Abstract
GATA-binding protein 2 (GATA2) and LIM domain only 2 (Lmo2) form common transcription complexes during hematopoietic differentiation. Here we show that these two transcription factors also play a key role in endothelial cells (EC) and lymphatic EC (LEC) function. Primary EC and tumor-associated blood vessels expressed GATA2 and Lmo2. VEGF-induced sprouting angiogenesis in both differentiating embryonic stem cells (embryoid bodies) and primary EC increased GATA2 and Lmo2 levels. Conversely, silencing of GATA2 and Lmo2 expression in primary EC inhibited VEGF-induced angiogenic activity, including EC migration and sprouting in vitro, two key steps of angiogenesis in vivo. This inhibition of EC function was associated with downregulated expression of neuropilin-2 (NRP2), a co-receptor for VEGF, at the protein, mRNA and promoter levels. NRP2 overexpression partially rescued the impaired angiogenic sprouting in the GATA2/Lmo2 knockdown EC, confirming that GATA2 and Lmo2 mediated EC function, at least in part, by directly regulating NRP2 gene expression. Furthermore, it was found that primary LEC expressed GATA2 and Lmo2 as well. Silencing of GATA2 and Lmo2 expression in LEC inhibited VEGF-induced LEC sprouting, also in a NRP2-dependent manner. In conclusion, our results demonstrate that GATA2 and Lmo2 cooperatively regulate VEGF-induced angiogenesis and lymphangiogenesis via NRP2.
Keywords: GATA2, Lmo2, Neuropilin-2, Transcriptional Regulation, Angiogenesis, Lymphangiogenesis
INTRODUCTION
During normal vertebrate development, endothelial cells (EC) and hematopoietic cells (HPC) are thought to originate from a common precursor, the hemangioblast [1–3]. Given their common origin, blood and endothelial cells share expression of a number of different genes, and several transcription factors involved in the commitment and differentiation of hemangioblasts to HPC also control the differentiation of hemangioblasts to EC [4–6]. Among these is GATA-binding protein 2 (GATA2), a transcription factor expressed in early progenitors with the potential to generate both HPC and EC.
GATA2 belongs to an evolutionarily conserved family of C4 zinc finger transcription factors that play demonstrably critical roles in development [7]. There are six members of the GATA family in vertebrates and they have historically been subdivided into two subfamilies. GATA1/2/3 are preferentially expressed in HPC and regulate proliferation and differentiation during hematopoiesis [8–10]. GATA4/5/6 have been shown to be involved in cardiac, genitourinary and endodermal development [11–13]. GATA2 was first demonstrated to be critical for the proliferation and differentiation of early hematopoietic progenitors [10,14–16]. However, GATA2 is also expressed in EC and regulates the promoter activity of several endothelium-specific genes, such as platelet endothelial cell adhesion molecule-1 (PECAM-1) and endothelin-1 [17–19]. Furthermore, it has been shown that GATA2 regulates the expression of vascular endothelial growth factor receptor-2 (VEGFR-2) during both vascular development and angiogenesis, the process by which EC form new blood vessels from an existing vascular network [20,21]. Whether GATA2 regulates the expression of neuropilin-1 and -2 (NRP1 and NRP2), two key co-receptors of VEGFRs for VEGF, was not investigated in these studies.
Like all the known members of the GATA family, GATA2 has two zinc finger domains and binds the DNA sequence GATA. It has been reported that GATA2 interacts with other transcription factors to form multimeric transcription complexes. Among these are LIM domain only 2 (Lmo2), LIM domain binding protein (Ldb1), and the basic helix-loop-helix transcription factors T-cell acute lymphoblastic leukemia 1/Stem cell leukemia (Tal1/Scl) and lymphoblastic leukemia derived sequence 1 (Lyl1).
Lmo2 is a key player in the assembly of these transcription complexes through its LIM-domain zinc-finger-like structures [22,23]. It has been proposed that Lmo2 acts as a bridging molecule that facilitates the formation of different multimeric complexes that regulate transcription of different genes at various stages of hematopoiesis [22–25]. For example, the ability of the Lmo2 protein to interact with other proteins led to the identification of a multimeric protein complex in erythroid cells, comprising Tal1/Scl, E47, Ldb1, and GATA1 in addition to Lmo2, which binds specifically to a bipartite DNA sequence [26]. In EC, Lmo2 also functions by interaction with specific partners in DNA-binding transcription complexes. In this regard, it has been shown that, in EC, VE-cadherin expression is regulated by Lmo2-complexes with GATA2, Tal1/Scl, Ldb1 and E47, whereas Angiopoietin-2 is regulated by several multi-protein complexes including Lmo2, GATA2, Tal1/Scl and/or Lyl1 [27,28]. Lmo2 is an essential protein in both the primitive and definitive hematopoietic pathways [29,30]. In mouse embryogenesis, Lmo2 is essential for initiation of yolk sac and definitive hematopoiesis, but it is not needed for the novo capillary formation from mesoderm [31]. However, it has been shown that Lmo2 is necessary for angiogenic remodeling of the existing capillary network into mature vasculature, and it is a key regulator of tumor angiogenesis [31,32].
Thus, while the crucial roles of GATA2 and Lmo2 in early hematopoiesis are well established, their functions in EC, especially in vascular development and angiogenesis, are less understood. Elucidating the molecular signaling pathways that result in EC differentiation and control EC function is not only important for understanding normal vascular development but also for understanding the deregulation of angiogenesis in many diseases, including cancer, arthritis and blindness [33,34]. VEGF-A, the major angiogenesis stimulator identified so far, along with its major receptor VEGFR-2, are of particular importance in angiogenesis because they are essential for normal blood vessel development. Deregulation of these factors leads to various pathological conditions, including proliferative retinopathy, arthritis and cancer.
Lymphangiogenesis, the formation of new lymphatic vessels from pre-existing lymphatic vessels, is also actively involved in many diseases, including cancer and lymphedema [35]. VEGF-C, acting via VEGFR-3 and VEGFR-2, is the major mediator of lymphatic development and tumor lymphangiogenesis [36]. Very little is known about the roles of GATA2 and Lmo2 in lymphatic endothelial cells (LEC). GATA2 is present at high levels in the leaflets of lymphatic vessel valves, and it controls the expression of genes that have key roles in programming lymphatic valve development [37]. Moreover, GATA2 function is required for proper lymphatic vascular development during embryogenesis [38]. Recently, several groups reported that human patients with GATA2 mutations presented symptoms of primary lymphedema [37,39,40], underscoring the vital role of transcription factor GATA2 in lymphatic development. Even though expression of Lmo2 has been shown in lung lymphatic microvascular EC [28], its role during lymphatic development and tumor lymphangiogenesis is still unknown.
NRP1 and NRP2, first described as receptors for class-3 semaphorins (SEMA3A-G) that regulate axon guidance during nervous system development, are also essential regulators of vascular development [41–47]. During development, NRP1 is mostly expressed in arteries and NRP2 in veins and lymphatics. In EC and LEC, NRP1s function as co-receptors of VEGFRs for VEGF [48–50]. Indeed, NRP1/2 expression in EC increases VEGF binding to VEGFRs, VEGFR phosphorylation and VEGF-dependent VEGFR activities such as EC survival and chemotaxis [48,51]. Furthermore, it has been shown that NRP2 mediates VEGF-C induced lymphatic sprouting together with VEGFR-3 [52].
In this report we have demonstrated that VEGF-induced angiogenic and lymphangiogenic activities, including EC migration and sprouting, are GATA2- and Lmo2-dependent. GATA2 and Lmo2 control angiogenesis and lymphangiogenesis via direct transcriptional regulation of NRP2. We conclude that GATA2 and Lmo2, shown previously to be active in regulating hematopoietic development, are novel contributors to the regulation of angiogenesis and lymphangiogenesis via NRP2. Thus, GATA2 and Lmo2 may be promising targets for antiangiogenic and anti-lymphangiogenic therapies.
