Abstract
The title compound, (NH4)2[Zn(NCS)4]·2C12H24O6·H2O, the result of the reaction of ammonium thiocyanate, 18-crown-6 and zinc(II) chloride in aqueous solution, exhibits an unusual supramolecular structure. The Zn atom, two of the thiocyanate chains and a water molecule, disordered over two positions, lie on a mirror plane. The macrocycle adopts a conformation with approximate D 3d symmetry. The ammonium molecules are contained within the bowl of the macrocycle via extensive N—H⋯O hydrogen bonds and the complex molecules are linked via N—H⋯S hydrogen bonds, forming chains along the c-axis direction. The macrocycle is disordered over two positions [refined occupancy ratio = 0.666 (8):0.334 (8)]. The S atoms of two isothiocyanate ligands are disordered within and about the mirror plane.
Related literature
For background to crown ether/ammonium ion complexes, see: Fender et al. (2002 ▶); Kryatova et al. (2004 ▶); Akutagawa et al. (2002 ▶); Ramesh et al. (2012 ▶).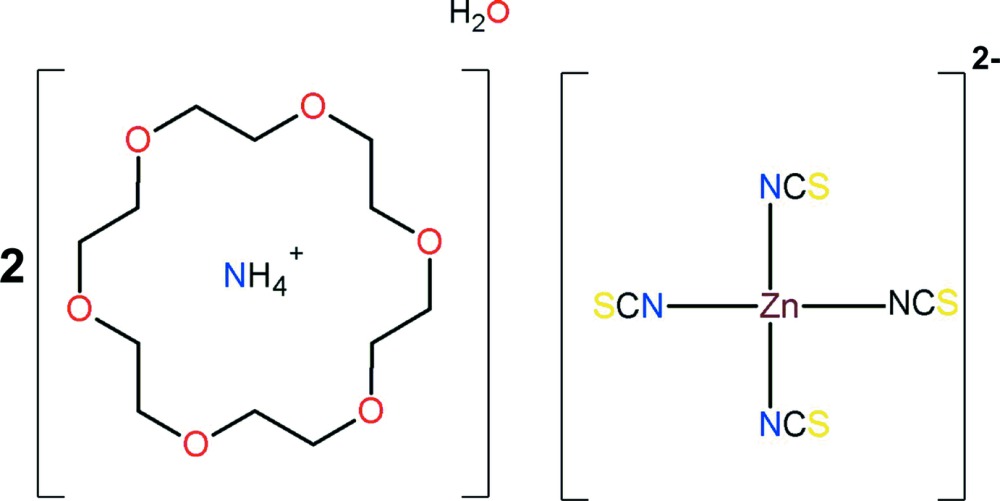
Experimental
Crystal data
(NH4)2[Zn(NCS)4]·2C12H24O6·H2O
M r = 880.41
Orthorhombic,
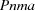
a = 22.7875 (12) Å
b = 23.6254 (12) Å
c = 8.5593 (5) Å
V = 4608.0 (4) Å3
Z = 4
Mo Kα radiation
μ = 0.77 mm−1
T = 293 K
0.30 × 0.25 × 0.20 mm
Data collection
Bruker Kappa APEXII CCD diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2004 ▶) T min = 0.802, T max = 0.861
24101 measured reflections
4641 independent reflections
2467 reflections with I > 2σ(I)
R int = 0.058
Refinement
R[F 2 > 2σ(F 2)] = 0.052
wR(F 2) = 0.175
S = 1.00
4641 reflections
454 parameters
276 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.72 e Å−3
Δρmin = −0.24 e Å−3
Data collection: APEX2 (Bruker, 2004 ▶); cell refinement: APEX2 and SAINT (Bruker, 2004 ▶); data reduction: SAINT and XPREP (Bruker, 2004 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 2012 ▶) and Mercury (Macrae et al., 2008 ▶); software used to prepare material for publication: WinGX (Farrugia, 2012 ▶) and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536813019739/su2617sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813019739/su2617Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N4—H4H⋯O1 | 0.88 (1) | 2.44 (4) | 3.069 (8) | 129 (4) |
| N4—H4E⋯O2 | 0.87 (1) | 2.06 (1) | 2.934 (8) | 176 (5) |
| N4—H4G⋯O4 | 0.88 (1) | 1.97 (2) | 2.829 (7) | 166 (4) |
| N4—H4G⋯O5 | 0.88 (1) | 2.53 (4) | 3.010 (8) | 115 (3) |
| N4—H4H⋯O6 | 0.88 (1) | 2.05 (3) | 2.850 (8) | 150 (5) |
| N4—H4F⋯S1i | 0.87 (1) | 2.63 (2) | 3.441 (4) | 156 (4) |
Symmetry code: (i) 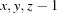 .
.
Acknowledgments
The authors are thankful to the SAIF, IIT Madras, for the data collection. KR thanks the University Grants Commission, Government of India, for financial support granted under a Major Research Project [F. No.41–1008/2012 (SR)].
supplementary crystallographic information
Comment
There is currently significant interest in crown ethers because of their ability to form non-covalent, hydrogen bonding complexes with ammonium cations both in the solid state and in solution (Fender et al., 2002; Kryatova et al., 2004; Akutagawa et al., 2002). Recently, the crystal structure of catena-Poly[ammonium(cadmium-tri-lthiocyanato κ4S:N; κ2N:S) -1,4,10,13,16- hexaoxacyclooctadecane (1/1)] (I), obtained in our laboratory, has been reported (Ramesh et al., 2012). In continuation of our studies of compounds containing 18-crown-6 macrocycles and ammonium cations NH4+, we describe herein the crystal structure of the title compound (II), which is isostructural with (I).
