Abstract
The asymmetric unit of the triclinic polymorph of the title compound, C21H25N3O3, consists of two molecules, whereas for the monoclinic polymorph Z′ = 1 [Fun et al. (2009 ▶). Acta Cryst. E65, o445]. The two molecules exhibit an E configuration with respect to the C=N bond. The molecules are linked into dimers by N—H⋯O and C—H⋯O hydrogen bonds forming R 2 2(8) ring motifs. In addition, π–π interactions occur between nitrophenyl groups [minimum centroid–centroid distance 3.940 (2) Å], stacking the molecules along the ac plane.
Related literature
For the structure of the monoclinic polymorph of the title compound, see: Fun et al. (2009 ▶). For graph-set notation, see: Bernstein et al. (1995 ▶). For the pharmacological activity of hydrazones, see: Bedia et al. (2006 ▶); Rollas et al. (2002 ▶); Terzioglu & Gursoy (2003 ▶).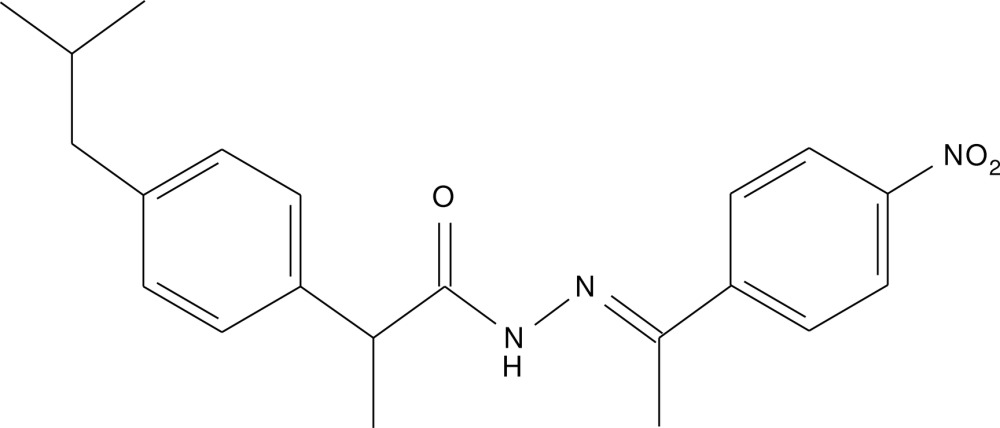
Experimental
Crystal data
C21H25N3O3
M r = 367.44
Triclinic,
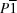
a = 12.201 (5) Å
b = 13.429 (5) Å
c = 13.932 (5) Å
α = 90.470 (7)°
β = 110.099 (6)°
γ = 107.321 (6)°
V = 2030.9 (13) Å3
Z = 4
Mo Kα radiation
μ = 0.08 mm−1
T = 300 K
0.23 × 0.22 × 0.22 mm
Data collection
Oxford Diffraction Xcalibur Eos diffractometer
21740 measured reflections
8844 independent reflections
4673 reflections with I > 2σ(I)
R int = 0.039
Refinement
R[F 2 > 2σ(F 2)] = 0.068
wR(F 2) = 0.193
S = 1.02
8844 reflections
495 parameters
H-atom parameters constrained
Δρmax = 0.19 e Å−3
Δρmin = −0.16 e Å−3
Data collection: CrysAlis PRO (Oxford Diffraction, 2009 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis PRO; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: Mercury (Macrae et al., 2008 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536813019892/gk2579sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813019892/gk2579Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813019892/gk2579Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1A—H1A⋯O1B i | 0.86 | 2.14 | 2.977 (3) | 165 |
| N1B—H1B⋯O1A ii | 0.86 | 2.15 | 2.919 (3) | 149 |
| C15A—H15C⋯O1B i | 0.96 | 2.41 | 3.252 (4) | 147 |
Symmetry codes: (i)  ; (ii)
; (ii) 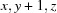 .
.
Acknowledgments
SMK thanks UGC-BRS and the University of Mysore for awarding a fellowship. MPS acknowledges the University Grants Commission, New Delhi, India.
supplementary crystallographic information
Comment
Hydrazone derivatives show divers pharmacological activities (Bedia et al., 2006; Rollas et al., 2002; Terzioglu & Gursoy, 2003).
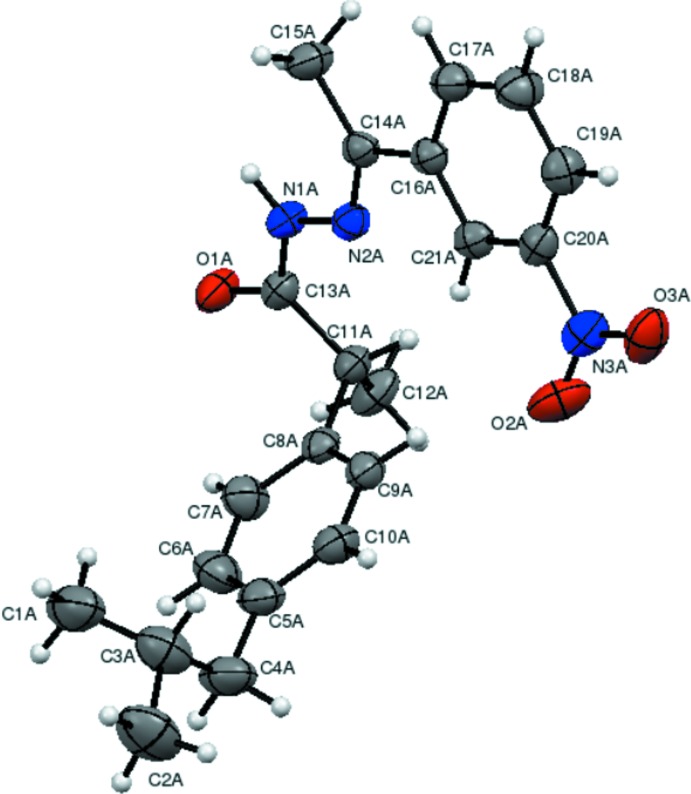
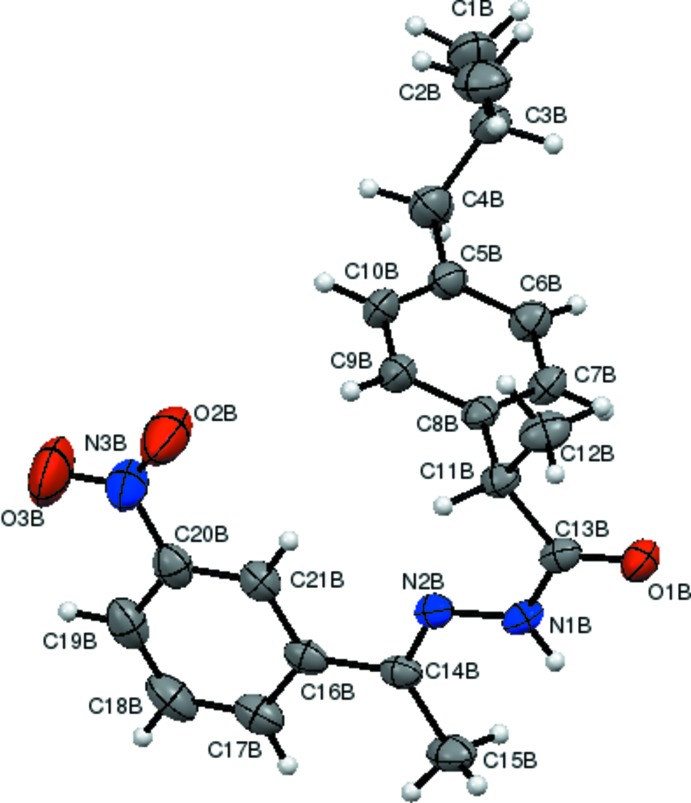
The asymmetric unit of the title compound consists of the A and B molecules (Fig. 1 & Fig. 2) and they show E configuration with respect to the C14=N2 bond. The molecular fragment composed of the atoms C11,C13,O1,N1,N2,C14,C15 is nearly planar, with the maximum deviation of 0.041 (3) Å for C15. It makes dihedral angles of 87.50 (14)°, 5.26 (14) ° and 42.43 (12) ° and 13.94 (12) ° with the terminal benzene rings in molecules B and A, respectively. The dihedral angle between nitrophenyl and phenyl groups are 87.64 (12)° and 74.31 ° for molecule B and A, respectively. These dihedral angles show that the two molecules differ in conformation. The bond lengths and bond angles are comparable to those in the monoclinic polymorph (Fun et al., 2009).
