growth medium (Fig. 6). However, TIME cells were not shown to develop capillary-like tubule networks beneath the surface monolayer (Fig. 6a, b). Rather, sprouting was characterized by isolated points of endothelial invasion with radially branching filopodia (Fig. 6c, d). When TIME cells cultured with the MCF7 bioengineered tumors were provided complete endothelial growth medium, angiogenic sprouting was still not observed. The inability to reproduce a similar response to the control group, with identical concentrations of supplemented VEGF and bFGF, supports the possibility that MCF7 cells may secrete anti-angiogenic factors.
FIG. 5.
Angiogenic sprouting of TIME cells cultured on MDA-MB-231 bioengineered tumors in the absence of exogenous VEGF and bFGF. (a) TIME cells grown to confluence after 7 days on a 2 mg/mL acellular collagen hydrogel layer of the bioengineered tumor (b) formed a capillary-like tubule network beneath the surface. (c) Select tubules were shown to develop patent lumens (day 10; 4 mg/mL acellular collagen layer). The top and side panels of (c) illustrate cross-sectional cuts of a single tubule in the x-z and y-z directions. * denotes patent lumens within the cross-sectional cuts of a single tubule. Green, F-actin; blue, nuclei. Scale bars represent 50 μm. bFGF, basic fibroblast growth factor. Color images available online at www.liebertpub.com/tec
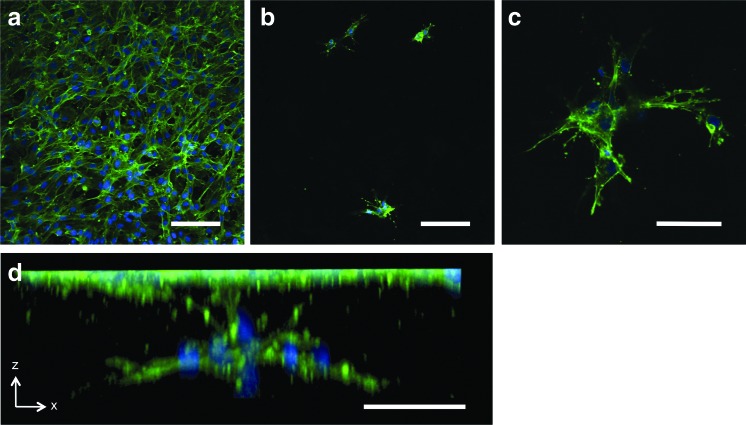
FIG. 6.
Angiogenic sprouting of TIME cells in the control group. (a) TIME cells grown to confluence after 7 days on a 2 mg/mL acellular collagen hydrogel layer of the control group demonstrated (b) isolated points of invasion with (c) radially branching filopodia beneath the surface. (d) Three-dimensional image reconstruction of an isolated sprouting event. Green, F-actin; blue, nuclei. Scale bars represent (a, b) 100 μm and (c, d) 50 μm. Color images available online at www.liebertpub.com/tec
Acellular collagen matrix concentration governs angiogenic sprouting
The concentration of the acellular collagen hydrogel layer was a limiting factor in supporting angiogenic sprouting in the MDA-MB-231 bioengineered tumors. After 7 days of co-culture, sprouting with branching tubule formation was only observed when TIME cells were cultured on a 2 mg/mL acellular collagen layer (Fig. 7a). Three-dimensional image reconstruction accentuated the complexity of the capillary-like tubule network that was achieved. Separate tubules were shown to anastomose and extend far beneath the confluent monolayer (Fig. 7b). TIME cells cultured on a 4 mg/mL acellular collagen hydrogel began invading beneath the surface, but these sprouting tubules were scarce, shorter in length, and showed no signs of branching (Fig. 7a). As mentioned earlier, invasive tubule formation was not observed within the 8 mg/mL acellular collagen hydrogel. The observed correlation between matrix concentration and the degree of sprouting indicates that collagen degradation may be a rate-limiting step in regulating invasive sprouting, as previously reported.33