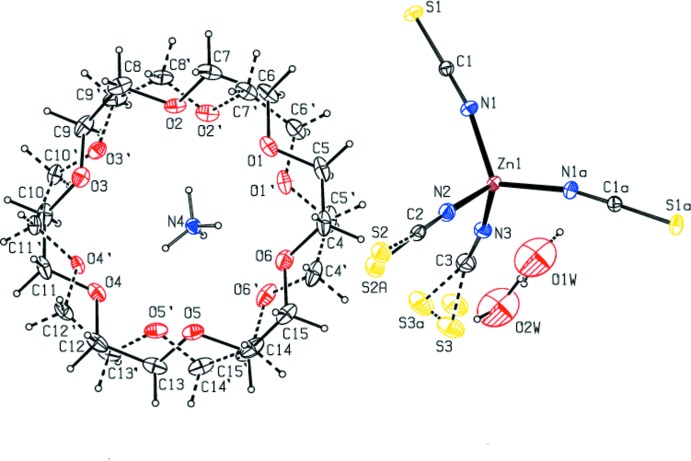
The reaction of ammonium thiocyanate, 18-crown-6 and Zinc (II) chloride in aqueous solution yields the title compound, Fig. 1. All bond lengths and angles are normal and correspond to those reported for (I) (Ramesh et al., 2012). The C—S [average value of 1.658 (2) Å] and C—N [average value of 1.116 (2) Å] bond lengths indicate the presence of double-bond character. The zinc atom, Zn1, two of the thiocyanate chains [N2—C2—S2 and N3—C3—S3; symmetry code: x, -y + 1/2, z] and the water molecule lie in a mirror plane, except one of the disordered component sulfur atoms, which is inclined at quite an angle to the ac plane. The thiocyanate (N1—C1—S1 = 178.2 (4) °) ligands are almost linear.
The macrocycle adopts a conformation with approximate D3d symmetry, with all O—C—C—O torsion angles being gauche and alternating in sign, and all C—O—C—C torsion angles being anti.
The sulfur atoms (S2 and S3) of the thiocyanate chains are disordered with large displacement parameters for the S atoms and short C—S bond lengths. The disorder over two positions was modelled and the site occupancies refined to 0.39 (9) and 0.61 (9) for atom S2 and 0.376 (9) and 0.248 (18) for atom S3. The entire crown either molecule is disordered, as detectable from the large displacement parameters for C and O atoms and short C—C and C—O bond lengths. The disorder over two positions was modelled and the site occupancies refined to 0.666 (9) and 0.334 (9) for carbon and oxygen atoms. The water molecule is disordered, as detectable from the large displacement parameters for the O atoms. The disorder over two positions was modelled and the site occupancies refined to 0.425 (15) and 0.575 (15). for carbon and oxygen atoms. The geometry was regularized by soft restraints.
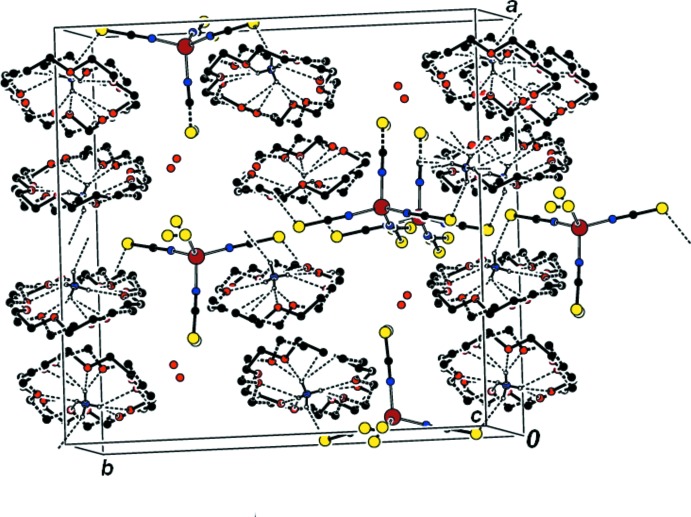
The ammonium cations are contained within the bowl of the macrocycle via extensive N—H···O hydrogen bonding. The N—H···O [2.830 (7) to 3.074 (7) Å] and N—H···S [3.442 (4) Å] hydrogen bond lengths are within the usual range (Table 1 and Fig. 2).
In the crystal, the complex molecules are linked via N—H···S hydrogen bonds forming chains along the c axis (Table 1 and Fig. 2).
Experimental
A mixture of 18-crown-6, ammonium thiocyanate and Zinc (II) chloride were dissolved in an aqueous solution in the molar ratio 2:4:1 and thoroughly mixed for two hours to obtain a homogeneous mixture. The solution was allowed to evaporate slowly at ambient temperature. Colourless single crystals suitable for single-crystal X-ray diffraction analysis were obtained in a week.
Refinement
The sulfur atoms (S2 and S3) of the thiocyanate chain are disordered over two positions with refined occupancies of 0.39 (9) and 0.61 (9) for atom S2, and 0.376 (9) and 0.248 (18) for atom S3. The entire crown either molecule is disordered over two positions with refined occupancies of 0.666 (9) and 0.334 (9). The water molecule is disordered over two positions with refined occupancies of 0.425 (15) and 0.575 (15). The corresponding bond distances involving the disordered atoms were restrained to be equal.
The N-bound H (N—H = 0.87 Å) atoms was located in difference map and refined in the riding mode approximation. C-bound H-atoms were placed in calculated positions [C—H 0.97 Å, Uiso(H) 1.2Ueq(C)] and were included in the refinement in the riding model approximation. The water H-atom, whose O atom lies on a mirror plane, was similar treated [O—H 0.61–0.90 Å].
Figures
Fig. 1.
Crystal structure of the title compound with atom labelling. Displacement ellipsoids are drawn at the 30% probability level [symmetry code: (a) x, -y+1/2, z; the disordered fraction is shown with dashed bonds and atom labels with prefix '].
Fig. 2.
A view along the c axis of the crystal packing of the title compound. Hydrogen bonds are shown as dashed lines (see Table 1 for details).