The molecules are connected by N—H···O and C—H···O hydrogen bonds with R22(8) ring motifs (Bernstein et al., 1995) (Table 1 and Fig. 3). An intermolecular π···π interaction (Cg2 and Cg4; Cg4 and Cg4) is observed. The distance between Cg2 and Cg4 is 3.940 (3) Å and between Cg4 and Cg4 is 3.979 (3) Å. (Cg2 is C16A/C17A/C18A/C19A/C20A/C21A centroid and Cg4 is C16B/C17B/C18B/C19B/C20B/C21B centroid). This interaction generates stacking of molecules along the ac plane.
Experimental
The title compound is prepared by heating 2-(4-isobutylphenyl)propanehydrazide (0.01 mol) with p-nitroacetophenone(0.01 mol), in the presence of catalytic amount of acetic acid, in ethanol (20 ml) at reflux temperature for 5 h. Solid compound was obtained by filtration, washed with ice cold water and dried. The title compound was crystallized by slow evaporation of ethanol and acetonitrile (m.p. 442 K).
Refinement
All the H atoms were placed in calculated positions, with N—H = 0.86 Å, Uiso (H) = 1.2 Ueq(N) for NH, C–H = 0.93 Å, Uiso (H) = 1.2 Ueq(C) for aromatic and C–H = 0.97 Å, Uiso (H) = 1.2 Ueq(C) for CH2, Uiso (H) = 1.5 Ueq(C) for CH3 atoms.
Figures
Fig. 1.
Molecule A of the title compound with displacement ellipsoids shown at the 50% probability level.
Fig. 2.
Molecule B of the title compound with displacement ellipsoids shown at the 50% probability level.
Fig. 3.
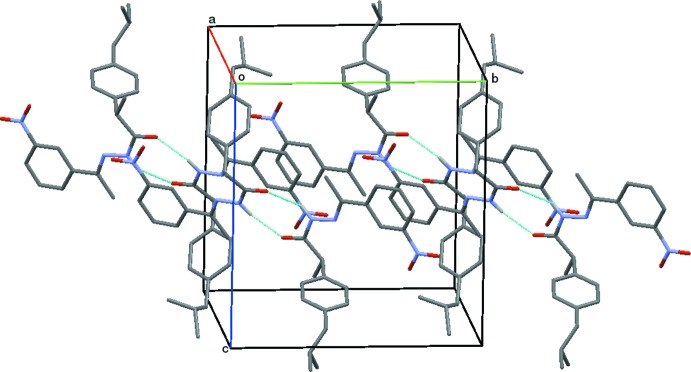
Packing diagram viewed along the crystallographic a axis. Dotted lines represent intermolecular N-H···O hydrogen bonding. Hydrogen atoms are not involved in the interactions were removed for clarity.
Crystal data
| C21H25N3O3 | Z = 4 |
| Mr = 367.44 | F(000) = 784 |
| Triclinic, P1 | Dx = 1.202 Mg m−3 |
| Hall symbol: -P 1 | Melting point: 442 K |
| a = 12.201 (5) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 13.429 (5) Å | Cell parameters from 8844 reflections |
| c = 13.932 (5) Å | θ = 1.6–27.1° |
| α = 90.470 (7)° | µ = 0.08 mm−1 |
| β = 110.099 (6)° | T = 300 K |
| γ = 107.321 (6)° | Block, colorless |
| V = 2030.9 (13) Å3 | 0.23 × 0.22 × 0.22 mm |
Data collection
| Oxford Diffraction Xcalibur Eos diffractometer | 4673 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.039 |
| Graphite monochromator | θmax = 27.1°, θmin = 1.6° |
| Detector resolution: 16.0839 pixels mm-1 | h = −15→15 |
| ω scans | k = −17→17 |
| 21740 measured reflections | l = −17→17 |
| 8844 independent reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.068 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.193 | H-atom parameters constrained |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0752P)2 + 0.3062P] where P = (Fo2 + 2Fc2)/3 |
| 8844 reflections | (Δ/σ)max = 0.004 |
| 495 parameters | Δρmax = 0.19 e Å−3 |
| 0 restraints | Δρmin = −0.16 e Å−3 |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell e.s.d.'s are taken into account in the estimation of distances, angles and torsion angles |
| Refinement. Refinement on F2 for ALL reflections except those flagged by the user for potential systematic errors. Weighted R-factors wR and all goodnesses of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The observed criterion of F2 > σ(F2) is used only for calculating -R-factor-obs etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1A | 0.84024 (16) | −0.14913 (15) | 0.55714 (13) | 0.0761 (7) | |
| O2A | 0.7121 (2) | 0.3312 (2) | 0.70085 (17) | 0.1031 (10) | |
| O3A | 0.6171 (2) | 0.44110 (17) | 0.63494 (17) | 0.0948 (9) | |
| N1A | 0.71527 (17) | −0.05635 (16) | 0.48633 (14) | 0.0577 (7) | |
| N2A | 0.67981 (18) | 0.03078 (15) | 0.49209 (14) | 0.0551 (7) | |
| N3A | 0.6410 (2) | 0.3595 (2) | 0.6291 (2) | 0.0756 (10) | |
| C1A | 0.6003 (4) | −0.1837 (3) | 0.9456 (3) | 0.1231 (18) | |
