Abstract
In the title solvate, C29H21ClN2O2·C3H6O, a prop-2-en-1-one bridge links two quinolinyl residues; the latter are almost perpendicular [dihedral angle = 78.27 (6)°]. The dihedral angle between the quinonyl ring system and its pendant phenyl group is 59.78 (8)°. A small twist in the bridging prop-2-en-1-one group is noted [O=C—C=C torsion angle = −10.6 (3)°]. In the crystal, a three-dimensional architecture arises as a result of C—H⋯O and π–π stacking [centroid–centroid distances = 3.5504 (12)–3.6623 (12) Å].
Related literature
For background details and the biological applications of quinolinyl derivatives, see: Joshi et al. (2011 ▶); Prasath et al. (2013a
▶). For a related structure, see: Prasath et al. (2013b
▶).
Experimental
Crystal data
C29H21ClN2O2·C3H6O
M r = 523.01
Monoclinic,
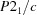
a = 17.1714 (3) Å
b = 10.7099 (2) Å
c = 14.5248 (2) Å
β = 100.021 (2)°
V = 2630.42 (8) Å3
Z = 4
Cu Kα radiation
μ = 1.58 mm−1
T = 100 K
0.30 × 0.25 × 0.20 mm
Data collection
Agilent SuperNova Dual diffractometer with an Atlas detector
Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2013 ▶) T min = 0.665, T max = 1.000
11367 measured reflections
5408 independent reflections
4574 reflections with I > 2σ(I)
R int = 0.032
Refinement
R[F 2 > 2σ(F 2)] = 0.058
wR(F 2) = 0.167
S = 1.03
5408 reflections
346 parameters
H-atom parameters constrained
Δρmax = 1.47 e Å−3
Δρmin = −0.46 e Å−3
Data collection: CrysAlis PRO (Agilent, 2013 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis PRO; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 2012 ▶) and DIAMOND (Brandenburg, 2006 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) general, I. DOI: 10.1107/S1600536813020217/hg5334sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813020217/hg5334Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813020217/hg5334Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C26—H26⋯O2i | 0.95 | 2.47 | 3.319 (3) | 149 |
| C30—H30A⋯O1i | 0.98 | 2.52 | 3.373 (4) | 146 |
| C28—H28⋯O3 | 0.95 | 2.57 | 3.467 (3) | 158 |
Symmetry code: (i) 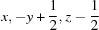 .
.
Acknowledgments
RP gratefully acknowledges the Council of Scientific and Industrial Research (CSIR), India, for a Senior Research Fellowship (09/919/(0014)/2012 EMR-I). We also thank the Ministry of Higher Education (Malaysia) for funding structural studies through the High-Impact Research scheme (UM.C/HIR-MOHE/SC/03).
supplementary crystallographic information
Comment
The the title compound, (I), was investigated in connection with on-going studies of quinolinyl chalcones (Prasath et al., 2013a), motivated by their potential anti-bacterial, anti-fungal, anti-malarial and anti-cancer activity (Joshi et al., 2011).
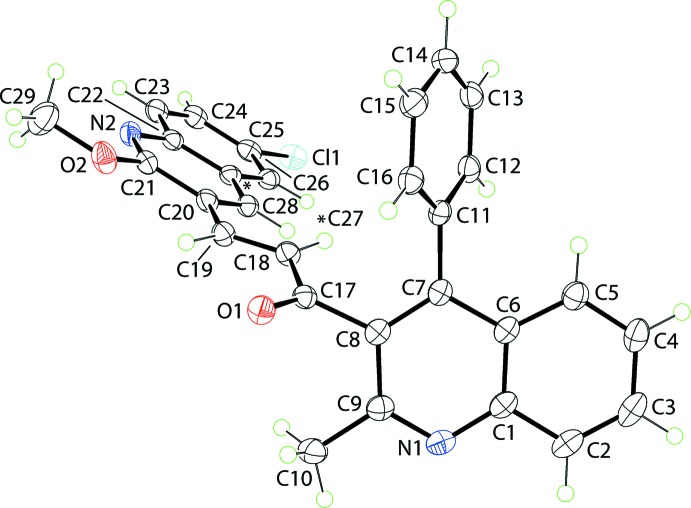
The molecular structure of the quinolinyl derivative, (I), Fig. 1, comprises two quinolinyl residues connected by the ends of a prop-2-en-1-one bridge, in an almost perpendicular relationship; the dihedral angle between the quinolinyl residues is 78.27 (6)°. The phenyl ring is inclined with respect to the quinolinyl residue to which it is attached, forming a dihedral angle of 59.78 (8)°. The conformation about the ethylene bond [C18═C19 = 1.336 (3) Å] is E. A small twist in the bridging prop-2-en-1-one group is manifested in the O1—C17—C18—C19 torsion angle of -10.6 (3)°. An distinct conformation was reported recently for a related structure, namely (2E)-3-(6-chloro-2-methoxyquinolin-3-yl)-1-(2,4-dimethylquinolin-3 - y)prop-2-en-1-one (Prasath et al., 2013b) where the nitrogen atoms are approximately syn as opposed to approximately anti in (I).