Crystal data
| (NH4)2[Zn(NCS)4]·2C12H24O6·H2O | F(000) = 1864 |
| Mr = 880.41 | Dx = 1.269 Mg m−3 |
| Orthorhombic, Pnma | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ac 2n | Cell parameters from 5119 reflections |
| a = 22.7875 (12) Å | θ = 2.6–26.7° |
| b = 23.6254 (12) Å | µ = 0.77 mm−1 |
| c = 8.5593 (5) Å | T = 293 K |
| V = 4608.0 (4) Å3 | Block, colourless |
| Z = 4 | 0.30 × 0.25 × 0.20 mm |
Data collection
| Bruker Kappa APEXII CCD diffractometer | 4641 independent reflections |
| Radiation source: fine-focus sealed tube | 2467 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.058 |
| ω and φ scan | θmax = 26.0°, θmin = 2.5° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 2004) | h = −24→28 |
| Tmin = 0.802, Tmax = 0.861 | k = −19→29 |
| 24101 measured reflections | l = −10→9 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.052 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.175 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.00 | w = 1/[σ2(Fo2) + (0.0932P)2 + 0.2607P] where P = (Fo2 + 2Fc2)/3 |
| 4641 reflections | (Δ/σ)max = 0.001 |
| 454 parameters | Δρmax = 0.72 e Å−3 |
| 276 restraints | Δρmin = −0.24 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| C1 | 0.51074 (15) | 0.13492 (16) | 0.9699 (5) | 0.0534 (10) | |
| C2 | 0.6728 (3) | 0.2500 | 0.7518 (7) | 0.0668 (16) | |
| C3 | 0.4978 (4) | 0.2500 | 0.4932 (11) | 0.094 (2) | |
| O1 | 0.6093 (3) | 0.0412 (3) | 0.5640 (8) | 0.086 (2) | 0.666 (8) |
| O2 | 0.6025 (4) | −0.0710 (3) | 0.4650 (8) | 0.082 (2) | 0.666 (8) |
| O3 | 0.6681 (3) | −0.1007 (3) | 0.1940 (9) | 0.079 (2) | 0.666 (8) |
| O4 | 0.6950 (3) | −0.0163 (3) | −0.0249 (8) | 0.0798 (19) | 0.666 (8) |
| O5 | 0.6998 (3) | 0.0974 (4) | 0.0798 (7) | 0.083 (2) | 0.666 (8) |
| O6 | 0.6318 (3) | 0.1232 (3) | 0.3415 (9) | 0.092 (2) | 0.666 (8) |
| C4 | 0.6209 (4) | 0.1375 (4) | 0.4993 (11) | 0.103 (3) | 0.666 (8) |
| H4A | 0.6025 | 0.1744 | 0.5053 | 0.124* | 0.666 (8) |
| H4B | 0.6576 | 0.1388 | 0.5567 | 0.124* | 0.666 (8) |
| C5 | 0.5811 (4) | 0.0935 (4) | 0.5696 (12) | 0.106 (3) | 0.666 (8) |
| H5A | 0.5721 | 0.1034 | 0.6770 | 0.127* | 0.666 (8) |
| H5B | 0.5446 | 0.0918 | 0.5115 | 0.127* | 0.666 (8) |
| C6 | 0.5761 (5) | 0.0013 (5) | 0.6449 (14) | 0.097 (4) | 0.666 (8) |
| H6A | 0.5363 | 0.0007 | 0.6046 | 0.116* | 0.666 (8) |
| H6B | 0.5745 | 0.0110 | 0.7549 | 0.116* | 0.666 (8) |
| C7 | 0.6040 (4) | −0.0556 (5) | 0.6243 (9) | 0.102 (3) | 0.666 (8) |
| H7A | 0.6443 | −0.0544 | 0.6603 | 0.122* | 0.666 (8) |
| H7B | 0.5831 | −0.0835 | 0.6859 | 0.122* | 0.666 (8) |
| C8 | 0.6256 (5) | −0.1260 (4) | 0.4391 (12) | 0.103 (3) | 0.666 (8) |
| H8A | 0.6009 | −0.1539 | 0.4898 | 0.123* | 0.666 (8) |
| H8B | 0.6646 | −0.1286 | 0.4840 | 0.123* | 0.666 (8) |
| C9 | 0.6284 (4) | −0.1378 (4) | 0.2670 (12) | 0.094 (3) | 0.666 (8) |
| H9A | 0.6407 | −0.1766 | 0.2500 | 0.113* | 0.666 (8) |
| H9B | 0.5897 | −0.1331 | 0.2214 | 0.113* | 0.666 (8) |
| C10 | 0.6751 (5) | −0.1127 (5) | 0.0337 (12) | 0.093 (4) | 0.666 (8) |
| H10A | 0.6377 | −0.1093 | −0.0199 | 0.111* | 0.666 (8) |
| H10B | 0.6895 | −0.1510 | 0.0198 | 0.111* | 0.666 (8) |
| C11 | 0.7185 (4) | −0.0709 (4) | −0.0321 (10) | 0.091 (3) | 0.666 (8) |
| H11A | 0.7547 | −0.0724 | 0.0273 | 0.109* | 0.666 (8) |
| H11B | 0.7273 | −0.0805 | −0.1397 | 0.109* | 0.666 (8) |
| C12 | 0.7343 (5) | 0.0238 (5) | −0.0886 (15) | 0.094 (4) | 0.666 (8) |
| H12A | 0.7426 | 0.0148 | −0.1969 | 0.113* | 0.666 (8) |
| H12B | 0.7709 | 0.0237 | −0.0308 | 0.113* | 0.666 (8) |
| C13 | 0.7052 (4) | 0.0806 (5) | −0.0767 (10) | 0.098 (3) | 0.666 (8) |
| H13A | 0.7282 | 0.1084 | −0.1333 | 0.118* | 0.666 (8) |