MATERIALS AND METHODS
Cell culture
Human umbilical vein EC (HUVEC) were cultured in EBM2 medium supplemented with EGM-2 SingleQuots (Lonza Inc.). Human microvascular EC (HMVEC) and dermal-derived human lymphatic EC (LEC) were cultured in EBM2 medium supplemented with EGM-2MV (Lonza Inc.). Human chronic myeloid leukemia K562 cells were cultured in RPMI (Invitrogen) supplemented with 5% fetal bovine serum (FBS, Denville). Human U87MG glioblastoma and A375SM melanoma cells and mouse B16F10 melanoma cells were cultured in MEM (Invitrogen) supplemented with 10% FBS. Mouse MS1 EC were cultured in DMEM (Invitrogen) supplemented with 5% FBS. Mouse dermal EC (MDEC) and prostate cancer TRAMP C1 cells were kindly provided by Dr. Andrew Dudley (University of North Carolina, Chapel Hill, NC, USA) [53].
Immunofluorescent staining of mouse tumors
Eight-week-old female nude mice were purchased from Massachusetts General Hospital (Boston, MA). The mice were maintained under specific pathogen-free conditions in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. The care and treatment of experimental animals were in accordance with the Institutional Animal Care and Use Committee guidelines at Children’s Hospital Boston. U87MG cells (106 cells per 100 μl of HBSS) were injected subcutaneously into the right dorsal flank of the mice. Mice were sacrificed 6 weeks after tumor cell inoculation. Frozen sections (8-μm thick) were fixed in acetone, followed by acetone/chloroform (1:1, v/v) and acetone. Sections were blocked in 5% horse serum (Vector) before staining. CD31 was detected using a rat anti-CD31 (BD Pharmingen) antibody followed by incubation with anti-rat Alexa 568 antibody. GATA2 and Lmo2 were detected with rabbit anti-GATA2 (Abcam) and goat anti-Lmo2 (Santa Cruz) antibodies in 5% horse serum and 0.5% triton X, followed by incubation with biotinylated secondary antibodies (Vector) and fluorescein-avidin (Vector). Nuclei were stained with 4,6′-diamidino-2-phenylindole. The mounted samples on slide glasses were imaged on a Leica TCS SP2 confocal laser-scanning microscope using a 40× objective (numerical aperture 1.25).
Embryoid bodies (EBs)
R1/SVJ 129 murine embryonic stem cells were kindly provided by Dr. Andras Nagy (Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, ON, Canada) and cultured as previously described [54]. At day 0, 200 cells were aggregated in hanging drops (20 μl) and induced to differentiate by omitting leukemia inhibitory factor as previously described [54]. Four-day-old EBs were seeded in a six-well plate in medium containing 50 ng/ml mouse VEGF (PeproTech). At day 9 of differentiation, RNA from EBs was extracted using the RNeasyMini Kit with on-column DNase digestion (Qiagen). One microgram of RNA was used for reverse transcription with 1 unit M-MLV reverse transcriptase (USB Corp.). Quantitative real-time PCR was carried out with Sybr green (Applied Biosystems), with mouse hypoxanthine-guanine phosphoribosyltransferase (HPRT) serving as internal control. The primer sequences are shown in Supplementary Table S1. All reactions were done in duplicate on an MX3005 instrument (Stratagene).
Isolation of mouse brain EC
Mouse brain EC were isolated from 8- to 10-week-old nude mice. Excised mouse brain tissues were processed to obtain single cell suspension. The cells were then incubated with anti-mouse CD31 antibody (eBioscience), and brain EC were isolated by MACS (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s protocol, using anti-rat IgG microbeads (Miltenyi Biotec). EC were plated onto 1.5% gelatin-coated culture plates and grown in EGM-2MV (Lonza). To improve the purity of brain EC, magnetic sorting was performed using two MACS columns set up in series.
Transfections
The full-length NRP2 construct has been previously described [55]. HUVEC were transfected using Superfect reagent (Qiagen).
siRNA knockdown
GATA2 siRNA has been previously described [20]. Lmo2 (sc-38027) and NRP2 (M-017721-01-0005) siRNAs were purchased from Santa Cruz Biotechnology, Inc. and Thermo Fisher Scientific, respectively. As a control, a siRNA duplex with an irrelevant sequence (Ambion Inc.) was used. Cells were transfected with 20 nM siRNA using SilentFect reagent (Bio-Rad Laboratories).
RNA isolation and analysis
Total RNA was isolated from the cells using the RNeasy kit (Qiagen). cDNA was prepared using Superscript II enzyme (Invitrogen Corp.) and 1 μg total RNA. For quantitative real-time PCR analysis, the IQ SYBR Green Supermix (Bio-Rad Laboratories) was used. Oligonucleotide primers are listed in Supplementary Table S1. Beta-2 microglobulin (B2M) was used as an internal control. Reactions were run on a LightCycler (Roche Applied Science). Each experiment was done in duplicate and repeated three times.
Immunoblot
Cells were lysed and immunoblotted as described previously [56]. Specific proteins were detected after incubation with anti-NRP1, -NRP2, (Santa Cruz Biotechnology, Inc.), -VEGFR-2 (Cell Signaling), -GATA2, -Lmo2 (Abcam) and -β-actin (Sigma-Aldrich Corp.) antibodies.
Cell migration
Transwells (Corning Inc.) with an 8.0-μm pore size were coated with a 0.5% gelatin solution. siRNA-treated HUVEC (2.5 × 104) in 0.5% FBS/EBM2 were added to the upper wells. EBM2 containing 0.5% FBS, 10 ng/ml VEGF-A (National Cancer Institute) and 1 μg/ml heparin were added to the lower wells. Cells that migrated through the filter after 16 hours were stained and counted by phase microscopy as described earlier [56]. The experiment was repeated three times in duplicate. The results represent the average of the three experiments.
Spheroid-based angiogenesis/lymphangiogenesis assay
Early passage mouse brain EC, HUVEC LEC subsequent, were suspended and then aggregated overnight in hanging drops (25 μl) to form cellular spheroids (500 cells/spheroid). Spheroids were embedded into collagen gels, and then 25 ng/ml VEGF-A or 500 ng/ml VEGF-C in EGM2 medium were added on top of the gel. After 24 hours, in vitro angiogenesis/lymphangiogenesis was quantified by measuring the number and the cumulative length of sprouts that had grown out of each spheroid using NIH ImageJ software. Ten to fifteen spheroids per experiment group were analyzed. For immunofluorescent staining, spheroids were fixed with 4% paraformaldehyde, followed by permeabilization with 0.2% Triton X-100 in PBS. F-actin and nuclei were stained with Alexa Fluor 488 phalloidin (Invitrogen) and 4,6′-diamidino-2-phenylindole, respectively. Spheroids were imaged on a LeicaTCS SP2 confocal laser-scanning microscope using a 10× objective (numerical aperture 1.4).
Promoter luciferase constructs
The human NRP2 promoter region was cloned from a human genomic PAC library as previously described [57]. A 1831 bp fragment spanning −2037 to −206 relative to the start codon at position + 1 was ligated into the pGL3 basic luciferase reporter vector [56]. A NRP2 promoter-luciferase deletion construct containing an internal deletion in the promoter was generated by PCR using the following forward primer: 5′-TCAAGTCAGAGATCTTCGGCCTGAAAAGGCAGTGG-3′ (622 bp fragment spanning −828 to −206). Site-specific mutagenesis was carried out with the Quick-Change XL-kit (Stratagen).
Luciferase reporter assay
HUVEC were transfected with 2 μg of the NRP2 promoter-luciferase constructs or pGL3 empty vector and 100 ng of a Renilla luciferase vector (used as a transfection efficiency control) (Promega Corp.). After 24 h, cells were transfected either with control or GATA2/Lmo2 siRNAs. After 48 h, cells were lysed and luciferase activities were measured with the dual-luciferase reporter Assay System (Promega Corp.), with the reporter activities normalized to Renilla luciferase activity. Each transfection was done in duplicate and repeated at least three times.
Chromatin immunoprecipitation (ChIP)
ChIP assays were carried out using the Chromatin Immunoprecipitation Assay Kit from Active Motif (Carlsbad). DNA from HUVEC was immunoprecipitated with the anti-GATA2 and anti-Lmo2 antibodies or normal IgG as a negative control, according to the manufacturer instructions. Immunoprecipitated DNA was amplified by PCR using specific primers for the GATA2 sequence present in the human NRP2 promoter: forward 5′-CGCCGCCTTAACTTCGATCC-3′; reverse 5′-CCTCGCTCGCGATCTCTGCC -3′ (247 bp PCR product).