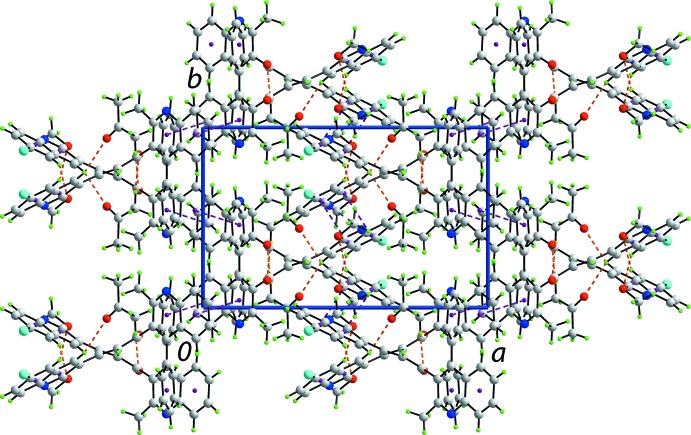
In the crystal packing, the quinolinyl and acetone molecules are connected by C—H···O interactions, Table 1. Additional C—H···O contacts and a number of π—π interactions, involving pyridyl, a quinolinyl-C6 ring and the phenyl group, connect molecules into a three-dimensional architecture [centroid···centroid distances = 3.5504 (12), 3.5747 (12) and 3.6623 (12) Å], Fig. 2.
Experimental
A mixture of 3-acetyl-2-methyl-4-phenylquinoline (260 mg, 0.001 M) and 2,6-dichloroquinoline-3-carbaldehyde (230 mg, 0.001 M) in methanol (20 ml) containing potassium hydroxide (0.2 g) was stirred at room temperature for 12 h. The reaction mixture was then neutralized with dilute acetic acid and the resultant solid was filtered, dried and purified by column chromatography using ethyl acetate - hexane (3:1) mixture to afford compound. Re-crystallization was by slow evaporation of an acetone solution of (I), which yielded blocks in 62% yield; M.pt: 366–368 K.
Refinement
Carbon-bound H-atoms were placed in calculated positions [C—H = 0.95–0.98 Å, Uiso(H) = 1.2–1.5Ueq(C)] and were included in the refinement in the riding model approximation. The maximum and minimum residual electron density peaks of 1.47 and 0.46 e Å-3, respectively, were located 0.85 Å and 0.68 Å from the O2 and Cl1 atoms, respectively.
Figures
Fig. 1.
The molecular structure of (I) showing the atom-labelling scheme and displacement ellipsoids at the 50% probability level.
Fig. 2.
View in projection down the c axis of the unit-cell contents of (I). The π—π and C—H···O interactions are shown as purple and orange dashed lines, respectively.
