Abstract
The Western diet (WD) is associated with a higher incidence of colorectal cancer (CRC) than the Mediterranean diet. Polyphenols extracted from Annurca apple showed chemopreventive properties in CRC cells. A multifactorial, four-arm study by using wild-type (wt) and ApcMin/+ mice was carried out to evaluate the effect on polyp number and growth of APE treatment (60 μmol/L) ad libitum in drinking water combined with a WD or a balanced diet (BD) for 12 weeks. Compared with APE treatment, we found a significant drop in body weight (P < 0.0001), severe rectal bleeding (P = 0.0076), presence of extra-intestinal tumors, and poorer activity status (P = 0.0034) in water-drinking ApcMin/+ mice, more remarkably in the WD arm. In the BD and WD groups, APE reduced polyp number (35% and 42%, respectively, P < 0.001) and growth (60% and 52%, respectively, P < 0.0001) in both colon and small intestine. Increased antioxidant activity was found in wt animals fed both diets and in ApcMin/+ mice fed WD and drinking APE. Reduced lipid peroxidation was found in ApcMin/+ mice drinking APE fed both diets and in wt mice fed WD. In normal mucosa, mice drinking water had lower global levels of DNA methylation than mice drinking APE. APE treatment is highly effective in reducing polyps in ApcMin/+ mice and supports the concept that a mixture of phytochemicals, as they are naturally present in foods, represent a plausible chemopreventive agent for CRC, particularly in populations at high risk for colorectal neoplasia.
Introduction
Colorectal cancer (CRC) is the fourth commonest cancer in the United States and Western countries (1). There is a great difference in CRC incidence among countries and, in particular, in some regions of the Mediterranean area, a significantly lower incidence is found (2). Increasing evidence suggests that dietary factors are the predominant components that modulate susceptibility to this disease. The Western diet (WD), rich in fats and proteins and poor in dietary fiber, calcium, and other constituents, is associated with a higher incidence of CRC than the Mediterranean diet (3), which encourages a balanced intake of macronutrients and health-promoting micronutrients (4).
Studies on migrations of populations from low- to high-risk areas have shown an increased incidence of colon cancer among descendents in a single generation (5–7). Furthermore, a sharp increase in CRC incidence has been registered in traditionally low-risk areas (i.e., Asia), possibly because of the adoption of Westernized style meals (8). On the contrary, epidemiologic data strongly support the hypothesis that the Mediterranean diet is associated with a lower risk of developing CRC. Gallus and colleagues evaluated case–control studies done in the Northern of Italy between 1983 and 2001 and found ORs between 0.3 and 0.7 for epithelial cancers among those with an increased consumption of fruit and vegetables (9). Furthermore, Trichopoulou and colleagues found an overall reduction in mortality among Greeks who strongly adhered to a Mediterranean diet (10). Accordingly, the Polyp Prevention Trial provided evidence that dry bean intake is inversely associated with advanced adenoma recurrence (11). Moreover, the EPIC study highlighted an inverse association between CRC incidence and the consumption of a diet rich in fruit and vegetables, especially among nonsmokers (12).
The Mediterranean diet differs from the Western counterpart in the quantity and quality of macronutrients (i.e., less fats, mainly polyunsaturated), and, also because, it is rich in certain plant-derived micronutrients, particularly phytochemicals. There is in vitro and in vivo evidence for chemopreventive effects of plant polyphenols. In particular, epigallocatechin gallate (EGCG) has gained attention for its ability to inhibit cell proliferation and metastasis and enhancing apoptosis (13). Recently, EGCG has been found to reduce polyp formation by approximately 30% in the ApcMin/+ mouse (14), whereas resveratrol and the synthetic analog DMU-212 induced a polyp reduction of 27% and 24%, respectively, in the same strain (15).
In spite of the promising laboratory findings, when the administration of single polyphenols is translated into clinical trials, the evidence is inconclusive (16, 17). Importantly, the clinical trials are mainly focused on supplementation of single compounds, whereas the epidemiologic evidence supports an interpretation that the mixture of bioactive compounds, as they naturally occur in the diet, may act synergistically to be effective (18). It is possible that when their consumption is associated with a balanced intake of proteins, carbohydrates, fatty acids, and micro-nutrients, as occurs in the Mediterranean diet, the overall health-promoting effect is more significant.
The Annurca is an apple variety with a “Protected Geographical Indication” from Southern Italy, and a whole extract from the fruit displays stronger antioxidant activity than other apple varieties (19). We previously showed that the whole Annurca apple polyphenol extract (APE) has in vitro chemopreventive properties by modulating cell viability, apoptosis, and reactivating tumor suppressor genes (20). On the basis of these observations, in this study, we aimed to evaluate the anticancer effects of APE in ApcMin/+ mice, which spontaneously develop multiple intestinal polyps within a few weeks of birth, fed either a BD or a WD.