RESULTS
GATA2 and Lmo2 are expressed by cultured primary EC and by tumor-associated blood vessels in vivo
GATA2 and Lmo2 are two transcription factors that were originally found to be expressed by HPC. However, these transcription factors were also expressed by cultured human primary EC, including HUVEC and HMVEC (Fig. 1A), consistent with previous reports [20,27,28]. Human chronic myeloid leukemia K562 cells, which are from hematopoietic origins, expressed levels of GATA2 and Lmo2 comparable to EC. In contrast, two tumor cell lines of non-hematopoietic origins, human U87MG glioblastoma and A375SM melanoma cells, expressed neither GATA2 nor Lmo2. Pancreatic microvascular EC (MS1) and mouse primary dermal EC (MDEC) expressed GATA2 and Lmo2 as well, whereas prostate cancer TRAMP C1 cells and B16F10 melanoma cells did not (Fig. 1B).
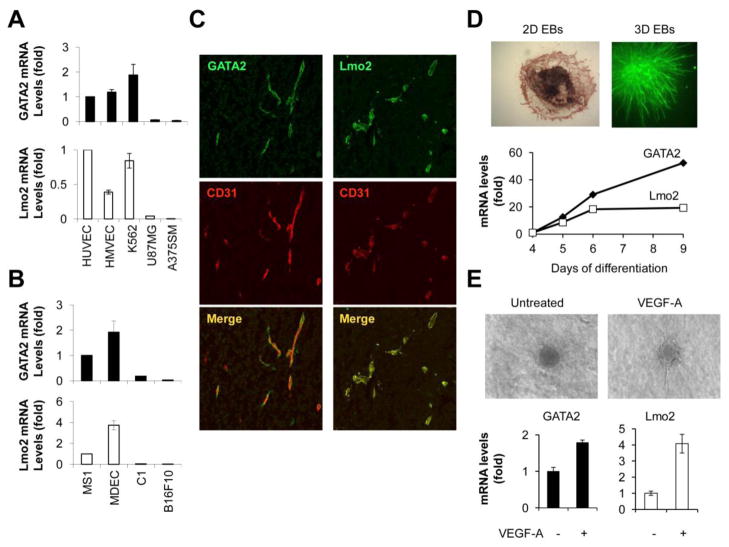
Figure 1. GATA2 and Lmo2 are expressed by primary EC and by tumor-associated blood vessels in vivo and their expression is upregulated during VEGF-A-induced sprouting.
(A) GATA2 (top) and Lmo2 (bottom) mRNA levels in human HUVEC, HMVEC, K562, U87MG and A375SM cells. (B) GATA2 (top) and Lmo2 (bottom) mRNA levels in mouse MS1, MDEC, C1 and B16F10 cells. (C) Confocal microscopy of immunostained sections from U87MG tumors stained with GATA2 or Lmo2 antibodies in addition to antibodies for the blood vessel marker CD31. (D) Top: Embryonic stem cells were induced to aggregate with the hanging drop method. At day 4, EBs were placed in a six-well plate (2D EBs) or in a collagen gel (3D EBs), and sprouting was induced by VEGF-A [59]. At day 9 (2D EBs) or day 12 (3D EBs) EBs were fixed and EC were visualized by staining for CD31. Bottom: 2D EBs were collected at different time points. mRNA was isolated and the expression levels of GATA2 and Lmo2 were analyzed by quantitative real-time PCR. GATA2 and Lmo2 expression was normalized to the levels at day 4. (E) Top: Spheroids from mouse brain EC either left untreated or treated with VEGF-A (25 ng/ml). Bottom: Spheroids were collected after 16 hours in collagen. mRNA was isolated and the expression levels of GATA2 and Lmo2 were analyzed by quantitative real-time PCR.
In addition to cultured primary EC, GATA2 and Lmo2 were expressed by tumor-associated blood vessels in mouse tumor models. U87MG cells were injected subcutaneously into nude mice and, after 4–6 weeks, tumor sections were analyzed for expression of GATA2 and Lmo2 (Fig. 1C). In order to identify the tumor vasculature, cryosections were immunostained with an antibody to the murine endothelial marker CD31. GATA2 (Fig. 1C, left) and Lmo2 (Fig. 1C, right) were detected in blood vessels present in U87MG tumors, as displayed in the merged image showing co-localization of GATA2 or Lmo2 immunostaining with CD31 expression. Blood vessels present in A375SM tumors expressed GATA2 and Lmo2 as well (data not shown). Collectively, these findings confirm that GATA2 and Lmo2 are two hematopoietic transcription factors expressed by cultured primary EC and by tumor-associated blood vessels in vivo.
GATA2 and Lmo2 expression is upregulated during VEGF-A-induced sprouting angiogenesis in differentiating embryonic stem cells and in primary EC
To delineate the roles of GATA2 and Lmo2 in vasculogenesis and angiogenesis, we analyzed GATA2 and Lmo2 expression in EBs, a model of VEGF-induced differentiating embryonic stem cells subjected to 2D or 3D cultures [58] (Fig. 1D, top). We have previously shown that VEGF-induced sprouting angiogenesis in EBs resulted in an upregulation of VEGF receptor expression, including VEGFR-2, NRP1 and NRP2 [59], consistent with the induction of EC differentiation and sprouting. GATA2 and Lmo2 expression in differentiating EBs was analyzed by quantitative real-time PCR. After 9 days of VEGF-induced differentiation, GATA2 (50-fold) and Lmo2 (20-fold) mRNA levels were significantly upregulated (Fig. 1D, bottom). The mRNA levels are relative to day 4, when EBs were plated and VEGF-A was added.
Since the increase in mRNA expression of GATA2 and Lmo2 in EBs could result from either differentiation, sprouting angiogenesis or both, we analyzed GATA2 and Lmo2 mRNA levels in primary EC in a 3D in vitro angiogenesis spheroid assay (Fig. 1E). Early passage mouse brain EC were suspended and then aggregated overnight in hanging drops to form cellular spheroids (500 cells/spheroid). Spheroids were then embedded in collagen gels and either left untreated or treated with VEGF-A (25 ng/ml) (Fig. 1E, top). After 16 hours of VEGF-induced sprouting, GATA2 (2-fold) and Lmo2 (4-fold) mRNA levels were significantly upregulated (Fig. 1E, bottom).
Together, these results show that VEGF-induced sprouting angiogenesis in differentiating EBs and in primary EC increases levels of GATA2 and Lmo2, suggesting that these two transcription factors may play a key role in EC function.
GATA2 and Lmo2 are necessary for VEGF-A-induced EC function
To elucidate the role of GATA2 and Lmo2 in EC function, we analyzed whether downregulation of GATA2 and Lmo2 expression would affect VEGF-induced EC migration and sprouting in vitro, two key steps of angiogenesis in vivo. GATA2, Lmo2 or both GATA2 and Lmo2 (GATA2/Lmo2) expression was knocked down in HUVEC using siRNAs. GATA2 and Lmo2 expression in HUVEC was downregulated efficiently 72 hours after siRNA transfection (Fig. 2A). Proliferation assays confirmed that siRNA treatment did not have any effect on cell survival (Supplementary Fig. S1), indicating that GATA2 and Lmo2 are not essential for EC survival and growth in vitro. Silencing of GATA2 or Lmo2 expression individually inhibited VEGF-induced EC migration by 70% and 60%, respectively (Fig. 2B). A complete inhibition of EC migration was observed when silencing expression of both GATA2/Lmo2. A complete inhibition of VEGF-induced EC migration was also observed when silencing expression of both GATA2/Lmo2 in HMVEC (Supplementary Fig. S2).
Figure 2. GATA2 and Lmo2 are necessary for VEGF-A-induced EC migration and sprouting in vitro.
(A) GATA2 (top) and Lmo2 (bottom) mRNA levels in HUVEC transfected with either control, GATA2, Lmo2 or GATA2/Lmo2 siRNAs. (B) Transwell cell migration of HUVEC transfected with either control, GATA2, Lmo2 or GATA2/Lmo2 siRNAs. Media containing VEGF-A and heparin were added to the lower wells. (C) Representative images (left) and statistical summary (right) of 3D spheroid-based in vitro angiogenesis assays, with spheroids generated from control (a, b), GATA2 (c, d), Lmo2 (e, f) or GATA2/Lmo2 (g, h) siRNAs-treated HUVEC, either left untreated or treated with VEGF-A (25 ng/ml).