| H13B | 0.6666 | 0.0788 | −0.1241 | 0.118* | 0.666 (8) |
| C14 | 0.6687 (5) | 0.1489 (5) | 0.0924 (14) | 0.107 (4) | 0.666 (8) |
| H14A | 0.6296 | 0.1447 | 0.0488 | 0.129* | 0.666 (8) |
| H14B | 0.6889 | 0.1785 | 0.0351 | 0.129* | 0.666 (8) |
| C15 | 0.6647 (5) | 0.1645 (4) | 0.2617 (13) | 0.107 (3) | 0.666 (8) |
| H15A | 0.7038 | 0.1669 | 0.3062 | 0.128* | 0.666 (8) |
| H15B | 0.6460 | 0.2012 | 0.2726 | 0.128* | 0.666 (8) |
| O1' | 0.6101 (6) | 0.0748 (7) | 0.5055 (18) | 0.095 (4) | 0.334 (8) |
| O2' | 0.5880 (8) | −0.0418 (7) | 0.489 (2) | 0.094 (4) | 0.334 (8) |
| O3' | 0.6481 (6) | −0.1061 (6) | 0.2641 (16) | 0.073 (4) | 0.334 (8) |
| O4' | 0.6913 (5) | −0.0500 (6) | 0.0013 (14) | 0.062 (3) | 0.334 (8) |
| O5' | 0.7128 (6) | 0.0659 (6) | 0.0284 (19) | 0.082 (4) | 0.334 (8) |
| O6' | 0.6437 (7) | 0.1284 (6) | 0.2342 (19) | 0.103 (5) | 0.334 (8) |
| C4' | 0.6350 (10) | 0.1591 (9) | 0.373 (2) | 0.115 (6) | 0.334 (8) |
| H4'1 | 0.6223 | 0.1974 | 0.3495 | 0.138* | 0.334 (8) |
| H4'2 | 0.6714 | 0.1613 | 0.4319 | 0.138* | 0.334 (8) |
| C5' | 0.5891 (10) | 0.1293 (8) | 0.468 (3) | 0.118 (7) | 0.334 (8) |
| H5'1 | 0.5809 | 0.1504 | 0.5623 | 0.142* | 0.334 (8) |
| H5'2 | 0.5531 | 0.1263 | 0.4079 | 0.142* | 0.334 (8) |
| C6' | 0.5720 (9) | 0.0449 (9) | 0.604 (2) | 0.103 (6) | 0.334 (8) |
| H6'1 | 0.5334 | 0.0432 | 0.5563 | 0.124* | 0.334 (8) |
| H6'2 | 0.5683 | 0.0649 | 0.7024 | 0.124* | 0.334 (8) |
| C7' | 0.5935 (12) | −0.0141 (10) | 0.634 (2) | 0.098 (7) | 0.334 (8) |
| H7'1 | 0.6340 | −0.0137 | 0.6686 | 0.118* | 0.334 (8) |
| H7'2 | 0.5697 | −0.0326 | 0.7132 | 0.118* | 0.334 (8) |
| C8' | 0.6053 (10) | −0.0980 (7) | 0.513 (2) | 0.094 (6) | 0.334 (8) |
| H8'1 | 0.5787 | −0.1159 | 0.5865 | 0.113* | 0.334 (8) |
| H8'2 | 0.6444 | −0.0987 | 0.5586 | 0.113* | 0.334 (8) |
| C9' | 0.6055 (9) | −0.1305 (8) | 0.363 (2) | 0.093 (6) | 0.334 (8) |
| H9'1 | 0.6148 | −0.1699 | 0.3822 | 0.112* | 0.334 (8) |
| H9'2 | 0.5671 | −0.1285 | 0.3139 | 0.112* | 0.334 (8) |
| C10' | 0.6499 (6) | −0.1330 (6) | 0.1179 (19) | 0.071 (4) | 0.334 (8) |
| H10C | 0.6134 | −0.1267 | 0.0621 | 0.085* | 0.334 (8) |
| H10D | 0.6553 | −0.1735 | 0.1312 | 0.085* | 0.334 (8) |
| C11' | 0.7003 (6) | −0.1085 (6) | 0.028 (3) | 0.058 (4) | 0.334 (8) |
| H11C | 0.7365 | −0.1139 | 0.0858 | 0.070* | 0.334 (8) |
| H11D | 0.7041 | −0.1279 | −0.0716 | 0.070* | 0.334 (8) |
| C12' | 0.7390 (6) | −0.0224 (7) | −0.0687 (18) | 0.080 (4) | 0.334 (8) |
| H12C | 0.7514 | −0.0431 | −0.1608 | 0.096* | 0.334 (8) |
| H12D | 0.7717 | −0.0209 | 0.0038 | 0.096* | 0.334 (8) |
| C13' | 0.7211 (12) | 0.0367 (9) | −0.114 (2) | 0.082 (6) | 0.334 (8) |
| H13C | 0.7515 | 0.0547 | −0.1755 | 0.098* | 0.334 (8) |
| H13D | 0.6850 | 0.0362 | −0.1738 | 0.098* | 0.334 (8) |
| C14' | 0.6902 (10) | 0.1195 (6) | −0.007 (3) | 0.105 (6) | 0.334 (8) |
| H14C | 0.6521 | 0.1152 | −0.0563 | 0.125* | 0.334 (8) |
| H14D | 0.7160 | 0.1382 | −0.0809 | 0.125* | 0.334 (8) |
| C15' | 0.6838 (11) | 0.1560 (10) | 0.135 (3) | 0.105 (7) | 0.334 (8) |
| H15C | 0.7213 | 0.1605 | 0.1871 | 0.127* | 0.334 (8) |
| H15D | 0.6692 | 0.1932 | 0.1067 | 0.127* | 0.334 (8) |
| N1 | 0.52011 (15) | 0.18067 (14) | 0.9297 (4) | 0.0703 (10) | |
| N2 | 0.6247 (3) | 0.2500 | 0.7865 (7) | 0.0768 (16) | |
| N3 | 0.5010 (3) | 0.2500 | 0.6166 (7) | 0.0796 (16) | |
| N4 | 0.61488 (17) | 0.01292 (18) | 0.2144 (5) | 0.0594 (9) | |
| S1 | 0.49673 (5) | 0.07035 (4) | 1.02060 (15) | 0.0704 (4) | |
| S2 | 0.7443 (4) | 0.2500 | 0.740 (7) | 0.100 (6) | 0.39 (9) |
| S2A | 0.7397 (6) | 0.2500 | 0.684 (5) | 0.098 (5) | 0.61 (9) |
| S3 | 0.5017 (4) | 0.2703 (4) | 0.3036 (5) | 0.138 (3) | 0.376 (9) |