| C2A | 0.6171 (4) | −0.0619 (4) | 1.0906 (3) | 0.134 (2) | |
| C3A | 0.6444 (4) | −0.0725 (3) | 0.9916 (3) | 0.1029 (16) | |
| C4A | 0.7774 (3) | −0.0140 (3) | 1.0121 (2) | 0.1044 (15) | |
| C5A | 0.8123 (3) | −0.0023 (3) | 0.9175 (2) | 0.0768 (10) | |
| C6A | 0.8563 (3) | −0.0728 (3) | 0.8837 (2) | 0.0910 (15) | |
| C7A | 0.8846 (3) | −0.0651 (2) | 0.7957 (2) | 0.0797 (11) | |
| C8A | 0.8700 (2) | 0.0160 (2) | 0.73670 (17) | 0.0563 (8) | |
| C9A | 0.8267 (2) | 0.0874 (2) | 0.77044 (19) | 0.0639 (9) | |
| C10A | 0.7972 (2) | 0.0782 (2) | 0.8581 (2) | 0.0729 (10) | |
| C11A | 0.9017 (2) | 0.0263 (2) | 0.64037 (18) | 0.0592 (8) | |
| C12A | 1.0355 (2) | 0.0331 (2) | 0.6613 (2) | 0.0848 (11) | |
| C13A | 0.8170 (2) | −0.0664 (2) | 0.55925 (18) | 0.0572 (9) | |
| C14A | 0.5906 (2) | 0.04170 (19) | 0.41559 (17) | 0.0530 (8) | |
| C15A | 0.5237 (3) | −0.0325 (2) | 0.31726 (19) | 0.0773 (10) | |
| C16A | 0.5552 (2) | 0.13587 (19) | 0.42770 (17) | 0.0524 (8) | |
| C17A | 0.4665 (2) | 0.1624 (2) | 0.34890 (19) | 0.0698 (10) | |
| C18A | 0.4373 (3) | 0.2526 (3) | 0.3600 (2) | 0.0838 (11) | |
| C19A | 0.4939 (3) | 0.3181 (2) | 0.4514 (2) | 0.0733 (11) | |
| C20A | 0.5797 (2) | 0.2901 (2) | 0.53021 (19) | 0.0600 (9) | |
| C21A | 0.6115 (2) | 0.2023 (2) | 0.52082 (17) | 0.0561 (8) | |
| O1B | 0.60116 (16) | 0.75492 (14) | 0.32931 (12) | 0.0678 (6) | |
| O2B | 0.7193 (3) | 0.2616 (2) | 0.2322 (2) | 0.1500 (15) | |
| O3B | 0.8201 (3) | 0.1626 (2) | 0.3011 (2) | 0.1580 (16) | |
| N1B | 0.70320 (18) | 0.65361 (16) | 0.42081 (14) | 0.0575 (7) | |
| N2B | 0.73709 (17) | 0.56469 (16) | 0.42424 (14) | 0.0544 (7) | |
| N3B | 0.7882 (3) | 0.2379 (2) | 0.3055 (2) | 0.0912 (12) | |
| C1B | 1.0431 (3) | 0.6854 (3) | −0.0762 (3) | 0.1043 (17) | |
| C2B | 0.8173 (3) | 0.6347 (3) | −0.1156 (2) | 0.1057 (15) | |
| C3B | 0.9435 (3) | 0.6789 (2) | −0.0323 (2) | 0.0760 (11) | |
| C4B | 0.9575 (3) | 0.6178 (3) | 0.0603 (2) | 0.0843 (12) | |
| C5B | 0.8631 (3) | 0.6109 (2) | 0.10965 (18) | 0.0642 (10) | |
| C6B | 0.8514 (2) | 0.6997 (2) | 0.14981 (19) | 0.0661 (10) | |
| C7B | 0.7625 (2) | 0.6943 (2) | 0.19134 (17) | 0.0601 (9) | |
| C8B | 0.6818 (2) | 0.59888 (19) | 0.19621 (15) | 0.0515 (8) | |
| C9B | 0.6943 (3) | 0.5099 (2) | 0.15785 (18) | 0.0674 (10) | |
| C10B | 0.7823 (3) | 0.5158 (2) | 0.11506 (19) | 0.0727 (11) | |
| C11B | 0.5795 (2) | 0.59323 (19) | 0.23640 (16) | 0.0552 (8) | |
| C12B | 0.4704 (2) | 0.6125 (2) | 0.15221 (19) | 0.0760 (10) | |
| C13B | 0.6271 (2) | 0.6736 (2) | 0.33137 (17) | 0.0544 (8) | |
| C14B | 0.8072 (2) | 0.5478 (2) | 0.51048 (17) | 0.0560 (8) | |
| C15B | 0.8537 (3) | 0.6172 (2) | 0.61121 (18) | 0.0788 (10) | |
| C16B | 0.8429 (2) | 0.4523 (2) | 0.50529 (18) | 0.0587 (9) | |
| C17B | 0.9217 (2) | 0.4223 (3) | 0.5904 (2) | 0.0757 (10) | |
| C18B | 0.9561 (3) | 0.3350 (3) | 0.5827 (3) | 0.0888 (14) | |
| C19B | 0.9135 (3) | 0.2737 (3) | 0.4908 (3) | 0.0844 (14) | |
| C20B | 0.8343 (2) | 0.3021 (2) | 0.4058 (2) | 0.0683 (10) | |
| C21B | 0.7998 (2) | 0.3889 (2) | 0.41132 (19) | 0.0616 (9) | |
| H1A | 0.67210 | −0.10530 | 0.43560 | 0.0690* | |
| H4A1 | 0.82740 | −0.05020 | 1.05870 | 0.1250* | |
| H3A | 0.59630 | −0.03620 | 0.94160 | 0.1230* | |
| H4A2 | 0.79750 | 0.05550 | 1.04680 | 0.1250* | |
| H6A | 0.86760 | −0.12810 | 0.92150 | 0.1090* | |
| H7A | 0.91390 | −0.11510 | 0.77560 | 0.0960* | |
| H9A | 0.81680 | 0.14350 | 0.73340 | 0.0770* | |
| H10A | 0.76650 | 0.12740 | 0.87770 | 0.0880* | |
| H1A1 | 0.61870 | −0.18750 | 0.88430 | 0.1850* | |
| H11A | 0.88910 | 0.09070 | 0.61300 | 0.0710* | |
| H1A2 | 0.51290 | −0.21200 | 0.92870 | 0.1850* | |
| H12D | 1.04760 | −0.03190 | 0.68230 | 0.1270* | |