Sprouting of EC is another important property of angiogenesis in vivo. Silencing of GATA2, Lmo2 or both GATA2/Lmo2 was tested for effects on EC sprouting in a 3D in vitro angiogenesis spheroid assay. HUVEC spheroids, with defined size and cell number, were embedded in collagen gels and then treated with VEGF-A (25 ng/ml). Outgrowth of capillary-like structures was assessed. Control siRNA-transfected HUVEC spheroids generated numerous sprouts after VEGF-A treatment (Fig. 2C a vs. b). In contrast, silencing of GATA2 (Fig. 2C b vs. d) or Lmo2 (Fig. 2C b vs. f) strongly inhibited VEGF-induced sprouting (75% and 50%, respectively). A complete inhibition of VEGF-induced EC sprouting was observed when silencing expression of both GATA2/Lmo2 in HUVEC (Fig. 2C b vs. h). Together, siRNA-mediated downregulation of GATA2/Lmo2 in cultured primary human EC demonstrated that VEGF-induced angiogenic activity, including EC migration and sprouting in vitro, is GATA2/Lmo2-dependent.
GATA2 and Lmo2 are necessary for VEGF-A/C-induced LEC function
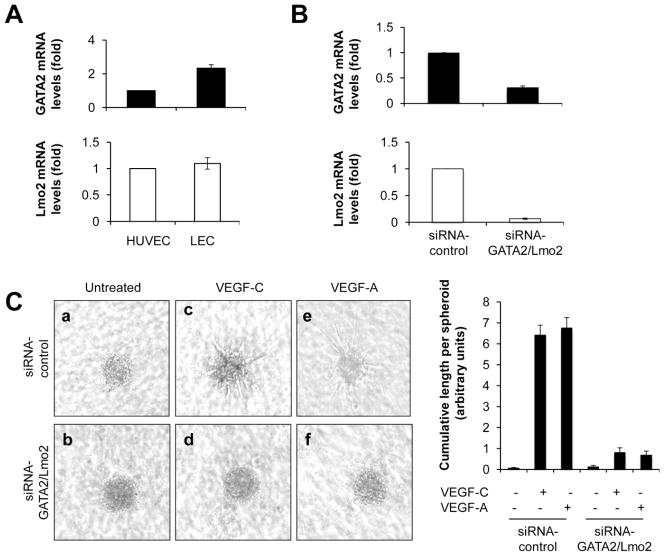
We found that dermal-derived human primary LEC in culture expressed GATA2 and Lmo2 at levels similar to those of EC (Fig. 3A). LEC differ from EC in that their cellular responses to VEGF are mainly regulated by VEGF-C instead of VEGF-A. Therefore, we investigated whether downregulation of GATA2 and Lmo2 would affect VEGF-C-induced LEC sprouting in vitro, a key step of lymphangiogenesis in vivo. GATA2 and Lmo2 expression was knocked down in LEC using siRNAs. GATA2 and Lmo2 mRNA levels in LEC were downregulated efficiently 72 hours after transfection (Fig. 3B). Proliferation assays confirmed that siRNA treatment did not have any effect on cell survival (Supplementary Fig. S3). Control siRNA-treated LEC spheroids generated numerous sprouts after VEGF-C treatment (Fig. 3C a vs. c). In contrast, silencing of both GATA2/Lmo2 in LEC spheroids almost completely inhibited the formation of lymphatic endothelial sprouts in vitro (Fig. 3C c vs. d). Silencing of GATA2 or Lmo2 expression individually inhibited VEGF-C-induced LEC migration by 75% and 50%, respectively (data not shown). VEGF-A also induced LEC sprouting in control siRNA-treated LEC spheroids (Fig. 3C a vs. e), and silencing of both GATA2/Lmo2 inhibited VEGF-A-induced LEC sprouting (Fig. 3C e vs. f).
Figure 3. GATA2 and Lmo2 are necessary for VEGF-A/C-induced LEC sprouting in vitro.
(A) GATA2 (top) and Lmo2 (bottom) mRNA levels in HUVEC and LEC. (B) GATA2 (top) and Lmo2 (bottom) mRNA levels in LEC transfected with either control or GATA2/Lmo2 siRNAs. (C) Representative images (left) and statistical summary (right) of 3D spheroid-based in vitro lymphangiogenesis assays, with spheroids generated from control (a, c, e) or GATA2/Lmo2 (b, d, f) siRNA-treated LEC, either left untreated or treated with VEGF-C (250 ng/ml) or VEGF-A (25 ng/ml).
Taken together, these results demonstrate that GATA2 and Lmo2 are expressed by cultured primary human LEC and that they are required for VEGF-C/A-induced lymphangiogenic activity, including LEC sprouting in vitro.
GATA2/Lmo2 knockdown downregulates the expression of the main VEGF receptors
To identify molecular mechanisms underlying the GATA2/Lmo2-dependent regulation of VEGF-induced angiogenesis and lymphangiogenesis, we analyzed whether GATA2 and Lmo2 regulated gene expression of the main VEGF receptors, including VEGFR-1-3 and NRP1, 2.
siRNA-mediated downregulation of both GATA2/Lmo2 in EC downregulated the expression of VEGFR-2, the major VEGFR in EC, as well as the expression of VEGFR-1, VEGFR-3, NRP1 and NRP2 (Fig. 4A, B, top). Silencing of GATA2/Lmo2 in LEC downregulated the expression of VEGFR-3, the major VEGFR in LEC, as well as the expression of VEGFR-1, VEGFR-2, NRP1 and NRP2 (Fig. 4A, B, bottom). These results suggest that GATA2 and Lmo2 act upstream of VEGFR1-3, NRP1 and NRP2 and that EC and LEC lose certain features of their identity with the reduction of GATA2 and Lmo2.
Figure 4. GATA2/Lmo2 knockdown downregulates the expression of the main VEGF receptors.
VEGFR-1, VEGFR-2 and VEGFR-3 mRNA levels (A) and NRP1 and NRP2 mRNA levels (B) in HUVEC (top) and LEC (bottom) transfected with either control or GATA2/Lmo2 siRNAs.
NRP2 knockdown causes the same defects of in vitro EC and LEC sprouting as GATA2/Lmo2 knockdown
It has been previously reported that GATA2 regulates VEGFR-2 expression during VEGF-induced angiogenesis [20]. However, NRP1 and NRP2, two key co-receptors of VEGFR for VEGF, were not analyzed in this study. Interestingly, we found that GATA2/Lmo2 knockdown downregulated NRP1 and NRP2 expression in both EC and LEC. However, only NRP2 was further investigated in this study since, in addition to being a key co-receptor of VEGFR-2 in vascular EC, NRP2 is the main co-receptor of VEGFR-3 in LEC [51,52,60]. Thus, in contrast to NRP1, which is mainly expressed in vascular EC, NRP2 has an important role in both vascular EC and LEC, all together suggesting that NRP2 might be a physiologically relevant GATA2/Lmo2 downstream target gene in vitro for both VEGF-induced angiogenesis and lymphangiogenesis.
To confirm this hypothesis, first we analyzed whether silencing of NRP2 expression in EC and LEC would inhibit EC and LEC function as GATA2/Lmo2 knockdown. Silencing of GATA2/Lmo2 expression in EC and LEC expression downregulated NRP2 expression at the protein level (Fig. 5A, top). Similarly, NRP2 expression in EC and LEC was downregulated efficiently 72 hours after siRNA transfection (Fig. 5A, bottom). Our above-described results demonstrated that GATA2/Lmo2 knockdown in EC and LEC spheroids almost completely inhibited the formation of endothelial sprouts in vitro. A decrease in NRP2 expression mediated by NRP2 siRNA inhibited VEGF-A-induced EC sprouting and VEGF-A/C-induced LEC sprouting similar to that of GATA2/Lmo2 knockdown (Fig. 5B), suggesting that GATA2 and Lmo2 mediate VEGF-induced angiogenesis and lymphangiogenesis by modulating the expression of NRP2.
Figure 5. Silencing of NRP2 inhibits VEGF-induced EC and LEC sprouting similar to that of GATA2/Lmo2 knockdown.