Materials and Methods
APE
APE was extracted from the frozen flesh of Annurca apples as previously described (21). Its polyphenol composition, as assessed by high-performance liquid chromatography/tandem mass spectrometry (HPLC/MS/MS), was as follows: 35.7% procyanidin B2; 32.7% chlorogenic acid; 22.6% epicatechin; 4.3% catechin; 3.6% phlorizin; 0.7% caffeic acid and 0.5% rutin. Because APE is a mixture of different phenolic compounds with different molecular weights, to give APE an arbitrary molar concentration, its polyphenol concentration was expressed as catechin equivalents (21). APE of 6.1 mmol/L catechin equivalents in a 60:40 water:ethanol solution was prepared, aliquoted, stored at −80°C to maintain stability, and diluted 1:100 every other day over the study period, just before providing it to the mice in the drinking water. A final APE concentration of approximately 60 μmol/L catechin equivalents, approximately 0.002% APE in drinking water, was given to APE-treated mice. The final alcohol concentration was 0.4%.
Animal treatments and tissue harvesting
This study was approved by the Institutional Animal Care and Use Committee at the Baylor Research Institute, Dallas, TX. Six-week-old wild-type (wt) female C57BL/6 mice were bred with male ApcMin/+ mice (The Jackson Laboratory). Animals were housed in a temperature and humidity controlled animal facility with a 12-hour light/dark cycle. The ApcMin/+ offspring were identified by an allele-specific PCR assay (22). Animals were either fed a standard balanced diet (BD) AIN-93G or a WD characterized by low dietary fiber, calcium, vitamin D, and high-fat content. Both diets were purchased from Research Diets. The characteristics of the diets are reported in Supplementary Table S1. APE of 60 μmol/L catechin equivalents in a 60:40 water:ethanol solution was provided to each animal. Control animals were provided 0.4% alcohol in their drinking water. At 5 weeks of age, animals were weaned and started the feeding protocol. ApcMin/+ and wt mice (n = 98) were randomized into an 8-group matrix based on the genotype (wt or ApcMin/+), the type of diet (BD or WD), and the drinking water they were receiving (water or APE; Supplementary Fig. S1). Mice were allowed to eat food and drink APE or water ad libitum. Food intake, body weight, animal activity, and physical changes were monitored weekly. Animal activity was measured manually and scored every 30 seconds over a 5-minute period as either very poor (continuously immobile, not responding to stimuli, score = 0), poor (immobile but responding to stimuli, score = 1), or normal (responding to stimuli and active, score = 2) by modifying a previously published protocol (23). The presence of visible rectal bleeding was recorded. After 12 weeks of treatment, mice were sacrificed by cervical dislocation and blood was collected by intracardiac puncture under anesthesia with Isofluorane (IsoFlo; Burns Vet Supply). The entire gastrointestinal tract was removed immediately after sacrifice, washed with PBS, cut into 5 segments [I–IV from proximal (I) to distal small intestine (IV), and colon], then cut longitudinally. Fresh tissues were collected for molecular studies. Segments were flattened on filter paper, fixed overnight in 10% buffered formalin and then stained with 0.2% methylene blue (Sigma Aldrich). The number, location, and size of visible tumors throughout the intestine were determined at 10× magnification by using an Olympus SZX-ILLB100 dissecting microscope (Center Valley) by 2 independent investigators. After counting, segments were transferred to 10% buffered formalin to be processed for histopathologic examination. Polyp size was determined by caliper measurement of the largest (Dim1) and the perpendicular diameter (Dim2). On the basis of Dim1, polyps throughout the intestinal tract were classified into 3 categories (1 mm, 1–3 mm, and >3 mm). Polyp area (A) was calculated according to the equation A = π × (Dim1/2) × (Dim2/2). Polyp load was expressed as the sum of all the polyp areas for each animal (mm2). After sacrifice, mice were also examined for the presence of extraintestinal tumors.
Histologic characterization of the polyps
One representative segment for the small intestine (IV segment) and the entire length of the colon were Swiss-rolled, formalin-fixed, and paraffin-embedded to be processed for histologic evaluation. One slide of each specimen was stained with hematoxylin and eosin and evaluated independently by 2 pathologists for histologic characterization of the polyps (grade of dysplasia and cancer).