| S3A | 0.4660 (14) | 0.2500 | 0.3119 (17) | 0.161 (7) | 0.248 (18) |
| Zn1 | 0.54016 (3) | 0.2500 | 0.82110 (8) | 0.0587 (3) | |
| O1W | 0.3179 (18) | 0.2500 | 0.361 (5) | 0.328 (15) | 0.425 (15) |
| O2W | 0.3443 (14) | 0.2500 | 0.199 (3) | 0.328 (15) | 0.575 (15) |
| H1W | 0.31 (2) | 0.2500 | 0.258 (11) | 0.492* | 0.425 (15) |
| H2W | 0.289 (15) | 0.2500 | 0.43 (4) | 0.492* | 0.425 (15) |
| H3W | 0.327 (15) | 0.2500 | 0.29 (2) | 0.492* | 0.575 (15) |
| H4W | 0.384 (3) | 0.2500 | 0.19 (5) | 0.492* | 0.575 (15) |
| H4E | 0.609 (2) | −0.0122 (17) | 0.288 (4) | 0.11 (2)* | |
| H4F | 0.5826 (12) | 0.016 (2) | 0.160 (5) | 0.099 (18)* | |
| H4G | 0.6438 (14) | 0.0081 (19) | 0.148 (4) | 0.097 (17)* | |
| H4H | 0.624 (2) | 0.0393 (16) | 0.282 (5) | 0.11 (2)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.048 (2) | 0.053 (2) | 0.059 (3) | 0.0033 (18) | 0.0027 (18) | 0.004 (2) |
| C2 | 0.089 (5) | 0.043 (3) | 0.069 (4) | 0.000 | 0.003 (4) | 0.000 |
| C3 | 0.110 (6) | 0.090 (5) | 0.083 (6) | 0.000 | −0.001 (5) | 0.000 |
| O1 | 0.080 (4) | 0.112 (5) | 0.067 (4) | 0.007 (4) | 0.016 (3) | −0.017 (4) |
| O2 | 0.097 (5) | 0.083 (5) | 0.065 (4) | −0.013 (4) | −0.009 (3) | 0.026 (4) |
| O3 | 0.085 (5) | 0.073 (4) | 0.081 (5) | 0.002 (3) | −0.018 (4) | −0.011 (4) |
| O4 | 0.061 (4) | 0.113 (5) | 0.065 (4) | 0.007 (4) | 0.013 (3) | −0.003 (4) |
| O5 | 0.082 (4) | 0.087 (5) | 0.080 (5) | −0.003 (4) | −0.014 (3) | 0.031 (4) |
| O6 | 0.110 (4) | 0.070 (4) | 0.097 (6) | 0.012 (3) | −0.008 (4) | −0.008 (4) |
| C4 | 0.108 (7) | 0.098 (7) | 0.102 (7) | 0.038 (6) | −0.010 (6) | −0.047 (6) |
| C5 | 0.094 (6) | 0.137 (8) | 0.086 (7) | 0.031 (7) | 0.011 (5) | −0.042 (7) |
| C6 | 0.096 (8) | 0.139 (10) | 0.056 (6) | −0.014 (7) | 0.023 (5) | −0.013 (6) |
| C7 | 0.114 (6) | 0.133 (8) | 0.059 (6) | −0.031 (6) | 0.004 (5) | 0.023 (6) |
| C8 | 0.120 (8) | 0.084 (6) | 0.105 (8) | −0.019 (6) | −0.026 (6) | 0.030 (6) |
| C9 | 0.105 (7) | 0.057 (5) | 0.119 (9) | 0.002 (5) | −0.032 (6) | −0.006 (6) |
| C10 | 0.092 (9) | 0.089 (7) | 0.097 (7) | 0.030 (6) | −0.022 (7) | −0.033 (6) |
| C11 | 0.078 (6) | 0.131 (9) | 0.063 (5) | 0.038 (7) | 0.002 (5) | −0.034 (6) |
| C12 | 0.061 (6) | 0.155 (10) | 0.068 (7) | −0.007 (6) | 0.017 (5) | 0.000 (8) |
| C13 | 0.085 (6) | 0.138 (9) | 0.072 (6) | −0.023 (6) | 0.007 (5) | 0.022 (7) |
| C14 | 0.122 (7) | 0.075 (7) | 0.124 (9) | −0.010 (6) | −0.040 (7) | 0.036 (7) |
| C15 | 0.129 (7) | 0.058 (5) | 0.133 (9) | 0.009 (5) | −0.017 (7) | 0.012 (6) |
| O1' | 0.087 (7) | 0.101 (10) | 0.097 (10) | 0.023 (8) | 0.017 (7) | −0.039 (8) |
| O2' | 0.098 (9) | 0.108 (11) | 0.075 (8) | −0.022 (8) | −0.004 (7) | 0.019 (8) |
| O3' | 0.072 (8) | 0.053 (6) | 0.094 (10) | −0.012 (6) | −0.007 (6) | 0.004 (7) |
| O4' | 0.047 (6) | 0.076 (8) | 0.063 (7) | 0.009 (6) | 0.018 (5) | −0.014 (7) |
| O5' | 0.086 (8) | 0.095 (9) | 0.066 (8) | −0.023 (7) | −0.022 (7) | 0.027 (8) |
| O6' | 0.123 (10) | 0.056 (7) | 0.129 (14) | 0.007 (6) | −0.046 (9) | −0.009 (8) |
| C4' | 0.141 (13) | 0.061 (10) | 0.143 (16) | 0.026 (10) | −0.024 (12) | −0.034 (11) |
| C5' | 0.112 (12) | 0.095 (11) | 0.147 (15) | 0.034 (11) | −0.015 (12) | −0.051 (11) |
| C6' | 0.106 (12) | 0.123 (14) | 0.081 (11) | −0.001 (12) | 0.026 (10) | −0.014 (11) |
| C7' | 0.102 (13) | 0.123 (14) | 0.070 (12) | −0.014 (12) | 0.020 (10) | 0.002 (12) |
| C8' | 0.104 (11) | 0.094 (12) | 0.084 (12) | −0.036 (10) | 0.006 (10) | 0.031 (10) |
| C9' | 0.113 (12) | 0.075 (10) | 0.091 (13) | −0.040 (9) | −0.017 (10) | 0.024 (10) |
| C10' | 0.063 (8) | 0.051 (7) | 0.099 (11) | 0.007 (6) | −0.011 (8) | −0.016 (8) |
| C11' | 0.044 (9) | 0.058 (8) | 0.074 (9) | 0.003 (7) | −0.007 (7) | −0.024 (7) |