(A) Top: NRP2, GATA2, Lmo2 and β-actin protein levels in HUVEC (left) and LEC (right) transfected with either control or GATA2/Lmo2 siRNAs. Bottom: NRP2 and β-actin protein levels in HUVEC (left) and LEC (right) transfected with either control or NRP2 siRNAs. (B) Representative images (left) and statistical summary (right) of 3D spheroid-based in vitro angiogenesis and lymphangiogenesis assays, with spheroids generated from control or NRP2 siRNA-treated HUVEC or LEC, either left untreated or treated with VEGF-A (25 ng/ml) or VEGF-C (250 ng/ml).
NRP2 overexpression rescues the impaired sprouting in GATA2/Lmo2 knockdown EC and LEC
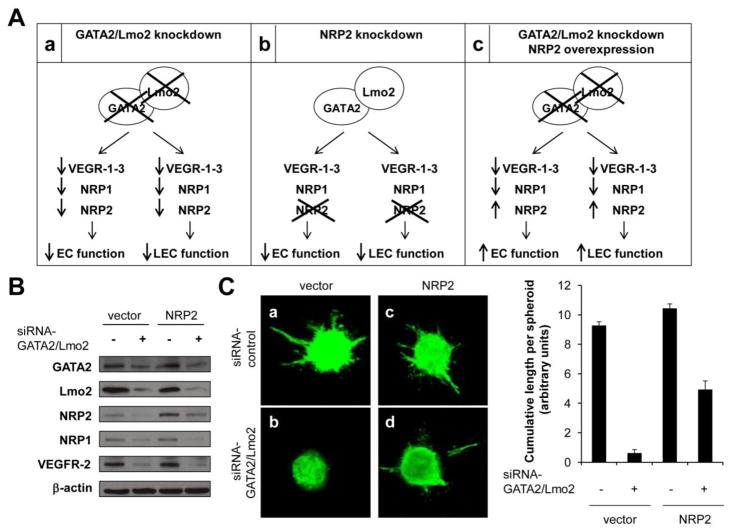
Our above-described results demonstrated that GATA2/Lmo2 knockdown (Fig. 6Aa) as well as NRP2 knockdown (Fig. 6Ab) inhibited EC and LEC function, measured as EC and LEC migration and sprouting in vitro. If GATA2 and Lmo2 mediate VEGF-induced angiogenesis and lymphangiogenesis by regulating NRP2 gene expression, our prediction was that ectopic expression of NRP2 in GATA2/Lmo2 knockdown EC and LEC would rescue the sprouting defects in GATA2/Lmo2 knockdown EC and LEC (Fig. 6Ac). To test this hypothesis, HUVEC were transfected with control or NRP2 constructs, and cells were then transfected with control or GATA2/Lmo2 siRNAs (Fig. 6A, B). As shown above, silencing of GATA2/Lmo2 gene expression in HUVEC cells downregulated NRP2 expression as well as NRP1 and VEGFR-2 expression (Fig. 6B, lane 2 vs. 3). However, when GATA2/Lmo2 knockdown HUVEC were transfected with a NRP2 construct, NRP2 levels were increased, whereas NRP1 and VEGFR-2 levels remained downregulated (Fig. 6B, lane 4 vs. 2). Proliferation assays confirmed that NRP2 overexpression did not have any effect on cell survival (Supplementary Fig. S4). Silencing of GATA2/Lmo2 expression in EC decreased the angiogenic responses of EC by 90% (Fig. 6C, a vs. b). In contrast, overexpression of NRP2 in GATA2/Lmo2 knockdown EC partially rescued VEGF-A-induced angiogenic activity, from a 90% inhibition to a 50% inhibition (Fig. 6C, c vs. d). Similar results were obtained when using LEC (Supplementary Fig. S5), all together suggesting that GATA2 and Lmo2 mediate angiogenesis and lymphangiogenesis, at least in part, by modulating the expression of NRP2.
Figure 6. NRP2 overexpression partially rescues the impaired sprouting in GATA2/Lmo2 knockdown EC.
(A) Schematic of regulation of EC and LEC function by GATA2 and Lmo2 via NRP2. a) GATA2/Lmo2 knockdown downregulates the expression of the main VEGF receptors and inhibits EC and LEC function, including VEGF-induced migration and sprouting. b) NRP2 knockdown inhibits EC and LEC function. c) Ectopic expression of NRP2 in GATA2/Lmo2 knockdown EC and LEC rescues the sprouting defects in GATA2/Lmo2 knockdown cells. (B) NRP2, NRP1, VEGFR-2, GATA2, Lmo2 and -actin protein levels in HUVEC transfected with a control construct and either with control or GATA2/Lmo2 siRNA, and transfected with a NRP2 construct and either with control or GATA2/Lmo2 siRNAs. (C) Representative images (left) and statistical summary (right) of 3D spheroid-based in vitro angiogenesis assays, with spheroids generated from HUVEC transfected with control or NRP2 constructs and either with control or GATA2/Lmo2 siRNAs and treated with VEGF-A (25 ng/ml).
Direct regulation of NRP2 transcription by GATA2 and Lmo2
To determine whether NRP2 is a direct downstream target of GATA2 and Lmo2, we isolated a fragment extending 1831 bp in the 5′-flanking region of the human NRP2 gene from a human genomic PAC library [56]. The NRP2 promoter contained a GATA binding site at position −863 upstream from the translation start site (Fig. 7A). Chromatin immunoprecipitation (ChIP) assays showed that NRP2 promoter immunoprecipitated with GATA2 or Lmo2 antibodies (Fig. 7B). The constructs containing a point mutation or a deletion in the GATA binding site displayed 90% and 60% reduction of its activity, respectively (Fig. 7C), confirming that this GATA binding site is necessary for NRP2 promoter activity. Furthermore, NRP2 promoter activity was decreased in HUVEC transfected with GATA2/Lmo2 siRNAs (Fig. 7D). Collectively, these results confirm that GATA2 and Lmo2 act as positive regulators to enhance NRP2 expression.
Figure 7. NRP2 is a direct target of GATA2 and Lmo2.
(A) Genomic organization of the NRP2 gene. The three luciferase constructs (full-length wild-type NRP2 promoter, NRP2 promoter containing a point mutation in the GATA binding site and NRP2 promoter lacking the GATA binding site) are also shown. The putative GATA binding site is indicated by a grey box. The GATA binding site with a point mutation is depicted as a grey box with a large X. (B) ChIP assays showing that NRP2 promoter immunoprecipitated with GATA2 and Lmo2 antibodies. (C) HUVEC were transfected with a luciferase construct (full-length wild-type NRP2 promoter, NRP2 promoter containing a point mutation for the GATA binding site or NRP2 promoter lacking the GATA binding site). (D) The full-length wild-type NRP2 luciferase promoter vector was co-transfected with either control or GATA2/Lmo2 siRNAs.
DISCUSSION
A common precursor for HPC and EC, the hemangioblast, has been postulated, given that many genes involved in hematopoiesis also participate in vascular development [1–6]. Examples are GATA2 and Lmo2, two transcription factors that form common transcription complexes during HPC differentiation. In this study, we have demonstrated that GATA2 and Lmo2 cooperatively regulate VEGF-induced angiogenesis and lymphangiogenesis. Thus, we reveal a crucial role for GATA2 and Lmo2 in EC and LEC function, suggesting molecular commonalities in the function of the vascular and hematopoietic systems.
To demonstrate a direct role for GATA2 and Lmo2 in VEGF-induced angiogenesis, we performed experiments on cultured primary EC. We found that GATA2 and Lmo2 are necessary for VEGF-induced EC migration and sprouting in vitro, two properties that contribute to angiogenesis in vivo. In addition, VEGF-induced sprouting angiogenesis in both differentiating EBs and in primary EC was accompanied by increased levels of GATA2 and Lmo2, supporting a role for GATA2 and Lmo2 in EC function. Whether GATA2 and Lmo2 regulate angiogenesis induced by other angiogenic factors was not analyzed.