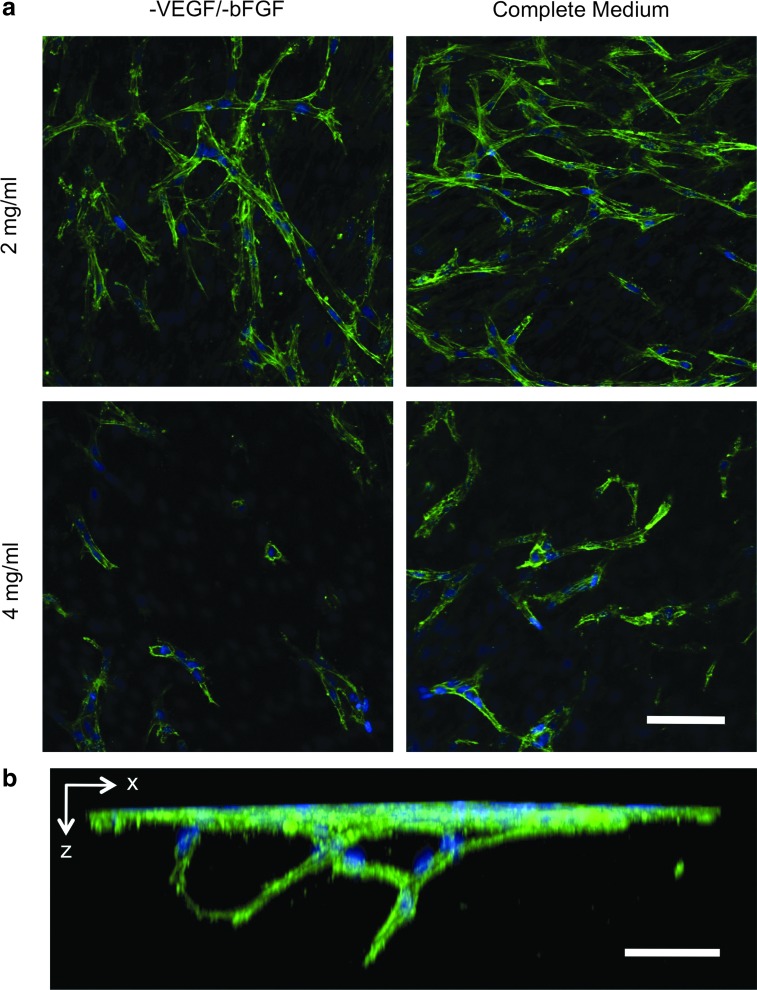
FIG. 7.
Influence of matrix concentration and supplemented bFGF on angiogenic sprouting of TIME cells cultured for 7 days on MDA-MB-231 bioengineered tumors. (a) A considerably greater degree of angiogenic sprouting was observed within the 2 mg/mL acellular collagen layers as compared with the 4 mg/mL acellular collagen. Sprouting was augmented within both collagen concentrations when complete medium containing bFGF (4 ng/mL) was supplemented to the co-culture TIME cells. (b) Three-dimensional image reconstruction shows two separate tubules anastomosing and extending down beneath the surface monolayer (2 mg/mL acellular collagen layer; 10 ng/mL bFGF). Green, F-actin; blue, nuclei. Scale bars represent 50 μm. Color images available online at www.liebertpub.com/tec
Angiogenic growth factor composition and concentration influence angiogenic sprouting
The influence of exogenous bFGF and endogenous VEGF on angiogenic sprouting was explored through changing the medium composition and altering the seeding density of the MDA-MB-231 bioengineered tumors, respectively. When co-culture TIME cells were provided complete endothelial growth medium (2 ng/mL VEGF and 4 ng/mL bFGF) for the extent of the study, angiogenic sprouting was enhanced in both the 2 and 4 mg/mL acellular collagen layers, as compared with the non-VEGF and bFGF supplemented co-cultures that only contained endogenous VEGF (Fig. 7a). It is probable that this increase was in response to the addition of bFGF, as an ELISA was unable to detect bFGF in the MDA-MB-231 bioengineered tumor-conditioned medium (data not shown). Furthermore, the endogenous concentration of VEGF secreted by the MDA-MB-231 bioengineered tumors was significantly larger than the amount provided in the complete medium; hence, it is unlikely that the exogenous VEGF from the medium provided an added stimulus to the VEGF already present within the system. Augmented in vitro angiogenic sprouting in response to VEGF and bFGF co-stimulation as compared with either growth factor alone has been previously shown using an endothelial cell monoculture assay.1 In that study, optimal sprouting was obtained when 30 ng/mL VEGF and 10 ng/mL bFGF were supplemented to the endothelial cells. However, when 10 ng/mL bFGF was added to TIME cells cultured with the MDA-MB-231 bioengineered tumors, angiogenic sprouting was not further increased (data not shown).