| H12E | 1.08950 | 0.08940 | 0.71510 | 0.1270* | |
| H12F | 1.05330 | 0.04610 | 0.59980 | 0.1270* | |
| H1A3 | 0.64070 | −0.22360 | 0.99420 | 0.1850* | |
| H2A1 | 0.66670 | −0.09230 | 1.14350 | 0.2010* | |
| H2A2 | 0.53160 | −0.09780 | 1.07700 | 0.2010* | |
| H15A | 0.51060 | 0.00710 | 0.25970 | 0.1160* | |
| H15B | 0.44560 | −0.07640 | 0.31740 | 0.1160* | |
| H15C | 0.57200 | −0.07560 | 0.31190 | 0.1160* | |
| H2A3 | 0.63620 | 0.01110 | 1.11300 | 0.2010* | |
| H17A | 0.42550 | 0.11830 | 0.28690 | 0.0840* | |
| H18A | 0.37880 | 0.26920 | 0.30510 | 0.1010* | |
| H19A | 0.47490 | 0.37890 | 0.45980 | 0.0880* | |
| H21A | 0.67030 | 0.18670 | 0.57610 | 0.0670* | |
| H4B1 | 0.95220 | 0.54710 | 0.03880 | 0.1010* | |
| H1B | 0.73030 | 0.69710 | 0.47570 | 0.0690* | |
| H4B2 | 1.03900 | 0.65070 | 0.11170 | 0.1010* | |
| H3B | 0.95290 | 0.75090 | −0.00840 | 0.0910* | |
| H2B1 | 0.81100 | 0.67760 | −0.17080 | 0.1590* | |
| H2B2 | 0.75540 | 0.63420 | −0.08760 | 0.1590* | |
| H6B | 0.90500 | 0.76520 | 0.14880 | 0.0790* | |
| H2B3 | 0.80540 | 0.56430 | −0.14140 | 0.1590* | |
| H7B | 0.75670 | 0.75610 | 0.21660 | 0.0720* | |
| H1B1 | 1.02760 | 0.61810 | −0.11160 | 0.1560* | |
| H1B2 | 1.12200 | 0.70520 | −0.02120 | 0.1560* | |
| H9B | 0.64230 | 0.44430 | 0.16080 | 0.0810* | |
| H1B3 | 1.04270 | 0.73700 | −0.12350 | 0.1560* | |
| H10B | 0.78740 | 0.45400 | 0.08910 | 0.0880* | |
| H11B | 0.55160 | 0.52280 | 0.25590 | 0.0660* | |
| H12A | 0.49630 | 0.68180 | 0.13330 | 0.1140* | |
| H12B | 0.40510 | 0.60630 | 0.17770 | 0.1140* | |
| H12C | 0.44150 | 0.56140 | 0.09300 | 0.1140* | |
| H15D | 0.90770 | 0.68430 | 0.60720 | 0.1180* | |
| H15E | 0.89770 | 0.58480 | 0.66580 | 0.1180* | |
| H15F | 0.78510 | 0.62670 | 0.62470 | 0.1180* | |
| H17B | 0.95180 | 0.46260 | 0.65410 | 0.0910* | |
| H18B | 1.00910 | 0.31750 | 0.64090 | 0.1070* | |
| H19B | 0.93680 | 0.21460 | 0.48530 | 0.1010* | |
| H21 | 0.74740 | 0.40610 | 0.35250 | 0.0740* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1A | 0.0714 (12) | 0.0606 (12) | 0.0770 (12) | 0.0225 (10) | 0.0029 (9) | −0.0123 (9) |
| O2A | 0.0921 (16) | 0.131 (2) | 0.0766 (14) | 0.0391 (15) | 0.0172 (12) | −0.0294 (13) |
| O3A | 0.1093 (17) | 0.0687 (15) | 0.1118 (16) | 0.0122 (13) | 0.0610 (14) | −0.0153 (12) |
| N1A | 0.0541 (12) | 0.0579 (13) | 0.0511 (11) | 0.0133 (10) | 0.0112 (9) | −0.0054 (9) |
| N2A | 0.0577 (12) | 0.0543 (13) | 0.0531 (11) | 0.0167 (10) | 0.0210 (10) | 0.0019 (9) |
| N3A | 0.0684 (16) | 0.0825 (19) | 0.0784 (17) | 0.0090 (14) | 0.0431 (14) | −0.0097 (14) |
| C1A | 0.120 (3) | 0.147 (4) | 0.077 (2) | 0.010 (3) | 0.033 (2) | 0.014 (2) |
| C2A | 0.175 (4) | 0.166 (4) | 0.109 (3) | 0.078 (3) | 0.090 (3) | 0.040 (3) |
| C3A | 0.119 (3) | 0.125 (3) | 0.084 (2) | 0.053 (3) | 0.048 (2) | 0.029 (2) |
| C4A | 0.112 (3) | 0.131 (3) | 0.0567 (17) | 0.028 (2) | 0.0239 (18) | 0.0022 (18) |
| C5A | 0.0759 (18) | 0.088 (2) | 0.0513 (15) | 0.0187 (17) | 0.0113 (13) | 0.0007 (15) |
| C6A | 0.112 (3) | 0.094 (3) | 0.0698 (19) | 0.046 (2) | 0.0249 (18) | 0.0283 (17) |
| C7A | 0.096 (2) | 0.078 (2) | 0.0737 (18) | 0.0449 (18) | 0.0266 (16) | 0.0120 (16) |
| C8A | 0.0483 (13) | 0.0552 (16) | 0.0519 (13) | 0.0136 (12) | 0.0047 (11) | −0.0004 (11) |
| C9A | 0.0660 (16) | 0.0578 (17) | 0.0594 (15) | 0.0199 (13) | 0.0127 (13) | 0.0023 (12) |
| C10A | 0.0740 (18) | 0.074 (2) | 0.0630 (16) | 0.0249 (15) | 0.0150 (14) | −0.0074 (14) |
| C11A | 0.0554 (14) | 0.0537 (16) | 0.0571 (14) | 0.0101 (12) | 0.0132 (11) | −0.0036 (11) |
| C12A | 0.0590 (17) | 0.084 (2) | 0.091 (2) | 0.0025 (15) | 0.0206 (15) | −0.0202 (16) |
| C13A | 0.0577 (15) | 0.0531 (16) | 0.0561 (14) | 0.0139 (12) | 0.0183 (12) | −0.0016 (11) |