There are several reports demonstrating that GATA2 and Lmo2 are key regulators of angiogenesis. For example, it has been shown that GATA2, in addition to mediating mechanosignaling-dependent angiogenesis, governs vascular integrity [20, 61]. On the other hand, it is known that Lmo2 regulates angiogenesis in mice [31,32]. We found that silencing of GATA2 inhibited VEGF-induced EC migration and sprouting by 70% and 75% respectively, whereas silencing of Lmo2 inhibited VEGF-induced EC migration and sprouting by 60% and 50%, respectively. However, a novel finding was that a complete inhibition of VEGF-induced EC migration and sprouting was observed when silencing both transcription factors in EC, indicating that GATA2 and Lmo2 cooperatively regulate EC function. These results are in good agreement with previous studies showing that GATA2 and Lmo2 form common transcription complexes in both HPC and EC [25–27]. We suggest that GATA2 and Lmo2 are novel contributors in the regulation of physiological angiogenesis. GATA2 and Lmo2 were also expressed by tumor-associated blood vessels in U87MG glioblastoma and A375SM melanoma mouse tumor models. Thus, these transcription factors may regulate tumor angiogenesis as well. It would be of interest to analyze GATA2 and Lmo2 expression in angiogenic human tumors as well as other angiogenic pathologies, such as vascular retinopathy.
GATA2 is also crucial for lymphatic valve development and also for proper lymphatic vascular development [37,38]. Recently, several groups reported that human patients with GATA2 mutations presented symptoms of primary lymphedema [37,39,40], underscoring the vital role of transcription factor GATA2 in lymphatic development. Here we demonstrate that, in addition to being expressed in HPC and EC, GATA2 and Lmo2 are also expressed by cultured primary human LEC. Furthermore, we find that GATA2 and Lmo2 are necessary for VEGF-induced LEC sprouting in vitro, a key step of lymphangiogenesis in vivo. Thus, we provide a crucial role for GATA2 and Lmo2 in LEC function, particularly in controlling LEC sprouting.
A novel finding is that GATA2 and Lmo2 are master regulators of the VEGF/NRP/VEGFR signaling axis. We found that siRNA-mediated downregulation of GATA2/Lmo2 in EC and LEC inhibited the expression of the main VEGR receptors, including VEGFR1-3 and NRP1, 2. Thus, we provide evidence that GATA2 and Lmo2 act upstream of the main VEGF receptors and that EC and LEC lose certain features of their identity with the reduction of GATA2 and Lmo2.
Even though both NRP receptors were downregulated in GATA2/Lmo2 knockdown EC and LEC, we focused our attention on NRP2 since, in addition to being a key co-receptor of VEGFR-2 in vascular EC, NRP2 is the main co-receptor of VEGFR-3 in LEC [51,52,60]. Thus, in contrast to NRP1, which is mainly expressed in vascular EC, NRP2 has an important role in both vascular EC and LEC. Indeed, silencing of NRP2 in EC and LEC almost completely inhibited VEGF-induced EC and LEC sprouting in vitro, similar to the complete inhibition of VEGF-induced EC and LEC sprouting observed when silencing GATA2/Lmo2 in EC and LEC. Here we demonstrate that GATA2 and Lmo2 mediate VEGF-induced angiogenesis and lymphangiogenesis via NRP2. As evidence, siRNA-mediated downregulation of both GATA2/Lmo2 expression inhibited NRP2 expression at both the mRNA and the protein levels, and NRP2 overexpression in GATA2/Lmo2 knockdown cells partially rescued the impaired sprouting observed in GATA2/Lmo2 knockdown cells. Partial rescue of the GATA2/Lmo2 knockdown phenotype by NRP2 expression indicates that the other VEGF receptors may also be involved, consistent with previous results showing that silencing of GATA2 expression in EC inhibited VEGF-induced angiogenesis via VEGFR-2 [20].
It has been reported that GATA2 and Lmo2 interact with other transcription factors to form multimeric transcription complexes. Among these is the basic helix-loop-helix transcription factor Tal1/Scl. Similar to GATA2 and Lmo2 knockdown in EC and LEC, silencing of Tal1/Scl in HUVEC inhibited in vitro tube formation [27]. Even though Tal1/Scl forms common transcriptional complexes with GATA2 and Lmo2 to regulate the expression of VE-cadherin and Angiopoietin-2 in HUVEC [27,28], whether knockdown of both GATA2 and Lmo2 inhibited in vitro tube formation was not analyzed in these studies. Thus, novel findings are that: i) GATA2 and Lmo2 cooperatively regulate VEGF-induced angiogenesis and lymphangiogenesis; and ii) GATA2 and Lmo2 mediate EC and LEC function, at least in part, by directly regulating NRP2 gene expression.
We have demonstrated that NRP2 is a direct target gene of the GATA2/Lmo2 transcription complex. Silencing of GATA2/Lmo2 in EC inhibited NRP2 promoter activity and expression and, as a functional consequence, inhibited VEGF-induced angiogenesis and lymphangiogenesis. Thus, these data demonstrate a previously unknown mechanism that regulates NRP2 gene expression in EC and LEC. However, GATA2 and Lmo2 are not the only transcriptional regulators of NRP2 expression. The homeobox-containing transcription factor Prox1, the mammalian homologue of the Drosophila gene prospero, is a marker for LEC. It has been shown previously that Prox1 downregulated NRP1 expression in EC [62], but whether Prox1 also regulates NRP2 expression remains unknown. COUPTFII, an orphan member of the nuclear receptor superfamily, is another marker for LEC. Indeed, knockdown of COUPTFII inhibited NRP2 expression in LEC [63] and increased NRP1 in vein vasculature [64]. We suggest that GATA2 and Lmo2 act jointly with COUPTFII and Prox1 in the pathway of LEC specification and function, since downregulation of either factor is able to suppress NRP2 expression and inhibit lymphangiogenesis.
In summary, we have identified GATA2 and Lmo2, shown previously to regulate hematopoietic differentiation, as key players in the blood vascular and lymphatic systems. GATA2 and Lmo2 control VEGF-induced angiogenic and lymphangiogenic activities via regulation of NRP2, making these two proteins potentially promising targets for various angiogenesis/lymphangiogenesis-dependent diseases, including proliferative retinopathy, arthritis and cancer, in the future.
Supplementary Material
Acknowledgments
We thank Dr. Lena Claesson-Welsh (Department of Immunology, Genetics and Pathology, Uppsala University) for critical reading of the manuscript, and Melissa Anderson and Kristin Johnson for preparation of the manuscript and for artwork. This study was supported by grants from the National Institutes of Health (NIH) - CA37392 and CA45548.
Footnotes
AUTHORSHIP CONTRIBUTION
S.C. designed and performed the experiments, interpreted the data and wrote the manuscript; M.AR. performed immunostaining; A.M. provided reagents and assisted with experimental design; T. A. performed spheroid assays with mouse brain EC; L.A.vM. performed embryoid body assays; and M.K. supervised all experiments and edited the manuscript.
DISCLOSURE OF CONFLICT OF INTEREST
The authors have no conflict of interest to disclose.