MDA-MB-231 bioengineered tumors seeded at a lower density (1×106 cells/mL) were shown to secrete a significantly lower concentration of VEGF (4-fold) (p<0.001) than the high seeding density (5×106 cells/mL) bioengineered tumors at each time point (Supplementary Fig. S5a). However, this concentration on day 7 was still 5-fold greater than the VEGF provided in the complete endothelial growth medium. TIME cells cultured with the low seeding density MDA-MB-231 bioengineered tumors proliferated at a similar rate to the high seeding density bioengineered tumors (Supplementary Fig. S5b) and developed identical elongated and aligned morphologies (Supplementary Fig. S5c). Invasive angiogenic sprouting was observed within both the 2 mg/mL (Supplementary Fig. S5d) and 4 mg/mL acellular collagen layers, with and without complete endothelial growth medium. While there was a minor decrease in the extent of angiogenic sprouting within the 4 mg/mL acellular collagen layers as compared with the high seeding density bioengineered tumors, it is clear that the MDA-MB-231 bioengineered tumors seeded at a density of 1×106 cells/mL provided sufficient VEGF signaling to induce a comparable angiogenic response.
Duration of co-culture determines induction but not extent of angiogenic sprouting
When co-cultured under optimal sprouting conditions (2 mg/mL acellular collagen; complete medium), capillary-like tubule formation was not observed until day 5 (Fig. 8a). In an attempt to accelerate tubule growth, TIME cells were seeded at confluence (1×105 cells/cm2), as determined from the proliferation experiment (Fig. 3). This approach was derived from previous in vitro angiogenesis studies that have allowed endothelial cells to reach confluence before inducing angiogenic activity,1,17–19 as it has been shown that tubule formation only occurs when endothelial cells have a low proliferative potential.16 While there was a minor increase in the number of sprouting events, capillary-like tubule formation was still not observed until day 5 (Fig. 8a). Another approach was to seed the TIME cells on the acellular collagen layer 5 days after initial culture of the MDA-MB-231 bioengineered tumors when there was a significantly greater concentration of secreted VEGF, as compared with day 1 (Fig. 2). Similar to increasing the TIME cell seeding density, a minor increase in the number of sprouting events was observed, but capillary-like tubule formation was not seen until day 5 (data not shown). Based on these results, it is possible that additional time-dependent crosstalk between the tumor and endothelial cells, besides VEGF signaling, is necessary to encourage angiogenic sprouting.34
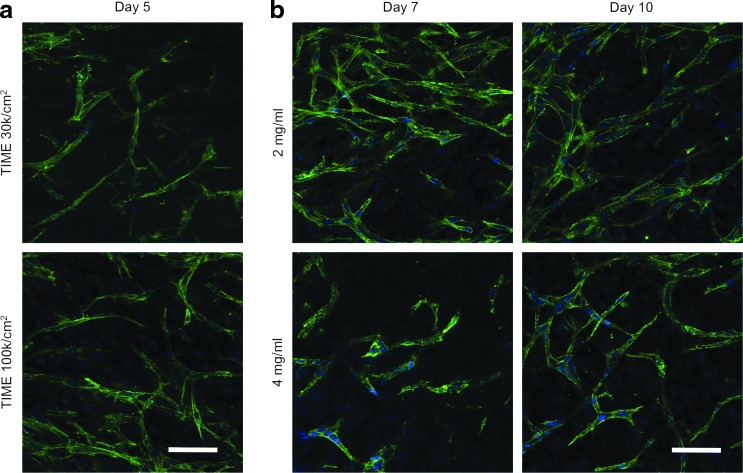
FIG. 8.