| C14A | 0.0516 (13) | 0.0595 (16) | 0.0462 (12) | 0.0106 (12) | 0.0218 (11) | 0.0069 (11) |
| C15A | 0.0811 (19) | 0.082 (2) | 0.0561 (15) | 0.0279 (16) | 0.0084 (13) | −0.0089 (13) |
| C16A | 0.0488 (13) | 0.0580 (15) | 0.0504 (13) | 0.0116 (11) | 0.0227 (11) | 0.0065 (11) |
| C17A | 0.0737 (17) | 0.074 (2) | 0.0569 (15) | 0.0262 (15) | 0.0158 (13) | 0.0056 (13) |
| C18A | 0.092 (2) | 0.086 (2) | 0.0727 (18) | 0.0438 (19) | 0.0161 (16) | 0.0094 (16) |
| C19A | 0.0778 (19) | 0.071 (2) | 0.0837 (19) | 0.0318 (16) | 0.0378 (16) | 0.0095 (15) |
| C20A | 0.0568 (15) | 0.0605 (17) | 0.0660 (15) | 0.0094 (13) | 0.0343 (13) | −0.0024 (12) |
| C21A | 0.0507 (13) | 0.0659 (17) | 0.0537 (13) | 0.0143 (12) | 0.0252 (11) | 0.0047 (12) |
| O1B | 0.0702 (11) | 0.0641 (12) | 0.0644 (10) | 0.0252 (10) | 0.0161 (9) | −0.0083 (9) |
| O2B | 0.205 (3) | 0.142 (3) | 0.0968 (18) | 0.106 (2) | 0.005 (2) | −0.0231 (17) |
| O3B | 0.201 (3) | 0.116 (2) | 0.168 (3) | 0.101 (2) | 0.038 (2) | −0.0118 (19) |
| N1B | 0.0633 (12) | 0.0630 (14) | 0.0426 (10) | 0.0180 (11) | 0.0169 (9) | −0.0075 (9) |
| N2B | 0.0570 (11) | 0.0577 (13) | 0.0471 (11) | 0.0141 (10) | 0.0210 (9) | 0.0034 (9) |
| N3B | 0.103 (2) | 0.074 (2) | 0.104 (2) | 0.0396 (17) | 0.0369 (18) | 0.0064 (17) |
| C1B | 0.135 (3) | 0.093 (3) | 0.117 (3) | 0.038 (2) | 0.083 (2) | 0.017 (2) |
| C2B | 0.118 (3) | 0.118 (3) | 0.0690 (19) | 0.022 (2) | 0.033 (2) | 0.0003 (18) |
| C3B | 0.100 (2) | 0.0682 (19) | 0.0791 (19) | 0.0332 (17) | 0.0504 (17) | 0.0104 (15) |
| C4B | 0.101 (2) | 0.094 (2) | 0.090 (2) | 0.0529 (19) | 0.0547 (18) | 0.0229 (17) |
| C5B | 0.0766 (17) | 0.072 (2) | 0.0564 (14) | 0.0358 (16) | 0.0289 (13) | 0.0105 (13) |
| C6B | 0.0628 (16) | 0.0652 (18) | 0.0709 (16) | 0.0157 (13) | 0.0292 (13) | 0.0028 (13) |
| C7B | 0.0598 (15) | 0.0557 (17) | 0.0620 (15) | 0.0143 (13) | 0.0228 (12) | −0.0090 (12) |
| C8B | 0.0555 (14) | 0.0536 (15) | 0.0383 (11) | 0.0134 (12) | 0.0121 (10) | −0.0031 (10) |
| C9B | 0.0882 (19) | 0.0559 (17) | 0.0632 (15) | 0.0196 (14) | 0.0366 (14) | 0.0018 (12) |
| C10B | 0.107 (2) | 0.065 (2) | 0.0667 (16) | 0.0440 (18) | 0.0423 (16) | 0.0071 (13) |
| C11B | 0.0551 (14) | 0.0557 (16) | 0.0476 (12) | 0.0094 (12) | 0.0174 (11) | −0.0043 (11) |
| C12B | 0.0565 (15) | 0.099 (2) | 0.0598 (15) | 0.0178 (15) | 0.0123 (13) | −0.0072 (14) |
| C13B | 0.0503 (13) | 0.0615 (17) | 0.0483 (13) | 0.0097 (12) | 0.0214 (11) | −0.0016 (11) |
| C14B | 0.0555 (14) | 0.0595 (16) | 0.0464 (13) | 0.0051 (12) | 0.0220 (11) | 0.0082 (11) |
| C15B | 0.089 (2) | 0.082 (2) | 0.0462 (13) | 0.0077 (16) | 0.0183 (13) | 0.0008 (13) |
| C16B | 0.0507 (13) | 0.0666 (18) | 0.0540 (14) | 0.0063 (12) | 0.0235 (11) | 0.0182 (12) |
| C17B | 0.0678 (18) | 0.088 (2) | 0.0623 (16) | 0.0152 (16) | 0.0208 (14) | 0.0247 (15) |
| C18B | 0.076 (2) | 0.104 (3) | 0.086 (2) | 0.034 (2) | 0.0236 (17) | 0.049 (2) |
| C19B | 0.081 (2) | 0.077 (2) | 0.111 (3) | 0.0325 (18) | 0.047 (2) | 0.042 (2) |
| C20B | 0.0647 (16) | 0.0629 (18) | 0.0821 (19) | 0.0182 (14) | 0.0341 (15) | 0.0219 (15) |
| C21B | 0.0592 (15) | 0.0637 (18) | 0.0622 (15) | 0.0196 (13) | 0.0221 (12) | 0.0158 (13) |
Geometric parameters (Å, º)
| O1A—C13A | 1.228 (3) | C12A—H12D | 0.9600 |
| O2A—N3A | 1.226 (4) | C15A—H15A | 0.9600 |
| O3A—N3A | 1.224 (4) | C15A—H15C | 0.9600 |
| O1B—C13B | 1.222 (3) | C15A—H15B | 0.9600 |
| O2B—N3B | 1.194 (4) | C17A—H17A | 0.9300 |
| O3B—N3B | 1.195 (4) | C18A—H18A | 0.9300 |
| N1A—N2A | 1.374 (3) | C19A—H19A | 0.9300 |
| N1A—C13A | 1.351 (3) | C21A—H21A | 0.9300 |
| N2A—C14A | 1.282 (3) | C1B—C3B | 1.519 (6) |
| N3A—C20A | 1.477 (4) | C2B—C3B | 1.512 (5) |
| N1A—H1A | 0.8600 | C3B—C4B | 1.522 (4) |
| N1B—C13B | 1.359 (3) | C4B—C5B | 1.513 (5) |