References
- 1.Lugus JJ, Park C, Choi K. Developmental relationship between hematopoietic and endothelial cells. Immunol Res. 2005;32 (1–3):57–74. doi: 10.1385/IR:32:1-3:057. IR:32:1-3:057 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Xiong JW. Molecular and developmental biology of the hemangioblast. Dev Dyn. 2008;237 (5):1218–1231. doi: 10.1002/dvdy.21542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125 (4):725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 4.Nishikawa SI. A complex linkage in the developmental pathway of endothelial and hematopoietic cells. Curr Opin Cell Biol. 2001;13(6):673–678. doi: 10.1016/s0955-0674(00)00270-2. S0955-0674(00)00270-2 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Kubo H, Alitalo K. The bloody fate of endothelial stem cells. Genes Dev. 2003;17 (3):322–329. doi: 10.1101/gad.1071203. [DOI] [PubMed] [Google Scholar]
- 6.Lugus JJ, Chung YS, Mills JC, Kim SI, Grass J, Kyba M, Doherty JM, Bresnick EH, Choi K. GATA2 functions at multiple steps in hemangioblast development and differentiation. Development. 2007;134 (2):393–405. doi: 10.1242/dev.02731. dev.02731 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Orkin SH. GATA-binding transcription factors in hematopoietic cells. Blood. 1992;80 (3):575–581. [PubMed] [Google Scholar]
- 8.Pandolfi PP, Roth ME, Karis A, Leonard MW, Dzierzak E, Grosveld FG, Engel JD, Lindenbaum MH. Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nat Genet. 1995;11 (1):40–44. doi: 10.1038/ng0995-40. [DOI] [PubMed] [Google Scholar]
- 9.Pevny L, Lin CS, D’Agati V, Simon MC, Orkin SH, Costantini F. Development of hematopoietic cells lacking transcription factor GATA-1. Development. 1995;121 (1):163–172. doi: 10.1242/dev.121.1.163. [DOI] [PubMed] [Google Scholar]
- 10.Tsai FY, Keller G, Kuo FC, Weiss M, Chen J, Rosenblatt M, Alt FW, Orkin SH. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371 (6494):221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- 11.Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11 (8):1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- 12.Molkentin JD, Tymitz KM, Richardson JA, Olson EN. Abnormalities of the genitourinary tract in female mice lacking GATA5. Mol Cell Biol. 2000;20 (14):5256–5260. doi: 10.1128/mcb.20.14.5256-5260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrisey EE, Tang Z, Sigrist K, Lu MM, Jiang F, Ip HS, Parmacek MS. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 1998;12 (22):3579–3590. doi: 10.1101/gad.12.22.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briegel K, Lim KC, Plank C, Beug H, Engel JD, Zenke M. Ectopic expression of a conditional GATA-2/estrogen receptor chimera arrests erythroid differentiation in a hormone-dependent manner. Genes Dev. 1993;7 (6):1097–1109. doi: 10.1101/gad.7.6.1097. [DOI] [PubMed] [Google Scholar]
- 15.Kitajima K, Masuhara M, Era T, Enver T, Nakano T. GATA-2 and GATA-2/ER display opposing activities in the development and differentiation of blood progenitors. EMBO J. 2002;21 (12):3060–3069. doi: 10.1093/emboj/cdf301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai FY, Orkin SH. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood. 1997;89 (10):3636–3643. [PubMed] [Google Scholar]
- 17.Gumina RJ, Kirschbaum NE, Piotrowski K, Newman PJ. Characterization of the human platelet/endothelial cell adhesion molecule-1 promoter: identification of a GATA-2 binding element required for optimal transcriptional activity. Blood. 1997;89 (4):1260–1269. [PubMed] [Google Scholar]
- 18.Kawana M, Lee ME, Quertermous EE, Quertermous T. Cooperative interaction of GATA-2 and AP1 regulates transcription of the endothelin-1 gene. Mol Cell Biol. 1995;15 (8):4225–4231. doi: 10.1128/mcb.15.8.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee ME, Temizer DH, Clifford JA, Quertermous T. Cloning of the GATA-binding protein that regulates endothelin-1 gene expression in endothelial cells. J Biol Chem. 1991;266 (24):16188–16192. [PubMed] [Google Scholar]
- 20.Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, Aderman CM, Mostoslavsky G, Smith LE, Ingber DE. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457 (7233):1103–1108. doi: 10.1038/nature07765. nature07765 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kappel A, Schlaeger TM, Flamme I, Orkin SH, Risau W, Breier G. Role of SCL/Tal-1, GATA, and ets transcription factor binding sites for the regulation of flk-1 expression during murine vascular development. Blood. 2000;96 (9):3078–3085. [PubMed] [Google Scholar]
- 22.Valge-Archer VE, Osada H, Warren AJ, Forster A, Li J, Baer R, Rabbitts TH. The LIM protein RBTN2 and the basic helix-loop-helix protein TAL1 are present in a complex in erythroid cells. Proc Natl Acad Sci U S A. 1994;91 (18):8617–8621. doi: 10.1073/pnas.91.18.8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wadman I, Li J, Bash RO, Forster A, Osada H, Rabbitts TH, Baer R. Specific in vivo association between the bHLH and LIM proteins implicated in human T cell leukemia. EMBO J. 1994;13 (20):4831–4839. doi: 10.1002/j.1460-2075.1994.tb06809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visvader JE, Mao X, Fujiwara Y, Hahm K, Orkin SH. The LIM-domain binding protein Ldb1 and its partner LMO2 act as negative regulators of erythroid differentiation. Proc Natl Acad Sci U S A. 1997;94 (25):13707–13712. doi: 10.1073/pnas.94.25.13707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lecuyer E, Herblot S, Saint-Denis M, Martin R, Begley CG, Porcher C, Orkin SH, Hoang T. The SCL complex regulates c-kit expression in hematopoietic cells through functional interaction with Sp1. Blood. 2002;100 (7):2430–2440. doi: 10.1182/blood-2002-02-0568. [DOI] [PubMed] [Google Scholar]
- 26.Wadman IA, Osada H, Grutz GG, Agulnick AD, Westphal H, Forster A, Rabbitts TH. The LIM-only protein Lmo2 is a bridging molecule assembling an erythroid, DNA-binding complex which includes the TAL1, E47, GATA-1 and Ldb1/NLI proteins. EMBO J. 1997;16 (11):3145–3157. doi: 10.1093/emboj/16.11.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deleuze V, Chalhoub E, El-Hajj R, Dohet C, Le Clech M, Couraud PO, Huber P, Mathieu D. TAL-1/SCL and its partners E47 and LMO2 up-regulate VE-cadherin expression in endothelial cells. Mol Cell Biol. 2007;27 (7):2687–2697. doi: 10.1128/MCB.00493-06. MCB.00493-06 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deleuze V, El-Hajj R, Chalhoub E, Dohet C, Pinet V, Couttet P, Mathieu D. Angiopoietin-2 is a direct transcriptional target of TAL1, LYL1 and LMO2 in endothelial cells. PLoS One. 2012;7(7):e40484. doi: 10.1371/journal.pone.0040484. PONE-D-12-07293 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warren AJ, Colledge WH, Carlton MB, Evans MJ, Smith AJ, Rabbitts TH. The oncogenic cysteine-rich LIM domain protein rbtn2 is essential for erythroid development. Cell. 1994;78(1):45–57. doi: 10.1016/0092-8674(94)90571-1. 0092-8674(94)90571-1 [pii] [DOI] [PubMed] [Google Scholar]
- 30.Yamada Y, Warren AJ, Dobson C, Forster A, Pannell R, Rabbitts TH. The T cell leukemia LIM protein Lmo2 is necessary for adult mouse hematopoiesis. Proc Natl Acad Sci U S A. 1998;95 (7):3890–3895. doi: 10.1073/pnas.95.7.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamada Y, Pannell R, Forster A, Rabbitts TH. The oncogenic LIM-only transcription factor Lmo2 regulates angiogenesis but not vasculogenesis in mice. Proc Natl Acad Sci U S A. 2000;97 (1):320–324. doi: 10.1073/pnas.97.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada Y, Pannell R, Forster A, Rabbitts TH. The LIM-domain protein Lmo2 is a key regulator of tumour angiogenesis: a new anti-angiogenesis drug target. Oncogene. 2002;21 (9):1309–1315. doi: 10.1038/sj.onc.1205285. [DOI] [PubMed] [Google Scholar]
- 33.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. nm0603-669 [pii] [DOI] [PubMed] [Google Scholar]
- 34.Ferrara N, Mass RD, Campa C, Kim R. Targeting VEGF-A to treat cancer and age-related macular degeneration. Annu Rev Med. 2007;58:491–504. doi: 10.1146/annurev.med.58.061705.145635. [DOI] [PubMed] [Google Scholar]
- 35.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140 (4):460–476. doi: 10.1016/j.cell.2010.01.045. S0092-8674(10)00115-7 [pii] [DOI] [PubMed] [Google Scholar]