Influence of (a) TIME cell seeding density and (b) duration in co-culture on angiogenic sprouting. (a) Angiogenic sprouting with noticeable capillary-like tubule formation was not observed within the 2 mg/mL acellular collagen until day 5, regardless of the TIME cell seeding density. However, seeding the TIME cells at confluence (1×105 cells/cm2) resulted in a minor increase in the number of sprouting events. (b) Sprouting reached a maximum by day 7 when TIME cells were cultured on the 2 mg/mL acellular collagen layer but continued to progress through day 10 when cultured on the 4 mg/mL acellular collagen layer. Green, F-actin; blue, nuclei. Scale bars represent 50 μm. Color images available online at www.liebertpub.com/tec
There was no noticeable increase in angiogenic sprouting between day 7 and day 10 from TIME cells cultured on the 2 mg/mL acellular collagen layer when supplemented with complete endothelial growth medium (Fig. 8b). Alternatively, sprouting continued to progress through day 10 within the 4 mg/mL acellular collagen with tubule growth and the formation of a branching network (Fig. 8b). When the co-culture was not supplemented with complete medium, sprouting also increased on both the 2 and 4 mg/mL acellular collagen layers (data not shown). This indicates that the rate of sprouting within this system is dependent on both matrix concentration and growth factor involvement, and the extent of sprouting is terminal. Given enough time in co-culture, sprouting will reach a maximum limit, as previously observed.18
Discussion
The first demonstration of in vitro angiogenesis was reported by Judah Folkman in 1980.15 In that study, capillary endothelial cells were cultured on gelatin and stimulated to form tubule networks when provided tumor-conditioned medium. Over the next decade, endothelial cell monoculture assays provided a fundamental understanding of the function of many pro-angiogenic factors, including bFGF,18 VEGF,19 and phorbol esters,17 individually as well as synergistically.1,20 More recently, co-culture angiogenesis assays have relied on paracrine signaling to promote angiogenic tubule formation. These systems can be separated into three categories: (1) tumor and endothelial cells co-cultured in a single monolayer,35,36 (2) endothelial cells embedded within a bulk hydrogel with the tumor cells cultured separately or in contact with the endothelial cells,24,26,28 and (3) endothelial cells cultured as a monolayer on the surface of a bulk hydrogel containing tumor cells.25,27 While these models allow for the study of multicellular interactions during tumor angiogenesis, each of these systems is flawed for one or more of the following reasons: the lack of an underlying matrix for endothelial remodeling,35,36 inaccurate spatial relationships between tumor/stromal and endothelial cells,25,28,35,36 incorrect endothelial polarity,26,28 and a reliance on exogenous pro-angiogenic growth factors.26,27,36 In this study, we establish an in vitro tumor angiogenesis model based on the hypothesis that paracrine signaling between tumor and endothelial cells co-cultured in a spatially relevant manner is sufficient for inducing an angiogenic response in the absence of exogenous pro-angiogenic growth factors. Microvascular endothelial cells cultured as an endothelium on the surface of aggressive MDA-MB-231 bioengineered tumors demonstrated a significant increase in proliferation, rapidly developed an elongated morphology, and invasively sprouted into an underlying acellular collagen I layer, forming a complex capillary-like tubule network with lumen formation and anastomosing branches.