Quantification of apple polyphenol metabolites in mice serum
Polyphenolic compounds were extracted from mice serum by using the procedure previously described (24), and their composition was studied by HPLC/MS/MS analysis. MS and MS/MS analyses of serum samples were done on an ABI 3000 Triple Quadrupole Mass Spectrometer (Applied Biosystems) equipped with a TurbolonSpray source working in the negative ion mode. The quantification was carried out by using a calibration curve obtained with pure epicatechin and chlorogenic acid (purchased from Sigma Aldrich). Analyses were done in multiple reactions monitoring mode, tracking the following precursor/product ion combinations: m/z 353 → 191 for chlorogenic acid and m/z 289 → 245 for epicatechin.
Determination of serum malondialdehyde and ferric reducing ability of plasma
Lipid peroxidation was evaluated in serum samples by malondialdehyde (MDA; ref. 25). The results were expressed as nmol per mL of serum. Reducing power of plasma samples was determined by the ferric reducing antioxidant power (FRAP) assay (26). The results were expressed as mmol/L Trolox Equivalents (TE) per mL of serum.
Bisulfite pyrosequencing for promoter and global DNA methylation analysis
DNA was extracted from frozen normal tissues and polyps with the QIAmp DNA extraction kit and subjected to bisulfite treatment by using the EpiTect Bisulfite Kit according to the manufacturer’s instructions (Qiagen). A quantitative bisulfite pyrosequencing method for all DNA methylation analyses was done with PSQ HS96Gold SNP Reagents on a PSQ 96HS machine (Biotage) as previously described (27). We analyzed methylation of LINE-1 (long interspersed nucleotide element-1, which is a good indicator of cellular 5-methylcytosine level), genes frequently methylated in human polyps (Cdkn2a, Rassf1a, Mlh1, Mgmt, Timp3, and Tmeff2), age-related hypomethylated genes (P2rx7, Igf2-DMR1), and age-related hypermethylated genes (Esr1, Nkx2–5, Gpr37, Prdm5, Myod1, Nptx2, and Igf2-DMR2). The complete nomenclature of each gene is reported in the Supplementary Material. Primer sequences and PCR conditions for bisulfite pyrosequencing assays are as previously reported (27). Each pyrosequencing reaction was done at least twice and the values averaged.
Statistical Analysis
Sample size was determined on the basis of average number of polyps and variances in the ApcMin/+ strain. We estimated that 12 mice in each group would be sufficient to detect a difference in means across the groups, with a desired power of 90% and a minimal reduction of 15%. This test is based on a 1-way ANOVA test. Relative weight gain was defined as: (weight at time t − weight at time t0)/weight at time t0. A generalized linear mixed model was used to evaluate the relative weight gain changes over time and among the different groups and to analyze number and size of polyps and polyp load. These variables were considered as a function of the liquid, food, and liquid food interactions as follows: Yijk = μ +αi + βj + γij + εijk, in which Yijk is the outcome, μ is the overall mean (intercept–constant), αi is the food effect, [i = 1 (WD) or 2 (BD)], βj liquid effect [j = 1 (water), 2 (apple)], γij is the food–liquid interactions [i = 1, 2 and j = 1, 2] and εijk is the random error.
Student’s t test with 2-tailed P was used to evaluate the mean differences between 2 groups for the continuous variables. Differences for methylation among groups of animals were assessed by the Kruskal–Wallis nonparametric test. χ2 test and Fisher exact tests were applied to analyze categorical variables. JMP version 8.02 and SAS version 9.2 were used for the statistical analyses. Data were expressed as mean ± SEM if not differently stated. Significance was assigned at P < 0.05.
Results
APE prevents cachexia in ApcMin/+ mice fed with the WD
In the wt group, although animals fed WD and drinking water showed a higher relative weight gain profile, no significant changes were found with respect to diet or APE supplementation (Fig. 1A). Importantly, a significant protective effect against cachexia was found for APE in ApcMin/+ animals fed with the WD (Fig. 1B). In fact, mice fed with the WD and drinking water showed a significant drop in their relative weight gain starting from week 9 compared with WD-APE (P < 0.0001), suggesting that APE counteracts the negative effects of the WD (Fig. 1B; Supplementary Tables S2 and S3).
Figure 1.
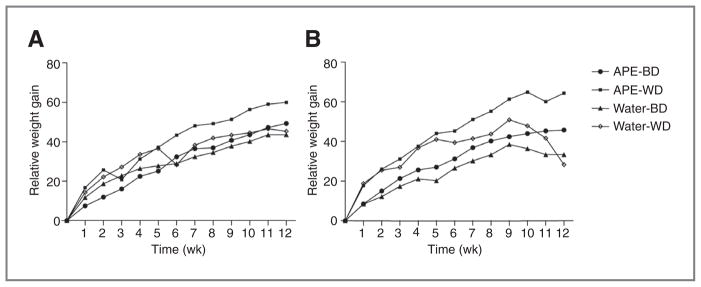
Effect of APE on the relative body weight in wt (A) and ApcMin/+ mice (B). A strong protective effect was found for APE in ApcMin/+ animals fed with a WD at week 9.