| N1B—N2B | 1.371 (3) | C5B—C6B | 1.379 (4) |
| N2B—C14B | 1.281 (3) | C5B—C10B | 1.383 (4) |
| N3B—C20B | 1.471 (4) | C6B—C7B | 1.379 (4) |
| N1B—H1B | 0.8600 | C7B—C8B | 1.383 (4) |
| C1A—C3A | 1.483 (6) | C8B—C11B | 1.517 (4) |
| C2A—C3A | 1.541 (6) | C8B—C9B | 1.375 (4) |
| C3A—C4A | 1.500 (6) | C9B—C10B | 1.380 (5) |
| C4A—C5A | 1.514 (4) | C11B—C13B | 1.526 (3) |
| C5A—C10A | 1.384 (4) | C11B—C12B | 1.534 (4) |
| C5A—C6A | 1.369 (5) | C14B—C15B | 1.509 (3) |
| C6A—C7A | 1.380 (4) | C14B—C16B | 1.481 (4) |
| C7A—C8A | 1.387 (4) | C16B—C21B | 1.399 (4) |
| C8A—C11A | 1.516 (3) | C16B—C17B | 1.396 (4) |
| C8A—C9A | 1.373 (4) | C17B—C18B | 1.373 (5) |
| C9A—C10A | 1.384 (4) | C18B—C19B | 1.363 (6) |
| C11A—C12A | 1.530 (4) | C19B—C20B | 1.386 (5) |
| C11A—C13A | 1.518 (4) | C20B—C21B | 1.362 (4) |
| C14A—C16A | 1.479 (4) | C1B—H1B1 | 0.9600 |
| C14A—C15A | 1.504 (3) | C1B—H1B2 | 0.9600 |
| C16A—C17A | 1.387 (4) | C1B—H1B3 | 0.9600 |
| C16A—C21A | 1.398 (3) | C2B—H2B1 | 0.9600 |
| C17A—C18A | 1.382 (5) | C2B—H2B2 | 0.9600 |
| C18A—C19A | 1.374 (4) | C2B—H2B3 | 0.9600 |
| C19A—C20A | 1.376 (4) | C3B—H3B | 0.9800 |
| C20A—C21A | 1.365 (4) | C4B—H4B1 | 0.9700 |
| C1A—H1A1 | 0.9600 | C4B—H4B2 | 0.9700 |
| C1A—H1A3 | 0.9600 | C6B—H6B | 0.9300 |
| C1A—H1A2 | 0.9600 | C7B—H7B | 0.9300 |
| C2A—H2A2 | 0.9600 | C9B—H9B | 0.9300 |
| C2A—H2A3 | 0.9600 | C10B—H10B | 0.9300 |
| C2A—H2A1 | 0.9600 | C11B—H11B | 0.9800 |
| C3A—H3A | 0.9800 | C12B—H12A | 0.9600 |
| C4A—H4A2 | 0.9700 | C12B—H12B | 0.9600 |
| C4A—H4A1 | 0.9700 | C12B—H12C | 0.9600 |
| C6A—H6A | 0.9300 | C15B—H15D | 0.9600 |
| C7A—H7A | 0.9300 | C15B—H15E | 0.9600 |
| C9A—H9A | 0.9300 | C15B—H15F | 0.9600 |
| C10A—H10A | 0.9300 | C17B—H17B | 0.9300 |
| C11A—H11A | 0.9800 | C18B—H18B | 0.9300 |
| C12A—H12F | 0.9600 | C19B—H19B | 0.9300 |
| C12A—H12E | 0.9600 | C21B—H21 | 0.9300 |
| N2A—N1A—C13A | 121.0 (2) | C16A—C17A—H17A | 119.00 |
| N1A—N2A—C14A | 118.38 (19) | C19A—C18A—H18A | 120.00 |
| O2A—N3A—O3A | 123.9 (3) | C17A—C18A—H18A | 120.00 |
| O2A—N3A—C20A | 117.8 (2) | C18A—C19A—H19A | 121.00 |
| O3A—N3A—C20A | 118.3 (2) | C20A—C19A—H19A | 121.00 |
| C13A—N1A—H1A | 119.00 | C20A—C21A—H21A | 120.00 |
| N2A—N1A—H1A | 120.00 | C16A—C21A—H21A | 120.00 |
| N2B—N1B—C13B | 120.56 (19) | C1B—C3B—C4B | 112.1 (3) |
| N1B—N2B—C14B | 118.6 (2) | C1B—C3B—C2B | 110.4 (3) |
| O2B—N3B—C20B | 119.3 (3) | C2B—C3B—C4B | 112.2 (3) |
| O3B—N3B—C20B | 118.3 (3) | C3B—C4B—C5B | 114.8 (3) |
| O2B—N3B—O3B | 122.5 (3) | C6B—C5B—C10B | 116.4 (3) |
| C13B—N1B—H1B | 120.00 | C4B—C5B—C10B | 122.1 (3) |
| N2B—N1B—H1B | 120.00 | C4B—C5B—C6B | 121.6 (3) |
| C1A—C3A—C4A | 115.7 (4) | C5B—C6B—C7B | 121.9 (3) |
| C1A—C3A—C2A | 111.7 (4) | C6B—C7B—C8B | 121.4 (2) |
| C2A—C3A—C4A | 109.5 (3) | C7B—C8B—C11B | 121.3 (2) |
| C3A—C4A—C5A | 115.1 (3) | C7B—C8B—C9B | 117.0 (3) |
| C4A—C5A—C6A | 122.0 (3) | C9B—C8B—C11B | 121.6 (2) |
| C6A—C5A—C10A | 116.2 (3) | C8B—C9B—C10B | 121.4 (3) |
| C4A—C5A—C10A | 121.8 (3) | C5B—C10B—C9B | 121.9 (3) |
| C5A—C6A—C7A | 122.6 (3) | C8B—C11B—C12B | 110.17 (18) |
| C6A—C7A—C8A | 121.0 (3) | C8B—C11B—C13B | 110.2 (2) |
| C7A—C8A—C9A | 116.7 (2) | C12B—C11B—C13B | 110.4 (2) |
| C7A—C8A—C11A | 121.8 (2) | O1B—C13B—N1B | 119.5 (2) |
| C9A—C8A—C11A | 121.5 (2) | N1B—C13B—C11B | 117.8 (2) |
| C8A—C9A—C10A | 121.8 (2) | O1B—C13B—C11B | 122.7 (2) |
| C5A—C10A—C9A | 121.7 (3) | N2B—C14B—C16B | 114.5 (2) |
| C12A—C11A—C13A | 109.0 (2) | C15B—C14B—C16B | 120.5 (2) |
| C8A—C11A—C13A | 110.1 (2) | N2B—C14B—C15B | 125.0 (2) |
| C8A—C11A—C12A | 112.6 (2) | C17B—C16B—C21B | 116.9 (3) |
| O1A—C13A—N1A | 119.5 (2) | C14B—C16B—C17B | 123.1 (2) |
| N1A—C13A—C11A | 118.9 (2) | C14B—C16B—C21B | 120.0 (2) |