- 36.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5(1):74–80. doi: 10.1038/ni1013. ni1013 [pii] [DOI] [PubMed] [Google Scholar]
- 37.Kazenwadel J, Secker GA, Liu YJ, Rosenfeld JA, Wildin RS, Cuellar-Rodriguez J, Hsu AP, Dyack S, Fernandez CV, Chong CE, Babic M, Bardy PG, Shimamura A, Zhang MY, Walsh T, Holland SM, Hickstein DD, Horwitz MS, Hahn CN, Scott HS, Harvey NL. Loss-of-function germline GATA2 mutations in patients with MDS/AML or MonoMAC syndrome and primary lymphedema reveal a key role for GATA2 in the lymphatic vasculature. Blood. 2011;119 (5):1283–1291. doi: 10.1182/blood-2011-08-374363. blood-2011-08-374363 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim KC, Hosoya T, Brandt W, Ku CJ, Hosoya-Ohmura S, Camper SA, Yamamoto M, Engel JD. Conditional Gata2 inactivation results in HSC loss and lymphatic mispatterning. J Clin Invest. 2012;122 (10):3705–3717. doi: 10.1172/JCI61619. 61619 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ostergaard P, Simpson MA, Connell FC, Steward CG, Brice G, Woollard WJ, Dafou D, Kilo T, Smithson S, Lunt P, Murday VA, Hodgson S, Keenan R, Pilz DT, Martinez-Corral I, Makinen T, Mortimer PS, Jeffery S, Trembath RC, Mansour S. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome) Nat Genet. 2011;43 (10):929–931. doi: 10.1038/ng.923. [pii] [DOI] [PubMed] [Google Scholar]
- 40.Ishida H, Imai K, Honma K, Tamura S, Imamura T, Ito M, Nonoyama S. GATA-2 anomaly and clinical phenotype of a sporadic case of lymphedema, dendritic cell, monocyte, B- and NK-cell (DCML) deficiency, and myelodysplasia. Eur J Pediatr. 2012;171 (8):1273–1276. doi: 10.1007/s00431-012-1715-7. [DOI] [PubMed] [Google Scholar]
- 41.Klagsbrun M, Eichmann A. A role for axon guidance receptors and ligands in blood vessel development and tumor angiogenesis. Cytokine Growth Factor Rev. 2005;16 (4–5):535–548. doi: 10.1016/j.cytogfr.2005.05.002. S1359-6101(05)00058-4 [pii] [DOI] [PubMed] [Google Scholar]
- 42.Ellis LM. The role of neuropilins in cancer. Mol Cancer Ther. 2006;5 (5):1099–1107. doi: 10.1158/1535-7163.MCT-05-0538. 5/5/1099 [pii] [DOI] [PubMed] [Google Scholar]
- 43.Guttmann-Raviv N, Kessler O, Shraga-Heled N, Lange T, Herzog Y, Neufeld G. The neuropilins and their role in tumorigenesis and tumor progression. Cancer Lett. 2006;231 (1):1–11. doi: 10.1016/j.canlet.2004.12.047. S0304-3835(05)00005-4 [pii] [DOI] [PubMed] [Google Scholar]
- 44.Gaur P, Bielenberg DR, Samuel S, Bose D, Zhou Y, Gray MJ, Dallas NA, Fan F, Xia L, Lu J, Ellis LM. Role of class 3 semaphorins and their receptors in tumor growth and angiogenesis. Clin Cancer Res. 2009;15 (22):6763–6770. doi: 10.1158/1078-0432.CCR-09-1810. 1078-0432.CCR-09-1810 [pii] [DOI] [PubMed] [Google Scholar]
- 45.Yuan L, Moyon D, Pardanaud L, Breant C, Karkkainen MJ, Alitalo K, Eichmann A. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129 (20):4797–4806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]
- 46.Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126 (21):4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- 47.Takashima S, Kitakaze M, Asakura M, Asanuma H, Sanada S, Tashiro F, Niwa H, Miyazaki Ji J, Hirota S, Kitamura Y, Kitsukawa T, Fujisawa H, Klagsbrun M, Hori M. Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci U S A. 2002;99(6):3657–3662. doi: 10.1073/pnas.022017899. 022017899 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92(6):735–745. doi: 10.1016/s0092-8674(00)81402-6. S0092-8674(00)81402-6 [pii] [DOI] [PubMed] [Google Scholar]
- 49.Geretti E, Shimizu A, Klagsbrun M. Neuropilin structure governs VEGF and semaphorin binding and regulates angiogenesis. Angiogenesis. 2008;11 (1):31–39. doi: 10.1007/s10456-008-9097-1. [DOI] [PubMed] [Google Scholar]
- 50.Kawamura H, Li X, Goishi K, van Meeteren LA, Jakobsson L, Cebe-Suarez S, Shimizu A, Edholm D, Ballmer-Hofer K, Kjellen L, Klagsbrun M, Claesson-Welsh L. Neuropilin-1 in regulation of VEGF-induced activation of p38MAPK and endothelial cell organization. Blood. 2008;112 (9):3638–3649. doi: 10.1182/blood-2007-12-125856. blood-2007-12-125856 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Favier B, Alam A, Barron P, Bonnin J, Laboudie P, Fons P, Mandron M, Herault JP, Neufeld G, Savi P, Herbert JM, Bono F. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood. 2006;108 (4):1243–1250. doi: 10.1182/blood-2005-11-4447. 2005-11-4447 [pii] [DOI] [PubMed] [Google Scholar]
- 52.Xu Y, Yuan L, Mak J, Pardanaud L, Caunt M, Kasman I, Larrivee B, Del Toro R, Suchting S, Medvinsky A, Silva J, Yang J, Thomas JL, Koch AW, Alitalo K, Eichmann A, Bagri A. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J Cell Biol. 2010;188 (1):115–130. doi: 10.1083/jcb.200903137. jcb.200903137 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dudley AC, Khan ZA, Shih SC, Kang SY, Zwaans BM, Bischoff J, Klagsbrun M. Calcification of multipotent prostate tumor endothelium. Cancer Cell. 2008;14 (3):201–211. doi: 10.1016/j.ccr.2008.06.017. S1535-6108(08)00227-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X, Claesson-Welsh L, Shibuya M. VEGF receptor signal transduction. Methods Enzymol. 2008;443:261–284. doi: 10.1016/S0076-6879(08)02013-2. S0076-6879(08)02013-2 [pii] [DOI] [PubMed] [Google Scholar]
- 55.Bielenberg DR, Hida Y, Shimizu A, Kaipainen A, Kreuter M, Kim CC, Klagsbrun M. Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, nonmetastatic tumor phenotype. J Clin Invest. 2004;114 (9):1260–1271. doi: 10.1172/JCI21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coma S, Shimizu A, Klagsbrun M. Hypoxia induces tumor and endothelial cell migration in a semaphorin 3F- and VEGF-dependent manner via transcriptional repression of their common receptor neuropilin 2. Cell Adh Migr. 2011;5(3):266–275. doi: 10.4161/cam.5.3.16294. 16294 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rossignol M, Gagnon ML, Klagsbrun M. Genomic organization of human neuropilin-1 and neuropilin-2 genes: identification and distribution of splice variants and soluble isoforms. Genomics. 2000;70(2):211–222. doi: 10.1006/geno.2000.6381. S0888-7543(00)96381-X [pii] [DOI] [PubMed] [Google Scholar]
- 58.Jakobsson L, Kreuger J, Claesson-Welsh L. Building blood vessels--stem cell models in vascular biology. J Cell Biol. 2007;177 (5):751–755. doi: 10.1083/jcb.200701146. jcb.200701146 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Geretti E, van Meeteren LA, Shimizu A, Dudley AC, Claesson-Welsh L, Klagsbrun M. A mutated soluble neuropilin-2 B domain antagonizes vascular endothelial growth factor bioactivity and inhibits tumor progression. Mol Cancer Res. 2010;8 (8):1063–1073. doi: 10.1158/1541-7786.MCR-10-0157. 1541-7786.MCR-10-0157 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caunt M, Mak J, Liang WC, Stawicki S, Pan Q, Tong RK, Kowalski J, Ho C, Reslan HB, Ross J, Berry L, Kasman I, Zlot C, Cheng Z, Le Couter J, Filvaroff EH, Plowman G, Peale F, French D, Carano R, Koch AW, Wu Y, Watts RJ, Tessier-Lavigne M, Bagri A. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell. 2008;13 (4):331–342. doi: 10.1016/j.ccr.2008.01.029. S1535-6108(08)00038-X [pii] [DOI] [PubMed] [Google Scholar]
- 61.Johnson KD, Hsu AP, Ryu MJ, Wang J, Gao X, Boyer ME, Liu Y, Lee Y, Calvo KR, Keles S, Zhang J, Holland SM, Bresnick EH. Cis-element mutated in GATA2-dependent immunodeficiency governs hematopoiesis and vascular integrity. J Clin Invest. 2012;122 (10):3692–3704. doi: 10.1172/JCI61623. [pii] 61623 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong YK, Harvey N, Noh YH, Schacht V, Hirakawa S, Detmar M, Oliver G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225 (3):351–357. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- 63.Lin FJ, Chen X, Qin J, Hong YK, Tsai MJ, Tsai SY. Direct transcriptional regulation of neuropilin-2 by COUP-TFII modulates multiple steps in murine lymphatic vessel development. J Clin Invest. 2010;120 (5):1694–1707. doi: 10.1172/JCI40101. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435 (7038):98–104. doi: 10.1038/nature03511. nature03511 [pii] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.