To the best of our knowledge, the bilayered bioengineered tumor angiogenesis model represents the first demonstration of tumor-endothelial cell paracrine signaling-induced invasive angiogenic sprouting into a 3D matrix. Previous groups have attempted to study this interaction but have been unable to achieve angiogenic sprouting without the addition of fibroblasts. In a similar co-culture setup, tumor cells cultured in a collagen hydrogel beneath an endothelial cell monolayer were reported to have proliferated too quickly, leading to high levels of acidity and endothelial toxicity.23 When fibroblasts were cultured in place of the tumor cells, the endothelial cells invasively sprouted into the collagen, forming capillary-like tubules. Similarly, endothelial cells cultured in direct contact with tumor cells as multicellular spheroids embedded in a collagen hydrogel were unable to sprout due to cell–cell contact-induced endothelial cell apoptosis.28 When the multicellular spheroids were cultured with fibroblasts and tumor cells, endothelial viability was maintained, and sprouting was observed. In studies in which endothelial cell health was not jeopardized by tumor cell co-culture, fibroblasts were still required for supporting endothelial invasive sprouting, in addition to the tumor cells.24,25 It is assumed that the fibroblasts provided an additional angiogenic growth factor stimulus which was necessary for inducing an angiogenic response. While fibroblasts are a central component of the tumor stroma, understanding the distinct influence of tumor cells on angiogenic sprouting will allow for decoupling of their role from fibroblasts, providing a clearer understanding of the complex paracrine signaling network that drives tumor angiogenesis.
Matrix concentration, growth factor involvement, and duration of co-culture were important aspects of our bilayered bioengineered tumor model that influenced the extent of in vitro angiogenic sprouting. Endothelial cell remodeling of collagen I hydrogels has recently been shown to progress in both a time- and concentration-dependent manner, with the magnitude of endothelial network formation increasing with time and the rate of network formation decreasing with collagen concentration,33 which was similar to our results. In addition, endothelial invasion in the lowest collagen hydrogel concentration (3 mg/mL) only progressed until day 14, while invasion in the higher concentrations (6 and 10 mg/mL) continued to increase until day 21.33 While we attributed this to the terminal nature of in vitro angiogenic sprouting within our system, it has been suggested in other studies that stiffer matrices may provide a more secure architecture for maintaining angiogenic sprouting longer with stable tubule formation.21,33,37 Since angiogenic sprouting was contingent on both matrix concentration and growth factor involvement in the bioengineered tumor model, the inability to induce invasive sprouting within the 8 mg/mL acellular collagen layer was likely due to an insufficient concentration and/or composition of paracrine angiogenic growth factors. This highlights an important distinction between monoculture and co-culture angiogenesis assays. Endothelial cells cultured alone can be forced to differentiate into an angiogenic phenotype in many different types and concentrations of matrices if supplemented with an appropriate cocktail of pro-angiogenic factors. Alternatively, co-culture models rely on paracrine signaling and, therefore, may produce an angiogenic response that is not as pronounced. Nevertheless, we believe that exploiting multicellular interactions to generate in vitro angiogenesis, rather than exogenous stimuli, will provide a more physiologically relevant response.
Angiogenic sprouting was strongly influenced by endogenous VEGF and exogenous bFGF. MDA-MB-231 bioengineered tumors secreted an ample concentration of VEGF, which resulted in a significant increase in TIME cell number and invasive sprouting. Bioengineered tumors cultured with a lower seeding density of MDA-MB-231 cells were shown to generate an identical angiogenic response, indicating that the concentration of VEGF required to provoke this level of angiogenic activity is <10 ng/mL and a four-fold increase in concentration does not enhance this response. Supplementing the co-culture TIME cells with exogenous bFGF (4 ng/mL) led to an increase in angiogenic sprouting. It is possible that this additive effect is related to an increase in plasminogen activator (PA) expression by the TIME cells as PA activity, which is necessary for endothelial invasion, is regulated by bFGF18 and not influenced by VEGF.19 bFGF has previously been shown to induce more developed capillary-like tubule formation at the same concentration as VEGF, and co-stimulation with both growth factors has led to accelerated tubule formation as compared