Crystal data
| C29H21ClN2O2·C3H6O | F(000) = 1096 |
| Mr = 523.01 | Dx = 1.321 Mg m−3 |
| Monoclinic, P21/c | Cu Kα radiation, λ = 1.54184 Å |
| Hall symbol: -P 2ybc | Cell parameters from 4229 reflections |
| a = 17.1714 (3) Å | θ = 2.6–76.5° |
| b = 10.7099 (2) Å | µ = 1.58 mm−1 |
| c = 14.5248 (2) Å | T = 100 K |
| β = 100.021 (2)° | Prism, pale-yellow |
| V = 2630.42 (8) Å3 | 0.30 × 0.25 × 0.20 mm |
| Z = 4 |
Data collection
| Agilent SuperNova Dual diffractometer with an Atlas detector | 5408 independent reflections |
| Radiation source: SuperNova (Cu) X-ray Source | 4574 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.032 |
| Detector resolution: 10.4041 pixels mm-1 | θmax = 76.7°, θmin = 2.6° |
| ω scan | h = −21→21 |
| Absorption correction: multi-scan (CrysAlis PRO; Agilent, 2013) | k = −9→13 |
| Tmin = 0.665, Tmax = 1.000 | l = −18→11 |
| 11367 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.058 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.167 | H-atom parameters constrained |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.0953P)2 + 1.726P] where P = (Fo2 + 2Fc2)/3 |
| 5408 reflections | (Δ/σ)max < 0.001 |
| 346 parameters | Δρmax = 1.47 e Å−3 |
| 0 restraints | Δρmin = −0.46 e Å−3 |
Special details
| Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | 0.62921 (3) | 0.12909 (5) | 0.32316 (4) | 0.03232 (17) | |
| O1 | 0.22537 (9) | 0.34889 (15) | 0.74016 (10) | 0.0264 (3) | |
| O2 | 0.47465 (9) | 0.10414 (17) | 0.80988 (11) | 0.0324 (4) | |
| N1 | 0.12879 (10) | 0.59002 (17) | 0.50819 (13) | 0.0258 (4) | |
| N2 | 0.55712 (10) | 0.06750 (17) | 0.70334 (12) | 0.0245 (4) | |
| C1 | 0.08058 (12) | 0.5228 (2) | 0.44105 (14) | 0.0235 (4) | |
| C2 | 0.03155 (13) | 0.5910 (2) | 0.36971 (15) | 0.0295 (5) | |
| H2 | 0.0332 | 0.6797 | 0.3702 | 0.035* | |
| C3 | −0.01806 (14) | 0.5301 (2) | 0.30037 (15) | 0.0338 (5) | |
| H3 | −0.0501 | 0.5767 | 0.2525 | 0.041* | |
| C4 | −0.02195 (13) | 0.3989 (2) | 0.29950 (15) | 0.0314 (5) | |
| H4 | −0.0567 | 0.3575 | 0.2510 | 0.038* | |
| C5 | 0.02415 (12) | 0.3303 (2) | 0.36832 (14) | 0.0264 (4) | |
| H5 | 0.0204 | 0.2418 | 0.3677 | 0.032* | |
| C6 | 0.07738 (11) | 0.3911 (2) | 0.44037 (13) | 0.0216 (4) | |
| C7 | 0.12817 (11) | 0.32582 (19) | 0.51344 (13) | 0.0201 (4) | |
| C8 | 0.17507 (11) | 0.39553 (19) | 0.58093 (13) | 0.0210 (4) | |
| C9 | 0.17372 (12) | 0.5289 (2) | 0.57624 (14) | 0.0241 (4) | |
| C10 | 0.22557 (14) | 0.6057 (2) | 0.64860 (17) | 0.0332 (5) | |
| H10A | 0.2229 | 0.6936 | 0.6293 | 0.050* | |
| H10B | 0.2803 | 0.5763 | 0.6551 | 0.050* | |
| H10C | 0.2074 | 0.5976 | 0.7087 | 0.050* | |
| C11 | 0.12677 (11) | 0.18704 (19) | 0.51703 (13) | 0.0211 (4) | |