| O1A—C13A—C11A | 121.6 (2) | C16B—C17B—C18B | 121.8 (3) |
| C15A—C14A—C16A | 119.7 (2) | C17B—C18B—C19B | 120.8 (3) |
| N2A—C14A—C15A | 124.7 (2) | C18B—C19B—C20B | 118.0 (3) |
| N2A—C14A—C16A | 115.7 (2) | N3B—C20B—C19B | 119.0 (3) |
| C14A—C16A—C21A | 120.4 (2) | N3B—C20B—C21B | 118.7 (2) |
| C17A—C16A—C21A | 117.3 (2) | C19B—C20B—C21B | 122.3 (3) |
| C14A—C16A—C17A | 122.3 (2) | C16B—C21B—C20B | 120.2 (2) |
| C16A—C17A—C18A | 121.8 (2) | C3B—C1B—H1B1 | 109.00 |
| C17A—C18A—C19A | 120.6 (3) | C3B—C1B—H1B2 | 109.00 |
| C18A—C19A—C20A | 117.4 (3) | C3B—C1B—H1B3 | 109.00 |
| C19A—C20A—C21A | 123.2 (2) | H1B1—C1B—H1B2 | 109.00 |
| N3A—C20A—C21A | 118.8 (2) | H1B1—C1B—H1B3 | 110.00 |
| N3A—C20A—C19A | 118.1 (2) | H1B2—C1B—H1B3 | 109.00 |
| C16A—C21A—C20A | 119.7 (2) | C3B—C2B—H2B1 | 109.00 |
| C3A—C1A—H1A1 | 110.00 | C3B—C2B—H2B2 | 109.00 |
| C3A—C1A—H1A2 | 109.00 | C3B—C2B—H2B3 | 109.00 |
| H1A1—C1A—H1A2 | 109.00 | H2B1—C2B—H2B2 | 109.00 |
| H1A2—C1A—H1A3 | 109.00 | H2B1—C2B—H2B3 | 109.00 |
| H1A1—C1A—H1A3 | 110.00 | H2B2—C2B—H2B3 | 110.00 |
| C3A—C1A—H1A3 | 109.00 | C1B—C3B—H3B | 107.00 |
| H2A1—C2A—H2A3 | 109.00 | C2B—C3B—H3B | 107.00 |
| C3A—C2A—H2A1 | 109.00 | C4B—C3B—H3B | 107.00 |
| C3A—C2A—H2A2 | 109.00 | C3B—C4B—H4B1 | 108.00 |
| C3A—C2A—H2A3 | 109.00 | C3B—C4B—H4B2 | 109.00 |
| H2A1—C2A—H2A2 | 110.00 | C5B—C4B—H4B1 | 109.00 |
| H2A2—C2A—H2A3 | 109.00 | C5B—C4B—H4B2 | 109.00 |
| C4A—C3A—H3A | 106.00 | H4B1—C4B—H4B2 | 108.00 |
| C2A—C3A—H3A | 106.00 | C5B—C6B—H6B | 119.00 |
| C1A—C3A—H3A | 106.00 | C7B—C6B—H6B | 119.00 |
| C3A—C4A—H4A1 | 108.00 | C6B—C7B—H7B | 119.00 |
| C3A—C4A—H4A2 | 109.00 | C8B—C7B—H7B | 119.00 |
| C5A—C4A—H4A2 | 109.00 | C8B—C9B—H9B | 119.00 |
| H4A1—C4A—H4A2 | 108.00 | C10B—C9B—H9B | 119.00 |
| C5A—C4A—H4A1 | 108.00 | C5B—C10B—H10B | 119.00 |
| C5A—C6A—H6A | 119.00 | C9B—C10B—H10B | 119.00 |
| C7A—C6A—H6A | 119.00 | C8B—C11B—H11B | 109.00 |
| C8A—C7A—H7A | 119.00 | C12B—C11B—H11B | 109.00 |
| C6A—C7A—H7A | 120.00 | C13B—C11B—H11B | 109.00 |
| C10A—C9A—H9A | 119.00 | C11B—C12B—H12A | 109.00 |
| C8A—C9A—H9A | 119.00 | C11B—C12B—H12B | 109.00 |
| C9A—C10A—H10A | 119.00 | C11B—C12B—H12C | 109.00 |
| C5A—C10A—H10A | 119.00 | H12A—C12B—H12B | 109.00 |
| C13A—C11A—H11A | 108.00 | H12A—C12B—H12C | 110.00 |
| C8A—C11A—H11A | 108.00 | H12B—C12B—H12C | 109.00 |
| C12A—C11A—H11A | 108.00 | C14B—C15B—H15D | 109.00 |
| H12D—C12A—H12F | 110.00 | C14B—C15B—H15E | 109.00 |
| C11A—C12A—H12E | 109.00 | C14B—C15B—H15F | 109.00 |
| H12D—C12A—H12E | 109.00 | H15D—C15B—H15E | 109.00 |
| C11A—C12A—H12D | 109.00 | H15D—C15B—H15F | 109.00 |
| H12E—C12A—H12F | 110.00 | H15E—C15B—H15F | 109.00 |
| C11A—C12A—H12F | 109.00 | C16B—C17B—H17B | 119.00 |
| H15A—C15A—H15C | 110.00 | C18B—C17B—H17B | 119.00 |
| H15B—C15A—H15C | 109.00 | C17B—C18B—H18B | 120.00 |
| C14A—C15A—H15B | 109.00 | C19B—C18B—H18B | 120.00 |
| C14A—C15A—H15C | 110.00 | C18B—C19B—H19B | 121.00 |
| C14A—C15A—H15A | 109.00 | C20B—C19B—H19B | 121.00 |
| H15A—C15A—H15B | 109.00 | C16B—C21B—H21 | 120.00 |
| C18A—C17A—H17A | 119.00 | C20B—C21B—H21 | 120.00 |
| C13A—N1A—N2A—C14A | 172.6 (2) | C21A—C16A—C17A—C18A | 2.2 (4) |
| N2A—N1A—C13A—O1A | 173.5 (2) | C17A—C16A—C21A—C20A | −1.2 (4) |
| N2A—N1A—C13A—C11A | −8.1 (3) | C14A—C16A—C21A—C20A | 178.7 (2) |
| N1A—N2A—C14A—C15A | −2.2 (4) | C16A—C17A—C18A—C19A | −1.6 (5) |
| N1A—N2A—C14A—C16A | 178.7 (2) | C17A—C18A—C19A—C20A | 0.0 (5) |
| O2A—N3A—C20A—C19A | 175.9 (3) | C18A—C19A—C20A—N3A | −179.8 (3) |
| O2A—N3A—C20A—C21A | −4.8 (4) | C18A—C19A—C20A—C21A | 1.0 (5) |
| O3A—N3A—C20A—C19A | −3.4 (4) | C19A—C20A—C21A—C16A | −0.4 (4) |
| O3A—N3A—C20A—C21A | 175.9 (3) | N3A—C20A—C21A—C16A | −179.6 (2) |
| N2B—N1B—C13B—C11B | 1.1 (4) | C1B—C3B—C4B—C5B | 178.4 (3) |