| C12' | 0.087 (10) | 0.102 (11) | 0.052 (9) | −0.019 (9) | 0.017 (7) | −0.023 (9) |
| C13' | 0.083 (12) | 0.114 (13) | 0.049 (10) | −0.023 (11) | 0.004 (9) | 0.022 (9) |
| C14' | 0.124 (12) | 0.084 (11) | 0.106 (13) | −0.024 (10) | −0.035 (11) | 0.035 (11) |
| C15' | 0.140 (13) | 0.057 (9) | 0.120 (16) | −0.020 (10) | −0.046 (12) | 0.011 (10) |
| N1 | 0.087 (2) | 0.0477 (19) | 0.076 (3) | −0.0038 (17) | 0.0057 (19) | 0.0091 (19) |
| N2 | 0.069 (3) | 0.063 (3) | 0.099 (5) | 0.000 | 0.015 (3) | 0.000 |
| N3 | 0.110 (5) | 0.068 (3) | 0.060 (4) | 0.000 | 0.009 (4) | 0.000 |
| N4 | 0.061 (3) | 0.067 (2) | 0.051 (3) | 0.007 (2) | −0.001 (2) | −0.002 (2) |
| S1 | 0.0688 (7) | 0.0521 (6) | 0.0903 (9) | −0.0072 (5) | −0.0075 (6) | 0.0253 (6) |
| S2 | 0.080 (5) | 0.099 (5) | 0.121 (17) | 0.000 | 0.029 (5) | 0.000 |
| S2A | 0.070 (3) | 0.104 (3) | 0.121 (12) | 0.000 | 0.025 (4) | 0.000 |
| S3 | 0.149 (6) | 0.198 (9) | 0.067 (3) | −0.007 (4) | −0.005 (3) | 0.016 (3) |
| S3A | 0.234 (17) | 0.175 (14) | 0.076 (7) | 0.000 | 0.019 (11) | 0.000 |
| Zn1 | 0.0725 (5) | 0.0362 (3) | 0.0674 (5) | 0.000 | 0.0085 (4) | 0.000 |
| O1W | 0.39 (3) | 0.34 (2) | 0.25 (3) | 0.000 | −0.20 (3) | 0.000 |
| O2W | 0.39 (3) | 0.34 (2) | 0.25 (3) | 0.000 | −0.20 (3) | 0.000 |
Geometric parameters (Å, º)
| C1—N1 | 1.154 (4) | O2'—C7' | 1.410 (10) |
| C1—S1 | 1.618 (4) | O3'—C10' | 1.405 (10) |
| C2—N2 | 1.136 (7) | O3'—C9' | 1.410 (10) |
| C2—S2A | 1.631 (9) | O4'—C12' | 1.403 (9) |
| C2—S2 | 1.632 (10) | O4'—C11' | 1.414 (10) |
| C3—N3 | 1.058 (8) | O5'—C14' | 1.403 (10) |
| C3—S3i | 1.695 (10) | O5'—C13' | 1.409 (10) |
| C3—S3 | 1.695 (10) | O6'—C15' | 1.406 (10) |
| C3—S3A | 1.714 (12) | O6'—C4' | 1.409 (10) |
| O1—C6 | 1.393 (9) | C4'—C5' | 1.497 (10) |
| O1—C5 | 1.394 (8) | C4'—H4'1 | 0.9700 |
| O2—C7 | 1.411 (8) | C4'—H4'2 | 0.9700 |
| O2—C8 | 1.420 (8) | C5'—H5'1 | 0.9700 |
| O3—C9 | 1.406 (8) | C5'—H5'2 | 0.9700 |
| O3—C10 | 1.410 (8) | C6'—C7' | 1.501 (10) |
| O4—C11 | 1.398 (7) | C6'—H6'1 | 0.9700 |
| O4—C12 | 1.411 (9) | C6'—H6'2 | 0.9700 |
| O5—C13 | 1.402 (8) | C7'—H7'1 | 0.9700 |
| O5—C14 | 1.413 (9) | C7'—H7'2 | 0.9700 |
| O6—C15 | 1.407 (8) | C8'—C9' | 1.500 (10) |
| O6—C4 | 1.414 (8) | C8'—H8'1 | 0.9700 |
| C4—C5 | 1.504 (8) | C8'—H8'2 | 0.9700 |
| C4—H4A | 0.9700 | C9'—H9'1 | 0.9700 |
| C4—H4B | 0.9700 | C9'—H9'2 | 0.9700 |
| C5—H5A | 0.9700 | C10'—C11' | 1.500 (10) |
| C5—H5B | 0.9700 | C10'—H10C | 0.9700 |
| C6—C7 | 1.499 (9) | C10'—H10D | 0.9700 |
| C6—H6A | 0.9700 | C11'—H11C | 0.9700 |
| C6—H6B | 0.9700 | C11'—H11D | 0.9700 |
| C7—H7A | 0.9700 | C12'—C13' | 1.506 (10) |
| C7—H7B | 0.9700 | C12'—H12C | 0.9700 |
| C8—C9 | 1.501 (8) | C12'—H12D | 0.9700 |
| C8—H8A | 0.9700 | C13'—H13C | 0.9700 |
| C8—H8B | 0.9700 | C13'—H13D | 0.9700 |
| C9—H9A | 0.9700 | C14'—C15' | 1.499 (10) |
| C9—H9B | 0.9700 | C14'—H14C | 0.9700 |
| C10—C11 | 1.507 (8) | C14'—H14D | 0.9700 |
| C10—H10A | 0.9700 | C15'—H15C | 0.9700 |
| C10—H10B | 0.9700 | C15'—H15D | 0.9700 |
| C11—H11A | 0.9700 | N1—Zn1 | 1.938 (3) |
| C11—H11B | 0.9700 | N2—Zn1 | 1.948 (6) |
| C12—C13 | 1.501 (8) | N3—Zn1 | 1.964 (7) |
| C12—H12A | 0.9700 | N4—H4E | 0.873 (10) |
| C12—H12B | 0.9700 | N4—H4F | 0.874 (10) |
| C13—H13A | 0.9700 | N4—H4G | 0.878 (10) |
| C13—H13B | 0.9700 | N4—H4H | 0.878 (10) |
| C14—C15 | 1.497 (9) | S3—S3i | 0.957 (17) |
| C14—H14A | 0.9700 | Zn1—N1i | 1.938 (3) |
| C14—H14B | 0.9700 | O1W—H1W | 0.900 (11) |
| C15—H15A | 0.9700 | O1W—H2W | 0.899 (10) |
| C15—H15B | 0.9700 | O1W—H3W | 0.61 (7) |
| O1'—C6' | 1.401 (10) | O2W—H1W | 0.9 (5) |
| O1'—C5' | 1.411 (10) | O2W—H3W | 0.900 (10) |