| C12 | 0.14436 (12) | 0.1150 (2) | 0.44272 (14) | 0.0242 (4) | |
| H12 | 0.1597 | 0.1550 | 0.3903 | 0.029* | |
| C13 | 0.13946 (13) | −0.0141 (2) | 0.44520 (16) | 0.0293 (5) | |
| H13 | 0.1517 | −0.0619 | 0.3946 | 0.035* | |
| C14 | 0.11663 (13) | −0.0742 (2) | 0.52142 (17) | 0.0317 (5) | |
| H14 | 0.1125 | −0.1627 | 0.5225 | 0.038* | |
| C15 | 0.10015 (13) | −0.0036 (2) | 0.59537 (16) | 0.0305 (5) | |
| H15 | 0.0852 | −0.0440 | 0.6479 | 0.037* | |
| C16 | 0.10515 (12) | 0.1257 (2) | 0.59373 (14) | 0.0250 (4) | |
| H16 | 0.0938 | 0.1729 | 0.6452 | 0.030* | |
| C17 | 0.23238 (11) | 0.33553 (18) | 0.65850 (13) | 0.0208 (4) | |
| C18 | 0.29959 (11) | 0.27093 (19) | 0.62832 (13) | 0.0217 (4) | |
| H18 | 0.2965 | 0.2518 | 0.5639 | 0.026* | |
| C19 | 0.36459 (11) | 0.23825 (19) | 0.68814 (14) | 0.0223 (4) | |
| H19 | 0.3649 | 0.2491 | 0.7531 | 0.027* | |
| C20 | 0.43552 (11) | 0.18660 (19) | 0.65883 (13) | 0.0218 (4) | |
| C21 | 0.49258 (12) | 0.11715 (19) | 0.72277 (14) | 0.0231 (4) | |
| C22 | 0.57217 (11) | 0.08240 (19) | 0.61423 (14) | 0.0221 (4) | |
| C23 | 0.64121 (12) | 0.0285 (2) | 0.59056 (15) | 0.0266 (4) | |
| H23 | 0.6762 | −0.0175 | 0.6360 | 0.032* | |
| C24 | 0.65784 (12) | 0.0425 (2) | 0.50195 (16) | 0.0273 (4) | |
| H24 | 0.7041 | 0.0059 | 0.4861 | 0.033* | |
| C25 | 0.60641 (12) | 0.1109 (2) | 0.43496 (15) | 0.0244 (4) | |
| C26 | 0.53820 (12) | 0.16319 (19) | 0.45448 (14) | 0.0229 (4) | |
| H26 | 0.5035 | 0.2075 | 0.4076 | 0.027* | |
| C27 | 0.52045 (11) | 0.15016 (18) | 0.54522 (14) | 0.0204 (4) | |
| C28 | 0.45154 (11) | 0.20257 (19) | 0.57037 (14) | 0.0215 (4) | |
| H28 | 0.4161 | 0.2491 | 0.5257 | 0.026* | |
| C29 | 0.52882 (18) | 0.0347 (3) | 0.87429 (17) | 0.0424 (6) | |
| H29A | 0.5101 | 0.0305 | 0.9342 | 0.064* | |
| H29B | 0.5807 | 0.0753 | 0.8832 | 0.064* | |
| H29C | 0.5332 | −0.0500 | 0.8501 | 0.064* | |
| O3 | 0.33767 (14) | 0.4389 (2) | 0.45197 (14) | 0.0573 (6) | |
| C30 | 0.23862 (15) | 0.4489 (3) | 0.31684 (17) | 0.0373 (6) | |
| H30A | 0.2371 | 0.3579 | 0.3235 | 0.056* | |
| H30B | 0.2551 | 0.4700 | 0.2574 | 0.056* | |
| H30C | 0.1859 | 0.4835 | 0.3178 | 0.056* | |
| C31 | 0.29599 (14) | 0.5025 (2) | 0.39548 (15) | 0.0331 (5) | |
| C32 | 0.29821 (18) | 0.6428 (3) | 0.40126 (19) | 0.0450 (6) | |
| H32A | 0.3467 | 0.6692 | 0.4427 | 0.068* | |
| H32B | 0.2521 | 0.6728 | 0.4262 | 0.068* | |
| H32C | 0.2973 | 0.6777 | 0.3387 | 0.068* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.0329 (3) | 0.0336 (3) | 0.0345 (3) | −0.0039 (2) | 0.0173 (2) | −0.0023 (2) |
| O1 | 0.0261 (7) | 0.0297 (8) | 0.0242 (7) | 0.0027 (6) | 0.0063 (6) | −0.0013 (6) |
| O2 | 0.0278 (8) | 0.0422 (10) | 0.0265 (7) | 0.0063 (7) | 0.0026 (6) | −0.0116 (7) |