As shown in Table 1, APE protected ApcMin/+ mice from rectal bleeding when fed with either the BD or WD. No rectal bleeding was observed in animals fed with the BD and drinking APE, whereas 62% of animals fed with the WD and drinking water experienced rectal bleeding. On the contrary, animals fed with the WD and drinking APE showed a significant reduction in rectal bleeding (P = 0.0076), an outcome similar to the impact on rectal bleeding observed in the animals fed with the BD and drinking water. Data on animal activity gave a concordant picture with the results for rectal bleeding. Indeed, 54% (7/13) of ApcMin/+ mice fed with the WD and drinking water showed either poor or very poor activity compared with the 8% (1/12) of their APE-drinking counterparts (P < 0.05). Interestingly, animals fed with the WD and drinking APE had better activity than mice fed with the BD and drinking water, suggesting that APE counteracts the effects of the WD. Of note, 2 animals fed with the WD and drinking water developed extra-intestinal tumors (trichoepithelioma and epithelial inclusion cyst).
Table 1.
General health parameters in ApcMin/+ mice
| Liquid | Diet | No. of mice/arm | Rectal bleeding [% (n mice)] | Animal activity [%(n mice)]
|
No. of extraintestinal tumors | ||
|---|---|---|---|---|---|---|---|
| Normal | Poor | Very poor | |||||
| APE | BD | 13 | 0 (0) | 100 (13) | 0 (0) | 0 (0) | 0 |
| Water | BD | 12 | 25 (3) | 83 (10) | 17 (2) | 0 (0) | 0 |
| APE | WD | 12 | 33 (4)a | 92 (11) | 8 (1)b | 0 (0) | 0 |
| Water | WD | 13 | 62 (8)a | 46 (6) | 39 (5)b | 15 (2)b | 2 |
P = 0.0076.
P < 0.05 for combined poor and very poor activity in Water-WD versus poor activity in APE-WD.
APE reduces polyp number and eliminates high-grade dysplasia in ApcMin/+ mice
As shown in Table 2, animals fed with the WD and drinking APE developed a smaller number of polyps in the small intestine and colon (21.92 ± 0.67) than animals fed with the WD and drinking water (38 ± 1.81; 42% reduction, P < 0.001). A significant reduction (35%, P < 0.001) was also observed among animals fed with the BD and drinking APE (17.23 ± 0.59) compared with those drinking water (26.58 ± 1.08). This was maintained when considering the small intestine and colon individually. The combination of the BD and APE resulted in a decrease in polyps of 55% compared with animals fed with the WD and drinking water (P < 0.0001). Interestingly, animals fed with the WD and drinking APE had a polyp reduction of 18% compared with animals fed with the BD and drinking water (P = 0.0079). Among APE-drinking animals, those fed with the BD developed 21% fewer polyps than animals fed with the WD (P = 0.0066). Considering the overall number of polyps, a multivariate regression analysis showed a strong effect of APE on polyp number (Supplementary Fig. S2).
Table 2.
Effect of APE on polyp number in ApcMin/+ mice (polyps/mouse)
| Water-WD (n = 13) | Water-BD (n = 12) | APE-WD (n = 12) | APE-BD (n = 13) | |
|---|---|---|---|---|
| Small intestine | 36.54 ± 1.72 | 25.67 ± 1.21 | 21.17 ± 0.73 | 16.92 ± 0.55 |
| Colon | 1.46 ± 0.27 | 0.92 ± 0.26 | 0.75 ± 0.22 | 0.31 ± 0.13 |
| Total | 38 ± 1.81a | 26.58 ± 1.08b | 21.92 ± 0.67a | 17.23 ± 0.59b |
P < 0.001.
P < 0.001.
Three animals fed with the WD and drinking water developed 2 invasive cancers in the rectum and 1 in the small intestine, whereas 1 fed with the BD and drinking water developed an invasive cancer in the small intestine. None of the APE-drinking animals developed cancers. Moreover, when looking at the histology of the polyps, although most of the polyps were tubular adenomas with low-grade dysplasia, 8 cases in the WD–water group (4 colons + 4 small intestines) and 9 cases in BD–water group (3 colons + 6 small intestines) had polyps with high-grade dysplasia (Supplementary Fig. S3). None of the APE-drinking animals developed polyps with high-grade dysplasia.