| O2'—C8' | 1.401 (10) | O2W—H4W | 0.898 (11) |
| N1—C1—S1 | 178.1 (4) | O6'—C4'—H4'1 | 110.1 |
| N2—C2—S2A | 174.3 (16) | C5'—C4'—H4'1 | 110.1 |
| N2—C2—S2 | 168 (2) | O6'—C4'—H4'2 | 110.1 |
| N3—C3—S3i | 162.2 (5) | C5'—C4'—H4'2 | 110.1 |
| N3—C3—S3 | 162.2 (5) | H4'1—C4'—H4'2 | 108.4 |
| N3—C3—S3A | 158.9 (13) | O1'—C5'—C4' | 108.5 (17) |
| C6—O1—C5 | 109.4 (8) | O1'—C5'—H5'1 | 110.0 |
| C7—O2—C8 | 112.2 (8) | C4'—C5'—H5'1 | 110.0 |
| C9—O3—C10 | 112.3 (7) | O1'—C5'—H5'2 | 110.0 |
| C11—O4—C12 | 111.0 (8) | C4'—C5'—H5'2 | 110.0 |
| C13—O5—C14 | 111.2 (9) | H5'1—C5'—H5'2 | 108.4 |
| C15—O6—C4 | 113.1 (9) | O1'—C6'—C7' | 112 (2) |
| O6—C4—C5 | 108.9 (7) | O1'—C6'—H6'1 | 109.3 |
| O6—C4—H4A | 109.9 | C7'—C6'—H6'1 | 109.3 |
| C5—C4—H4A | 109.9 | O1'—C6'—H6'2 | 109.3 |
| O6—C4—H4B | 109.9 | C7'—C6'—H6'2 | 109.3 |
| C5—C4—H4B | 109.9 | H6'1—C6'—H6'2 | 108.0 |
| H4A—C4—H4B | 108.3 | O2'—C7'—C6' | 104.5 (18) |
| O1—C5—C4 | 108.7 (9) | O2'—C7'—H7'1 | 110.8 |
| O1—C5—H5A | 110.0 | C6'—C7'—H7'1 | 110.8 |
| C4—C5—H5A | 110.0 | O2'—C7'—H7'2 | 110.8 |
| O1—C5—H5B | 110.0 | C6'—C7'—H7'2 | 110.8 |
| C4—C5—H5B | 110.0 | H7'1—C7'—H7'2 | 108.9 |
| H5A—C5—H5B | 108.3 | O2'—C8'—C9' | 110.9 (17) |
| O1—C6—C7 | 108.6 (8) | O2'—C8'—H8'1 | 109.5 |
| O1—C6—H6A | 110.0 | C9'—C8'—H8'1 | 109.5 |
| C7—C6—H6A | 110.0 | O2'—C8'—H8'2 | 109.5 |
| O1—C6—H6B | 110.0 | C9'—C8'—H8'2 | 109.5 |
| C7—C6—H6B | 110.0 | H8'1—C8'—H8'2 | 108.0 |
| H6A—C6—H6B | 108.4 | O3'—C9'—C8' | 107.9 (14) |
| O2—C7—C6 | 109.5 (9) | O3'—C9'—H9'1 | 110.1 |
| O2—C7—H7A | 109.8 | C8'—C9'—H9'1 | 110.1 |
| C6—C7—H7A | 109.8 | O3'—C9'—H9'2 | 110.1 |
| O2—C7—H7B | 109.8 | C8'—C9'—H9'2 | 110.1 |
| C6—C7—H7B | 109.8 | H9'1—C9'—H9'2 | 108.4 |
| H7A—C7—H7B | 108.2 | O3'—C10'—C11' | 107.8 (15) |
| O2—C8—C9 | 109.8 (7) | O3'—C10'—H10C | 110.1 |
| O2—C8—H8A | 109.7 | C11'—C10'—H10C | 110.1 |
| C9—C8—H8A | 109.7 | O3'—C10'—H10D | 110.1 |
| O2—C8—H8B | 109.7 | C11'—C10'—H10D | 110.1 |
| C9—C8—H8B | 109.7 | H10C—C10'—H10D | 108.5 |
| H8A—C8—H8B | 108.2 | O4'—C11'—C10' | 110.3 (12) |
| O3—C9—C8 | 110.4 (8) | O4'—C11'—H11C | 109.6 |
| O3—C9—H9A | 109.6 | C10'—C11'—H11C | 109.6 |
| C8—C9—H9A | 109.6 | O4'—C11'—H11D | 109.6 |
| O3—C9—H9B | 109.6 | C10'—C11'—H11D | 109.6 |
| C8—C9—H9B | 109.6 | H11C—C11'—H11D | 108.1 |
| H9A—C9—H9B | 108.1 | O4'—C12'—C13' | 109.3 (18) |
| O3—C10—C11 | 107.8 (8) | O4'—C12'—H12C | 109.8 |
| O3—C10—H10A | 110.2 | C13'—C12'—H12C | 109.8 |
| C11—C10—H10A | 110.2 | O4'—C12'—H12D | 109.8 |
| O3—C10—H10B | 110.2 | C13'—C12'—H12D | 109.8 |
| C11—C10—H10B | 110.2 | H12C—C12'—H12D | 108.3 |
| H10A—C10—H10B | 108.5 | O5'—C13'—C12' | 105.6 (15) |
| O4—C11—C10 | 109.8 (9) | O5'—C13'—H13C | 110.6 |
| O4—C11—H11A | 109.7 | C12'—C13'—H13C | 110.6 |
| C10—C11—H11A | 109.7 | O5'—C13'—H13D | 110.6 |
| O4—C11—H11B | 109.7 | C12'—C13'—H13D | 110.6 |
| C10—C11—H11B | 109.7 | H13C—C13'—H13D | 108.8 |
| H11A—C11—H11B | 108.2 | O5'—C14'—C15' | 112 (2) |
| O4—C12—C13 | 107.1 (9) | O5'—C14'—H14C | 109.1 |
| O4—C12—H12A | 110.3 | C15'—C14'—H14C | 109.1 |
| C13—C12—H12A | 110.3 | O5'—C14'—H14D | 109.1 |
| O4—C12—H12B | 110.3 | C15'—C14'—H14D | 109.1 |
| C13—C12—H12B | 110.3 | H14C—C14'—H14D | 107.9 |
| H12A—C12—H12B | 108.6 | O6'—C15'—C14' | 106.6 (19) |
| O5—C13—C12 | 110.9 (11) | O6'—C15'—H15C | 110.4 |
| O5—C13—H13A | 109.5 | C14'—C15'—H15C | 110.4 |
| C12—C13—H13A | 109.5 | O6'—C15'—H15D | 110.4 |
| O5—C13—H13B | 109.5 | C14'—C15'—H15D | 110.4 |
| C12—C13—H13B | 109.5 | H15C—C15'—H15D | 108.6 |
| H13A—C13—H13B | 108.0 | C1—N1—Zn1 | 168.0 (4) |
| O5—C14—C15 | 108.4 (9) | C2—N2—Zn1 | 173.6 (6) |
| O5—C14—H14A | 110.0 | C3—N3—Zn1 | 157.0 (7) |
| C15—C14—H14A | 110.0 | H4E—N4—H4F | 109 (5) |