| N1 | 0.0244 (9) | 0.0217 (9) | 0.0326 (9) | 0.0024 (7) | 0.0087 (7) | 0.0035 (7) |
| N2 | 0.0223 (8) | 0.0263 (9) | 0.0231 (8) | 0.0029 (7) | −0.0008 (6) | −0.0031 (7) |
| C1 | 0.0213 (9) | 0.0268 (11) | 0.0245 (9) | 0.0063 (8) | 0.0098 (8) | 0.0045 (8) |
| C2 | 0.0300 (11) | 0.0297 (11) | 0.0310 (11) | 0.0103 (9) | 0.0111 (9) | 0.0089 (9) |
| C3 | 0.0296 (11) | 0.0444 (14) | 0.0279 (11) | 0.0145 (10) | 0.0063 (9) | 0.0108 (10) |
| C4 | 0.0242 (10) | 0.0431 (14) | 0.0261 (10) | 0.0082 (9) | 0.0015 (8) | 0.0005 (9) |
| C5 | 0.0235 (10) | 0.0299 (11) | 0.0261 (10) | 0.0042 (8) | 0.0054 (8) | 0.0003 (8) |
| C6 | 0.0179 (9) | 0.0264 (10) | 0.0219 (9) | 0.0040 (7) | 0.0077 (7) | 0.0031 (8) |
| C7 | 0.0148 (8) | 0.0252 (10) | 0.0216 (9) | 0.0019 (7) | 0.0064 (7) | 0.0016 (7) |
| C8 | 0.0174 (9) | 0.0247 (10) | 0.0223 (9) | 0.0019 (7) | 0.0076 (7) | 0.0015 (7) |
| C9 | 0.0201 (9) | 0.0241 (10) | 0.0294 (10) | 0.0019 (8) | 0.0077 (8) | 0.0000 (8) |
| C10 | 0.0317 (11) | 0.0225 (11) | 0.0431 (13) | −0.0013 (9) | −0.0003 (10) | −0.0022 (9) |
| C11 | 0.0166 (8) | 0.0224 (10) | 0.0236 (9) | 0.0024 (7) | 0.0014 (7) | 0.0009 (7) |
| C12 | 0.0195 (9) | 0.0292 (11) | 0.0229 (9) | 0.0028 (8) | 0.0008 (7) | −0.0024 (8) |
| C13 | 0.0229 (10) | 0.0287 (11) | 0.0330 (11) | 0.0050 (8) | −0.0046 (8) | −0.0090 (9) |
| C14 | 0.0270 (10) | 0.0205 (10) | 0.0433 (12) | 0.0009 (8) | −0.0062 (9) | −0.0008 (9) |
| C15 | 0.0251 (10) | 0.0287 (11) | 0.0358 (11) | −0.0027 (8) | 0.0005 (9) | 0.0065 (9) |
| C16 | 0.0218 (9) | 0.0275 (11) | 0.0250 (10) | −0.0002 (8) | 0.0025 (7) | 0.0014 (8) |
| C17 | 0.0184 (9) | 0.0201 (9) | 0.0238 (9) | −0.0013 (7) | 0.0035 (7) | −0.0010 (7) |
| C18 | 0.0199 (9) | 0.0231 (10) | 0.0227 (9) | 0.0012 (7) | 0.0052 (7) | −0.0035 (7) |
| C19 | 0.0209 (9) | 0.0242 (10) | 0.0217 (9) | 0.0002 (7) | 0.0034 (7) | −0.0025 (7) |
| C20 | 0.0178 (9) | 0.0225 (10) | 0.0242 (9) | −0.0005 (7) | 0.0011 (7) | −0.0042 (8) |
| C21 | 0.0247 (10) | 0.0232 (10) | 0.0202 (9) | −0.0008 (8) | 0.0009 (7) | −0.0032 (7) |
| C22 | 0.0185 (9) | 0.0192 (9) | 0.0276 (10) | −0.0018 (7) | 0.0007 (7) | −0.0035 (8) |
| C23 | 0.0177 (9) | 0.0264 (10) | 0.0341 (11) | 0.0010 (8) | −0.0001 (8) | −0.0045 (9) |
| C24 | 0.0174 (9) | 0.0273 (11) | 0.0371 (11) | −0.0012 (8) | 0.0047 (8) | −0.0076 (9) |
| C25 | 0.0218 (9) | 0.0240 (10) | 0.0294 (10) | −0.0055 (8) | 0.0097 (8) | −0.0040 (8) |
| C26 | 0.0219 (9) | 0.0199 (10) | 0.0268 (10) | −0.0022 (7) | 0.0039 (7) | −0.0002 (8) |
| C27 | 0.0169 (9) | 0.0179 (9) | 0.0261 (9) | −0.0012 (7) | 0.0028 (7) | −0.0021 (7) |
| C28 | 0.0175 (9) | 0.0208 (9) | 0.0255 (9) | −0.0006 (7) | 0.0019 (7) | −0.0015 (7) |