with either ligand alone.1 Similar to VEGF, increasing the concentration of bFGF (10 ng/mL) in our co-culture did not lead to an enhanced response. The concentrations of VEGF (10 ng/mL) and bFGF (4 ng/mL) required to obtain a maximum synergistic effect in our system were considerably less than what has been reported in a monoculture angiogenesis assay (VEGF 30 ng/mL, bFGF 10 ng/mL).1 This supports the notion that the impact of an individual cytokine on tumor angiogenesis is contextual, that is, it relies on the presence of other factors in the microenvironment.1,20 However, within co-culture angiogenesis models, it is challenging to monitor the interplay among the multitude of growth factors secreted in relation to dynamic cellular and environmental interactions. In our study, the inability to induce angiogenic sprouting before 5 days in co-culture, regardless of seeding the endothelial cells at confluence or increasing the concentration of endogenous VEGF, strongly suggests that additional paracrine signaling between the tumor and endothelial cells occurs in a time-dependent manner and is necessary for stimulating in vitro angiogenesis. It is possible that the angiopoietins (ANG) have active roles in regulating angiogenic sprouting within our model, as we have previously reported that a shift in the ANG1:ANG2 ratio occurs in favor of an increase in ANG2 expression by MDA-MB-231 cells when co-cultured with microvascular endothelial cells.34
While in vitro angiogenesis assays have been used in cancer research for a few decades, these systems have relied on exogenous stimuli and incorrect culturing conditions to induce an angiogenic response. This presents a problem, because determining the therapeutic impact of potential angiogenic inhibitors is highly dependent on the pro-angiogenic stimulus. If the stimulus is incorrect (composition and/or concentration of exogenous growth factors) or the system is physiologically inaccurate (incorrect spatial relationships and endothelial polarity), any conclusions obtained using those models may be unreliable in an in vivo setting. Since the bilayered bioengineered tumor angiogenesis model presented here was driven solely by paracrine signaling between tumor and endothelial cells in a spatially relevant manner, it is expected that this system will provide a more clinically translatable drug response. However, it is important to recognize the limitations of this system. The integration of additional stromal cells, such as fibroblasts, pericytes, and immune cells, may enhance angiogenic activity, influencing the magnitude, rate, and duration of sprouting. Furthermore, the incorporation of flow will allow for an analysis of the effect of shear stress on the development of angiogenic sprouting. Although our system does not allow for vascular flow, it is still practical, as the tortuous nature of the in vivo tumor vasculature leads to many regions of static circulation where angiogenic sprouting may be more inclined to occur. Future studies will focus on incorporating further complexity into the in vitro tumor angiogenesis model as well as testing the functionality of our system through blocking angiogenic sprouting with known inhibitors.
Conclusion
A principal distinction between conventional in vitro angiogenesis assays and angiogenesis development in vivo is that exogenous angiogenic growth factors interact with the apical surface of endothelial cells cultured in vitro, while during in vivo angiogenesis, paracrine signaling from adjacent tumor cells interacts with the basal endothelial cell surface. To more accurately mimic in vivo angiogenesis, a co-culture in vitro tumor angiogenesis model was introduced with microvascular endothelial cells cultured as an endothelium on the surface of an acellular collagen I hydrogel under which MDA-MB-231 breast cancer cells were cultured in a separate collagen I hydrogel. Endothelial cells were shown to assemble into a capillary-like tubule network with lumen formation and anastomosing braches evident beneath the surface of the confluent monolayer. Angiogenic sprouting was stimulated in response to paracrine signaling between the tumor and endothelial cells in the absence of exogenous angiogenic factors, and the magnitude and rate of sprouting were dependent on endogenous VEGF secretion, matrix concentration, and duration of co-culture. This model may be well suited for conducting improved anti-angiogenesis drug screening in a controlled and reproducible manner.
Supplementary Material
Acknowledgments
The authors would like to thank Andrea Martin for generously donating the Sprague Dawley rat tails for collagen isolation. Funding for this study was provided by the National Science Foundation Early CAREER Award CBET 0955072 and the National Institute of Health grant 1R21CA158454-01A1.
Disclosure Statement
No competing financial interests exist.