| C13B—N1B—N2B—C14B | −178.8 (2) | C2B—C3B—C4B—C5B | −56.8 (4) |
| N2B—N1B—C13B—O1B | −177.7 (2) | C3B—C4B—C5B—C6B | −59.8 (4) |
| N1B—N2B—C14B—C15B | 1.9 (4) | C3B—C4B—C5B—C10B | 118.8 (3) |
| N1B—N2B—C14B—C16B | −177.5 (2) | C4B—C5B—C6B—C7B | 177.5 (2) |
| O2B—N3B—C20B—C21B | −2.0 (5) | C10B—C5B—C6B—C7B | −1.2 (4) |
| O2B—N3B—C20B—C19B | 179.5 (4) | C4B—C5B—C10B—C9B | −178.4 (3) |
| O3B—N3B—C20B—C21B | 179.1 (3) | C6B—C5B—C10B—C9B | 0.3 (4) |
| O3B—N3B—C20B—C19B | 0.6 (5) | C5B—C6B—C7B—C8B | 1.1 (4) |
| C2A—C3A—C4A—C5A | −170.2 (3) | C6B—C7B—C8B—C9B | 0.0 (3) |
| C1A—C3A—C4A—C5A | 62.6 (4) | C6B—C7B—C8B—C11B | −176.5 (2) |
| C3A—C4A—C5A—C6A | −92.9 (4) | C7B—C8B—C9B—C10B | −0.9 (4) |
| C3A—C4A—C5A—C10A | 84.7 (4) | C11B—C8B—C9B—C10B | 175.6 (2) |
| C4A—C5A—C6A—C7A | 177.8 (3) | C7B—C8B—C11B—C12B | 80.2 (3) |
| C10A—C5A—C6A—C7A | 0.1 (5) | C7B—C8B—C11B—C13B | −41.9 (3) |
| C4A—C5A—C10A—C9A | −178.6 (3) | C9B—C8B—C11B—C12B | −96.2 (3) |
| C6A—C5A—C10A—C9A | −0.9 (5) | C9B—C8B—C11B—C13B | 141.7 (2) |
| C5A—C6A—C7A—C8A | 0.3 (5) | C8B—C9B—C10B—C5B | 0.8 (4) |
| C6A—C7A—C8A—C9A | 0.2 (4) | C8B—C11B—C13B—O1B | 105.5 (3) |
| C6A—C7A—C8A—C11A | 179.4 (3) | C8B—C11B—C13B—N1B | −73.2 (3) |
| C7A—C8A—C9A—C10A | −1.0 (4) | C12B—C11B—C13B—O1B | −16.4 (3) |
| C7A—C8A—C11A—C13A | 64.8 (3) | C12B—C11B—C13B—N1B | 164.9 (2) |
| C9A—C8A—C11A—C12A | 122.2 (3) | N2B—C14B—C16B—C17B | 178.0 (3) |
| C9A—C8A—C11A—C13A | −115.9 (3) | N2B—C14B—C16B—C21B | −0.3 (4) |
| C11A—C8A—C9A—C10A | 179.8 (2) | C15B—C14B—C16B—C17B | −1.3 (4) |
| C7A—C8A—C11A—C12A | −57.0 (3) | C15B—C14B—C16B—C21B | −179.6 (3) |
| C8A—C9A—C10A—C5A | 1.4 (4) | C14B—C16B—C17B—C18B | −178.1 (3) |
| C12A—C11A—C13A—O1A | 37.8 (3) | C21B—C16B—C17B—C18B | 0.3 (5) |
| C8A—C11A—C13A—O1A | −86.1 (3) | C14B—C16B—C21B—C20B | 178.7 (2) |
| C8A—C11A—C13A—N1A | 95.5 (3) | C17B—C16B—C21B—C20B | 0.4 (4) |
| C12A—C11A—C13A—N1A | −140.6 (2) | C16B—C17B—C18B—C19B | −0.4 (6) |
| N2A—C14A—C16A—C21A | −4.6 (4) | C17B—C18B—C19B—C20B | −0.2 (6) |
| C15A—C14A—C16A—C17A | −3.9 (4) | C18B—C19B—C20B—N3B | 179.3 (3) |
| N2A—C14A—C16A—C17A | 175.3 (2) | C18B—C19B—C20B—C21B | 0.9 (5) |
| C15A—C14A—C16A—C21A | 176.2 (3) | N3B—C20B—C21B—C16B | −179.4 (3) |
| C14A—C16A—C17A—C18A | −177.7 (3) | C19B—C20B—C21B—C16B | −0.9 (4) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1A—H1A···O1Bi | 0.86 | 2.14 | 2.977 (3) | 165 |
| N1B—H1B···O1Aii | 0.86 | 2.15 | 2.919 (3) | 149 |
| C11A—H11A···N2A | 0.98 | 2.41 | 2.803 (4) | 103 |
| C15A—H15C···O1Bi | 0.96 | 2.41 | 3.252 (4) | 147 |
| C15A—H15C···N1A | 0.96 | 2.41 | 2.791 (4) | 103 |
Symmetry codes: (i) x, y−1, z; (ii) x, y+1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: GK2579).
References
- Bedia, K. K., Elcin, O., Seda, U., Fatma, K., Nathaly, S., Sevim, R. & Dimoglo, A. (2006). Eur. J. Med. Chem. 41, 1253–1261. [DOI] [PubMed]
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
- Fun, H.-K., Chantrapromma, S., Sujith, K. V. & Kalluraya, B. (2009). Acta Cryst. E65, o445. [DOI] [PMC free article] [PubMed]
- Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J. & Wood, P. A. (2008). J. Appl. Cryst. 41, 466–470.
- Oxford Diffraction (2009). CrysAlis PRO. Oxford Diffraction, Yarnton, Oxfordshire, England.
- Rollas, S., Gulerman, N. & Erdeniz, H. (2002). Il Farmaco, 57, 171–174. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Terzioglu, N. & Gursoy, A. (2003). Eur. J. Med. Chem. 38, 781–786. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536813019892/gk2579sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813019892/gk2579Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813019892/gk2579Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report