| C29 | 0.0550 (16) | 0.0437 (15) | 0.0290 (12) | 0.0158 (12) | 0.0087 (11) | 0.0058 (10) |
| O3 | 0.0579 (13) | 0.0665 (15) | 0.0453 (11) | 0.0049 (11) | 0.0028 (10) | 0.0181 (10) |
| C30 | 0.0363 (12) | 0.0427 (14) | 0.0359 (12) | −0.0130 (10) | 0.0146 (10) | −0.0117 (10) |
| C31 | 0.0324 (12) | 0.0415 (14) | 0.0269 (10) | −0.0060 (10) | 0.0098 (9) | 0.0035 (9) |
| C32 | 0.0560 (17) | 0.0404 (15) | 0.0407 (13) | −0.0156 (12) | 0.0142 (12) | −0.0066 (11) |
Geometric parameters (Å, º)
| Cl1—C25 | 1.746 (2) | C14—H14 | 0.9500 |
| O1—C17 | 1.221 (2) | C15—C16 | 1.388 (3) |
| O2—C21 | 1.360 (3) | C15—H15 | 0.9500 |
| O2—C29 | 1.411 (3) | C16—H16 | 0.9500 |
| N1—C9 | 1.317 (3) | C17—C18 | 1.476 (3) |
| N1—C1 | 1.369 (3) | C18—C19 | 1.336 (3) |
| N2—C21 | 1.304 (3) | C18—H18 | 0.9500 |
| N2—C22 | 1.373 (3) | C19—C20 | 1.467 (3) |
| C1—C6 | 1.411 (3) | C19—H19 | 0.9500 |
| C1—C2 | 1.419 (3) | C20—C28 | 1.371 (3) |
| C2—C3 | 1.366 (3) | C20—C21 | 1.435 (3) |
| C2—H2 | 0.9500 | C22—C23 | 1.414 (3) |
| C3—C4 | 1.407 (4) | C22—C27 | 1.418 (3) |
| C3—H3 | 0.9500 | C23—C24 | 1.374 (3) |
| C4—C5 | 1.375 (3) | C23—H23 | 0.9500 |
| C4—H4 | 0.9500 | C24—C25 | 1.401 (3) |
| C5—C6 | 1.422 (3) | C24—H24 | 0.9500 |
| C5—H5 | 0.9500 | C25—C26 | 1.372 (3) |
| C6—C7 | 1.433 (3) | C26—C27 | 1.410 (3) |
| C7—C8 | 1.375 (3) | C26—H26 | 0.9500 |
| C7—C11 | 1.488 (3) | C27—C28 | 1.414 (3) |
| C8—C9 | 1.431 (3) | C28—H28 | 0.9500 |
| C8—C17 | 1.505 (3) | C29—H29A | 0.9800 |
| C9—C10 | 1.499 (3) | C29—H29B | 0.9800 |
| C10—H10A | 0.9800 | C29—H29C | 0.9800 |
| C10—H10B | 0.9800 | O3—C31 | 1.202 (3) |
| C10—H10C | 0.9800 | C30—C31 | 1.488 (3) |
| C11—C16 | 1.398 (3) | C30—H30A | 0.9800 |
| C11—C12 | 1.401 (3) | C30—H30B | 0.9800 |
| C12—C13 | 1.386 (3) | C30—H30C | 0.9800 |
| C12—H12 | 0.9500 | C31—C32 | 1.505 (4) |
| C13—C14 | 1.395 (3) | C32—H32A | 0.9800 |
| C13—H13 | 0.9500 | C32—H32B | 0.9800 |
| C14—C15 | 1.383 (3) | C32—H32C | 0.9800 |
| C21—O2—C29 | 116.16 (17) | C18—C17—C8 | 114.89 (16) |
| C9—N1—C1 | 118.42 (19) | C19—C18—C17 | 122.50 (18) |
| C21—N2—C22 | 117.61 (17) | C19—C18—H18 | 118.7 |
| N1—C1—C6 | 123.29 (18) | C17—C18—H18 | 118.7 |
| N1—C1—C2 | 117.2 (2) | C18—C19—C20 | 123.50 (18) |
| C6—C1—C2 | 119.5 (2) | C18—C19—H19 | 118.3 |
| C3—C2—C1 | 120.5 (2) | C20—C19—H19 | 118.3 |
| C3—C2—H2 | 119.8 | C28—C20—C21 | 116.46 (18) |
| C1—C2—H2 | 119.8 | C28—C20—C19 | 122.50 (18) |
| C2—C3—C4 | 120.4 (2) | C21—C20—C19 | 121.03 (18) |
| C2—C3—H3 | 119.8 | N2—C21—O2 | 119.87 (18) |
| C4—C3—H3 | 119.8 | N2—C21—C20 | 125.56 (18) |
| C5—C4—C3 | 120.5 (2) | O2—C21—C20 | 114.56 (18) |
| C5—C4—H4 | 119.8 | N2—C22—C23 | 119.04 (18) |