References
- 1.Pepper M.S. Ferrara N. Orci L. Montesano R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun. 1992;189:824. doi: 10.1016/0006-291x(92)92277-5. [DOI] [PubMed] [Google Scholar]
- 2.Bikfalvi A. Significance of angiogenesis in tumour progression and metastasis. Eur J Cancer. 1995;31A:1101. doi: 10.1016/0959-8049(95)00169-j. [DOI] [PubMed] [Google Scholar]
- 3.Cross M.J. Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001;22:201. doi: 10.1016/s0165-6147(00)01676-x. [DOI] [PubMed] [Google Scholar]
- 4.Shojaei F. Ferrara N. Antiangiogenesis to treat cancer and intraocular neovascular disorders. Lab Invest. 2007;87:227. doi: 10.1038/labinvest.3700526. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D. Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 6.Naumov G.N. Akslen L.A. Folkman J. Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle. 2006;5:1779. doi: 10.4161/cc.5.16.3018. [DOI] [PubMed] [Google Scholar]
- 7.Brahimi-Horn M.C. Chiche J. Pouyssegur J. Hypoxia and cancer. J Mol Med. 2007;85:1301. doi: 10.1007/s00109-007-0281-3. [DOI] [PubMed] [Google Scholar]
- 8.Sfiligoi C. de Luca A. Cascone I. Sorbello V. Fuso L. Ponzone R., et al. Angiopoietin-2 expression in breast cancer correlates with lymph node invasion and short survival. Int J Cancer. 2003;103:466. doi: 10.1002/ijc.10851. [DOI] [PubMed] [Google Scholar]
- 9.Kerbel R.S. Tumor angiogenesis. N Engl J Med. 2008;358:2039. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D. Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 11.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 12.Wu H. Huang C. Chang D. Anti-angiogenic therapeutic drugs for treatment of human cancer. J Cancer Mol. 2008;4:37. [Google Scholar]
- 13.Ferrara N. Kerbel R.S. Angiogenesis as a therapeutic target. Nature. 2005;438:967. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 14.Shojaei F. Anti-angiogenesis therapy in cancer: current challenges and future perspectives. Cancer Lett. 2012;320:130. doi: 10.1016/j.canlet.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Folkman J. Haudenschild C. Angiogenesis in vitro. Nature. 1980;288:551. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- 16.Maciag T. Kadish J. Wilkins L. Stemerman M.B. Weinstein R. Organizational behavior of human umbilical vein endothelial cells. J Cell Biol. 1982;94:511. doi: 10.1083/jcb.94.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montesano R. Orci L. Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell. 1985;42:469. doi: 10.1016/0092-8674(85)90104-7. [DOI] [PubMed] [Google Scholar]
- 18.Montesano R. Vassalli J.D. Baird A. Guillemin R. Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci U S A. 1986;83:7297. doi: 10.1073/pnas.83.19.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bikfalvi A. Sauzeau C. Moukadiri H. Maclouf J. Busso N. Bryckaert M., et al. Interaction of vasculotropin/vascular endothelial cell growth factor with human umbilical vein endothelial cells: binding, internalization, degradation, and biological effects. J Cell Physiol. 1991;149:50. doi: 10.1002/jcp.1041490108. [DOI] [PubMed] [Google Scholar]
- 20.Mandriota S.J. Pepper M.S. Vascular endothelial growth factor-induced in vitro angiogenesis and plasminogen activator expression are dependent on endogenous basic fibroblast growth factor. J Cell Sci. 1997;110:2293. doi: 10.1242/jcs.110.18.2293. [DOI] [PubMed] [Google Scholar]
- 21.van Hinsbergh V.W. Collen A. Koolwijk P. Role of fibrin matrix in angiogenesis. Ann N Y Acad Sci. 2001;936:426. doi: 10.1111/j.1749-6632.2001.tb03526.x. [DOI] [PubMed] [Google Scholar]