| O5—C14—H14B | 110.0 | H4E—N4—H4G | 119 (5) |
| C15—C14—H14B | 110.0 | H4F—N4—H4G | 107 (5) |
| H14A—C14—H14B | 108.4 | H4E—N4—H4H | 92 (5) |
| O6—C15—C14 | 109.4 (11) | H4F—N4—H4H | 120 (5) |
| O6—C15—H15A | 109.8 | H4G—N4—H4H | 110 (5) |
| C14—C15—H15A | 109.8 | S3i—S3—C3 | 73.6 (3) |
| O6—C15—H15B | 109.8 | N1—Zn1—N1i | 115.4 (2) |
| C14—C15—H15B | 109.8 | N1—Zn1—N2 | 107.82 (13) |
| H15A—C15—H15B | 108.2 | N1i—Zn1—N2 | 107.82 (13) |
| C6'—O1'—C5' | 112.8 (17) | N1—Zn1—N3 | 108.70 (13) |
| C8'—O2'—C7' | 106.5 (15) | N1i—Zn1—N3 | 108.70 (13) |
| C10'—O3'—C9' | 111.7 (14) | N2—Zn1—N3 | 108.2 (2) |
| C12'—O4'—C11' | 114.2 (14) | H1W—O1W—H2W | 120 (2) |
| C14'—O5'—C13' | 107.7 (17) | H2W—O1W—H3W | 152 (10) |
| C15'—O6'—C4' | 111.2 (18) | H1W—O2W—H4W | 152 (10) |
| O6'—C4'—C5' | 108 (2) | H3W—O2W—H4W | 121 (2) |
| C15—O6—C4—C5 | 174.9 (8) | O1'—C6'—C7'—O2' | −68 (3) |
| C6—O1—C5—C4 | 173.1 (8) | C7'—O2'—C8'—C9' | −175.8 (18) |
| O6—C4—C5—O1 | 61.5 (10) | C10'—O3'—C9'—C8' | −178.1 (15) |
| C5—O1—C6—C7 | 174.5 (8) | O2'—C8'—C9'—O3' | 64 (2) |
| C8—O2—C7—C6 | −176.9 (8) | C9'—O3'—C10'—C11' | −174.2 (15) |
| O1—C6—C7—O2 | −63.5 (11) | C12'—O4'—C11'—C10' | 174.0 (14) |
| C7—O2—C8—C9 | −174.4 (8) | O3'—C10'—C11'—O4' | −62.7 (18) |
| C10—O3—C9—C8 | 176.1 (8) | C11'—O4'—C12'—C13' | 171.2 (15) |
| O2—C8—C9—O3 | 64.5 (10) | C14'—O5'—C13'—C12' | −174.0 (16) |
| C9—O3—C10—C11 | −179.8 (8) | O4'—C12'—C13'—O5' | 68 (2) |
| C12—O4—C11—C10 | −178.8 (9) | C13'—O5'—C14'—C15' | −175.8 (19) |
| O3—C10—C11—O4 | −65.3 (10) | C4'—O6'—C15'—C14' | 179.3 (18) |
| C11—O4—C12—C13 | 179.6 (8) | O5'—C14'—C15'—O6' | −62 (2) |
| C14—O5—C13—C12 | −175.7 (9) | S3i—C3—N3—Zn1 | 67 (3) |
| O4—C12—C13—O5 | 67.7 (11) | S3—C3—N3—Zn1 | −67 (3) |
| C13—O5—C14—C15 | −179.8 (8) | S3A—C3—N3—Zn1 | 180.0 |
| C4—O6—C15—C14 | −174.1 (8) | N3—C3—S3—S3i | 156 (3) |
| O5—C14—C15—O6 | −63.7 (11) | S3A—C3—S3—S3i | −62.2 (12) |
| C15'—O6'—C4'—C5' | 176.4 (18) | C1—N1—Zn1—N1i | 170.5 (15) |
| C6'—O1'—C5'—C4' | 175.5 (17) | C1—N1—Zn1—N2 | −69.0 (17) |
| O6'—C4'—C5'—O1' | 62 (2) | C1—N1—Zn1—N3 | 48.1 (17) |
| C5'—O1'—C6'—C7' | 176.4 (18) | C3—N3—Zn1—N1 | −116.85 (12) |
| C8'—O2'—C7'—C6' | −177.5 (18) | C3—N3—Zn1—N1i | 116.85 (12) |
Symmetry code: (i) x, −y+1/2, z.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N4—H4H···O1 | 0.88 (1) | 2.44 (4) | 3.069 (8) | 129 (4) |
| N4—H4E···O2 | 0.87 (1) | 2.06 (1) | 2.934 (8) | 176 (5) |
| N4—H4G···O4 | 0.88 (1) | 1.97 (2) | 2.829 (7) | 166 (4) |
| N4—H4G···O5 | 0.88 (1) | 2.53 (4) | 3.010 (8) | 115 (3) |
| N4—H4H···O6 | 0.88 (1) | 2.05 (3) | 2.850 (8) | 150 (5) |
| N4—H4F···S1ii | 0.87 (1) | 2.63 (2) | 3.441 (4) | 156 (4) |
Symmetry code: (ii) x, y, z−1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SU2617).
References
- Akutagawa, T., Hashimoto, A., Nishihara, S., Hasegawa, T. & Nakamura, T. (2002). J. Supramol. Chem. 2, 175–186.
- Bruker (2004). APEX2, SAINT and XPREP Bruker AXS Inc., Madison, Wisconsin, USA.
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Fender, N. S., Kahwa, I. A. & Fronczek, F. R. (2002). J. Solid State Chem. 163, 286–293.
- Kryatova, O. P., Korendovych, I. V. & Rybak-Akimova, E. V. (2004). Tetrahedron, 60, 4579–4588.
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Ramesh, V., Rajarajan, K., Kumar, K. S., Subashini, A. & NizamMohideen, M. (2012). Acta Cryst. E68, m335–m336. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2004). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536813019739/su2617sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813019739/su2617Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report