| C3—C4—H4 | 119.8 | N2—C22—C27 | 121.91 (18) |
| C4—C5—C6 | 120.3 (2) | C23—C22—C27 | 119.06 (19) |
| C4—C5—H5 | 119.8 | C24—C23—C22 | 120.2 (2) |
| C6—C5—H5 | 119.8 | C24—C23—H23 | 119.9 |
| C1—C6—C5 | 118.83 (18) | C22—C23—H23 | 119.9 |
| C1—C6—C7 | 117.66 (18) | C23—C24—C25 | 119.92 (19) |
| C5—C6—C7 | 123.51 (19) | C23—C24—H24 | 120.0 |
| C8—C7—C6 | 117.93 (19) | C25—C24—H24 | 120.0 |
| C8—C7—C11 | 121.90 (17) | C26—C25—C24 | 121.83 (19) |
| C6—C7—C11 | 120.12 (17) | C26—C25—Cl1 | 118.97 (17) |
| C7—C8—C9 | 120.37 (18) | C24—C25—Cl1 | 119.20 (16) |
| C7—C8—C17 | 121.82 (18) | C25—C26—C27 | 118.94 (19) |
| C9—C8—C17 | 117.72 (18) | C25—C26—H26 | 120.5 |
| N1—C9—C8 | 122.29 (19) | C27—C26—H26 | 120.5 |
| N1—C9—C10 | 116.9 (2) | C26—C27—C28 | 121.92 (18) |
| C8—C9—C10 | 120.79 (19) | C26—C27—C22 | 120.04 (18) |
| C9—C10—H10A | 109.5 | C28—C27—C22 | 118.04 (18) |
| C9—C10—H10B | 109.5 | C20—C28—C27 | 120.41 (18) |
| H10A—C10—H10B | 109.5 | C20—C28—H28 | 119.8 |
| C9—C10—H10C | 109.5 | C27—C28—H28 | 119.8 |
| H10A—C10—H10C | 109.5 | O2—C29—H29A | 109.5 |
| H10B—C10—H10C | 109.5 | O2—C29—H29B | 109.5 |
| C16—C11—C12 | 118.5 (2) | H29A—C29—H29B | 109.5 |
| C16—C11—C7 | 120.37 (18) | O2—C29—H29C | 109.5 |
| C12—C11—C7 | 121.04 (18) | H29A—C29—H29C | 109.5 |
| C13—C12—C11 | 120.4 (2) | H29B—C29—H29C | 109.5 |
| C13—C12—H12 | 119.8 | C31—C30—H30A | 109.5 |
| C11—C12—H12 | 119.8 | C31—C30—H30B | 109.5 |
| C12—C13—C14 | 120.5 (2) | H30A—C30—H30B | 109.5 |
| C12—C13—H13 | 119.7 | C31—C30—H30C | 109.5 |
| C14—C13—H13 | 119.7 | H30A—C30—H30C | 109.5 |
| C15—C14—C13 | 119.2 (2) | H30B—C30—H30C | 109.5 |
| C15—C14—H14 | 120.4 | O3—C31—C30 | 122.8 (3) |
| C13—C14—H14 | 120.4 | O3—C31—C32 | 121.4 (2) |
| C14—C15—C16 | 120.7 (2) | C30—C31—C32 | 115.8 (2) |
| C14—C15—H15 | 119.6 | C31—C32—H32A | 109.5 |
| C16—C15—H15 | 119.6 | C31—C32—H32B | 109.5 |
| C15—C16—C11 | 120.6 (2) | H32A—C32—H32B | 109.5 |
| C15—C16—H16 | 119.7 | C31—C32—H32C | 109.5 |
| C11—C16—H16 | 119.7 | H32A—C32—H32C | 109.5 |
| O1—C17—C18 | 123.90 (18) | H32B—C32—H32C | 109.5 |
| O1—C17—C8 | 120.99 (18) | ||
| C9—N1—C1—C6 | −0.9 (3) | C7—C11—C16—C15 | 176.94 (18) |
| C9—N1—C1—C2 | 178.66 (18) | C7—C8—C17—O1 | −117.8 (2) |
| N1—C1—C2—C3 | 179.97 (19) | C9—C8—C17—O1 | 65.6 (3) |
| C6—C1—C2—C3 | −0.5 (3) | C7—C8—C17—C18 | 67.3 (2) |
| C1—C2—C3—C4 | 0.8 (3) | C9—C8—C17—C18 | −109.3 (2) |
| C2—C3—C4—C5 | −0.1 (3) | O1—C17—C18—C19 | −10.6 (3) |
| C3—C4—C5—C6 | −1.1 (3) | C8—C17—C18—C19 | 164.11 (19) |
| N1—C1—C6—C5 | 178.86 (18) | C17—C18—C19—C20 | −173.03 (19) |
| C2—C1—C6—C5 | −0.7 (3) | C18—C19—C20—C28 | 20.8 (3) |
| N1—C1—C6—C7 | −0.8 (3) | C18—C19—C20—C21 | −159.9 (2) |