- 22.Ghajar C.M. Bissell M.J. Tumor engineering: the other face of tissue engineering. Tissue Eng Part A. 2010;16:2153. doi: 10.1089/ten.tea.2010.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montesano R. Pepper M.S. Orci L. Paracrine induction of angiogenesis in vitro by Swiss 3T3 fibroblasts. J Cell Sci. 1993;105:1013. doi: 10.1242/jcs.105.4.1013. [DOI] [PubMed] [Google Scholar]
- 24.Janvier R. Sourla A. Koutsilieris M. Doillon C.J. Stromal fibroblasts are required for PC-3 human prostate cancer cells to produce capillary-like formation of endothelial cells in a three-dimensional co-culture system. Anticancer Res. 1997;17:1551. [PubMed] [Google Scholar]
- 25.Walter-Yohrling J. Pratt B.M. Ledbetter S. Teicher B.A. Myofibroblasts enable invasion of endothelial cells into three-dimensional tumor cell clusters: a novel in vitro tumor model. Cancer Chemother Pharmacol. 2003;52:263. doi: 10.1007/s00280-003-0664-2. [DOI] [PubMed] [Google Scholar]
- 26.Chen Z. Htay A. Dos Santos W. Gillies G.T. Fillmore H.L. Sholley M.M., et al. In vitro angiogenesis by human umbilical vein endothelial cells (HUVEC) induced by three-dimensional co-culture with glioblastoma cells. J Neurooncol. 2009;92:121. doi: 10.1007/s11060-008-9742-y. [DOI] [PubMed] [Google Scholar]
- 27.Verbridge S.S. Choi N.W. Zheng Y. Brooks D.J. Stroock A.D. Fischbach C. Oxygen-controlled three-dimensional cultures to analyze tumor angiogenesis. Tissue Eng Part A. 2010;16:2133. doi: 10.1089/ten.tea.2009.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Correa de Sampaio P. Auslaender D. Krubasik D. Failla A.V. Skepper J.N. Murphy G., et al. A heterogeneous in vitro three dimensional model of tumour-stroma interactions regulating sprouting angiogenesis. PLoS One. 2012;7:e30753. doi: 10.1371/journal.pone.0030753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szot C.S. Buchanan C.F. Freeman J.W. Rylander M.N. 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials. 2011;32:7905. doi: 10.1016/j.biomaterials.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kebers F. Lewalle J.M. Desreux J. Munaut C. Devy L. Foidart J.M., et al. Induction of endothelial cell apoptosis by solid tumor cells. Exp Cell Res. 1998;240:197. doi: 10.1006/excr.1998.3935. [DOI] [PubMed] [Google Scholar]
- 31.Szot C.S. Buchanan C.F. Gatenholm P. Rylander M.N. Freeman J.W. Investigation of cancer cell behaviour on nanofibrous scaffolds. Mater Sci Eng C. 2011;31:37. [Google Scholar]
- 32.Erkan M. Reiser-Erkan C. Michalski C.W. Deucker S. Sauliunaite D. Streit S., et al. Cancer-stellate cell interactions perpetuate the hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma. Neoplasia. 2009;11:497. doi: 10.1593/neo.81618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cross V.L. Zheng Y. Won Choi N. Verbridge S.S. Sutermaster B.A. Bonassar L.J., et al. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials. 2010;31:8596. doi: 10.1016/j.biomaterials.2010.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchanan C.F. Szot C.S. Wilson T.D. Akman S. Metheny-Barlow L.J. Robertson J.L., et al. Cross-talk between endothelial and breast cancer cells regulates reciprocal expression of angiogenic factors in vitro. J Cell Biochem. 2012;113:1142. doi: 10.1002/jcb.23447. [DOI] [PubMed] [Google Scholar]
- 35.Kozien D. Gerol M. Hendey B. RayChaudhury A. A novel in vitro model of tumor angiogenesis. In Vitro Cell Dev Biol Anim. 2000;36:555. doi: 10.1007/BF02577521. [DOI] [PubMed] [Google Scholar]
- 36.Venetsanakos E. Mirza A. Fanton C. Romanov S.R. Tlsty T. McMahon M. Induction of tubulogenesis in telomerase-immortalized human microvascular endothelial cells by glioblastoma cells. Exp Cell Res. 2002;273:21. doi: 10.1006/excr.2001.5424. [DOI] [PubMed] [Google Scholar]
- 37.Chung S. Sudo R. Mack P.J. Wan C.R. Vickerman V. Kamm R.D. Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab Chip. 2009;9:269. doi: 10.1039/b807585a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.