| C2—C1—C6—C7 | 179.69 (18) | C22—N2—C21—O2 | 179.46 (18) |
| C4—C5—C6—C1 | 1.5 (3) | C22—N2—C21—C20 | −0.1 (3) |
| C4—C5—C6—C7 | −178.94 (19) | C29—O2—C21—N2 | −0.5 (3) |
| C1—C6—C7—C8 | 1.8 (3) | C29—O2—C21—C20 | 179.0 (2) |
| C5—C6—C7—C8 | −177.81 (18) | C28—C20—C21—N2 | −0.8 (3) |
| C1—C6—C7—C11 | 179.30 (17) | C19—C20—C21—N2 | 179.8 (2) |
| C5—C6—C7—C11 | −0.3 (3) | C28—C20—C21—O2 | 179.62 (18) |
| C6—C7—C8—C9 | −1.3 (3) | C19—C20—C21—O2 | 0.3 (3) |
| C11—C7—C8—C9 | −178.73 (17) | C21—N2—C22—C23 | −179.30 (19) |
| C6—C7—C8—C17 | −177.82 (17) | C21—N2—C22—C27 | 0.6 (3) |
| C11—C7—C8—C17 | 4.7 (3) | N2—C22—C23—C24 | −179.71 (19) |
| C1—N1—C9—C8 | 1.5 (3) | C27—C22—C23—C24 | 0.3 (3) |
| C1—N1—C9—C10 | −179.85 (19) | C22—C23—C24—C25 | 0.3 (3) |
| C7—C8—C9—N1 | −0.4 (3) | C23—C24—C25—C26 | −1.3 (3) |
| C17—C8—C9—N1 | 176.27 (18) | C23—C24—C25—Cl1 | 179.27 (16) |
| C7—C8—C9—C10 | −179.01 (19) | C24—C25—C26—C27 | 1.6 (3) |
| C17—C8—C9—C10 | −2.3 (3) | Cl1—C25—C26—C27 | −179.00 (15) |
| C8—C7—C11—C16 | 59.1 (3) | C25—C26—C27—C28 | 179.44 (18) |
| C6—C7—C11—C16 | −118.3 (2) | C25—C26—C27—C22 | −0.9 (3) |
| C8—C7—C11—C12 | −123.1 (2) | N2—C22—C27—C26 | 179.99 (18) |
| C6—C7—C11—C12 | 59.5 (3) | C23—C22—C27—C26 | −0.1 (3) |
| C16—C11—C12—C13 | 0.7 (3) | N2—C22—C27—C28 | −0.3 (3) |
| C7—C11—C12—C13 | −177.16 (18) | C23—C22—C27—C28 | 179.64 (18) |
| C11—C12—C13—C14 | 0.3 (3) | C21—C20—C28—C27 | 1.1 (3) |
| C12—C13—C14—C15 | −1.0 (3) | C19—C20—C28—C27 | −179.53 (18) |
| C13—C14—C15—C16 | 0.8 (3) | C26—C27—C28—C20 | 179.06 (19) |
| C14—C15—C16—C11 | 0.2 (3) | C22—C27—C28—C20 | −0.6 (3) |
| C12—C11—C16—C15 | −0.9 (3) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C26—H26···O2i | 0.95 | 2.47 | 3.319 (3) | 149 |
| C30—H30A···O1i | 0.98 | 2.52 | 3.373 (4) | 146 |
| C28—H28···O3 | 0.95 | 2.57 | 3.467 (3) | 158 |
Symmetry code: (i) x, −y+1/2, z−1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HG5334).
References
- Agilent (2013). CrysAlis PRO Agilent Technologies Inc., Santa Clara, CA, USA.
- Brandenburg, K. (2006). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Joshi, R. S., Mandhane, P. G., Khan, W. & Gill, C. H. (2011). J. Heterocycl. Chem. 48, 872–876.
- Prasath, R., Bhavana, P., Ng, S. W. & Tiekink, E. R. T. (2013a). J. Organomet. Chem. 726, 62–70.
- Prasath, R., Sarveswari, S., Ng, S. W. & Tiekink, E. R. T. (2013b). Acta Cryst. E69, o1274. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) general, I. DOI: 10.1107/S1600536813020217/hg5334sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813020217/hg5334Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813020217/hg5334Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report