APE treatment significantly affects polyp growth
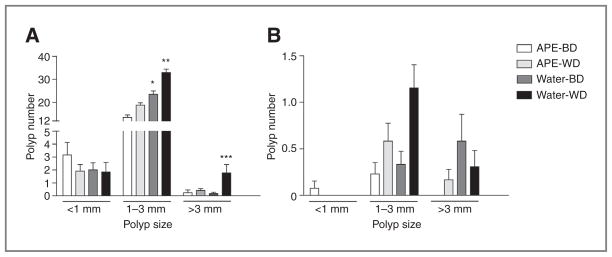
We also examined the effect of APE treatment on polyp growth in ApcMin/+ mice fed with the BD or WD. Looking at polyp distribution by size (Fig. 2), in the small intestine, polyps measuring less than 1 mm were more common in the APE–BD group, whereas 1 to 3 mm and larger than 3 mm polyps were significantly associated with the water-drinking arms, in particular in those fed with the WD. A similar distribution was found in the colon. In particular, polyps less than 1 mm were found only in the APE–BD group, which had no polyps larger than 3 mm. Statistical analysis in the colon was hampered by the paucity of polyps in this segment, as expected in the ApcMin/+ mouse.
Figure 2.
Polyp size distribution in the small intestine (A) and colon (B) of ApcMin/+ mice. A, polyps smaller than 1 mm were more common in the APE–BD group, whereas polyps sized 1 to 3 mm and larger than 3 mm were significantly associated with the water-drinking arms. *, P < 0.0001 for Water-BD versus APE-BD; **, P < 0.0001 for Water-WD versus APE-WD; ***, P = 0.0122 for Water-WD versus APE-WD. B, polyps smaller than 1 mm were found only in APE-BD, which lacked polyps larger than 3 mm.
Considering the polyp load (Table 3), in the WD groups, the polyp load per mouse was 44.11 ± 6.64 mm2 for APE-drinking mice and 91.1 ± 6.39 mm2 for their water-drinking counterparts, corresponding to a reduction of 52% (P < 0.001). Similarly, in the BD group, the polyp load per mouse was 17.92 ± 6.39 mm2 for APE-drinking mice and 44.65 ± 6.65 mm2 for their water-drinking counterparts, corresponding to a reduction of 60% (P < 0.001). A strong effect of APE on polyp load was confirmed by multivariate regression analysis (Supplementary Fig. S4). No differences in polyp load were found between water-BD and APE-WD mice, suggesting that APE treatment was effective in completely reverting the diet-induced enhancement on polyp growth. However, mice eating the BD and drinking APE had a reduction in polyp load of 80% compared with animals eating the WD and drinking water (P < 0.001).
Table 3.
Effect of APE on polyp load in ApcMin/+ mice (polyp load/mouse)
| Water-WD (n = 13) | Water-BD (n = 12) | APE-WD (n = 12) | APE-BD (n = 13) | |
|---|---|---|---|---|
| Small intestine | 84.5 ± 8.79 | 38.14 ± 5.88 | 38.97 ± 3.67 | 17.18 ± 2.63 |
| Colon | 6.6 ± 2.21 | 6.51 ± 2.82 | 5.14 ± 1.83 | 0.74 ± 0.42 |
| Total | 91.1 ± 6.39a | 44.65 ± 6.64b | 44.11 ± 6.65a | 17.92 ± 6.39b |
P < 0.001.
P < 0.001.
APE has antioxidant and antilipoperoxidation properties
Polyphenol metabolites such as epigallocatechin-glucuronide, parent-epicathechin, dimethylated-epicathechin, glucuronidated-epicathechin, and sulfated-epicatechin, as well as chlorogenic acid, were found only in serum samples from APE-drinking mice. The total serum concentration of polyphenol metabolites expressed as the sum of all detected compounds was significantly higher (P < 0.05) in BD animals than in those fed with the WD, being 109.0 ± 63.6 versus 11.4 ± 6.7 nmol/L, respectively, in wt mice, and 196.8 ± 135.4 versus 0.006 ± 0.003 nmol/L in ApcMin/+ mice.
To establish whether APE influences the systemic oxidative balance in treated animals, the FRAP of serum and serum MDA concentration, as a marker of lipid peroxidation, were measured. In wt animals, serum FRAP values were significantly higher in APE-treated than in water-drinking animals (Fig. 3A), whereas among ApcMin/+ mice, the difference did not reach significance. Serum MDA concentrations were significantly lower in APE-drinking than in water-drinking animals, except for wt mice fed with BD (Fig. 3B).
Figure 3.
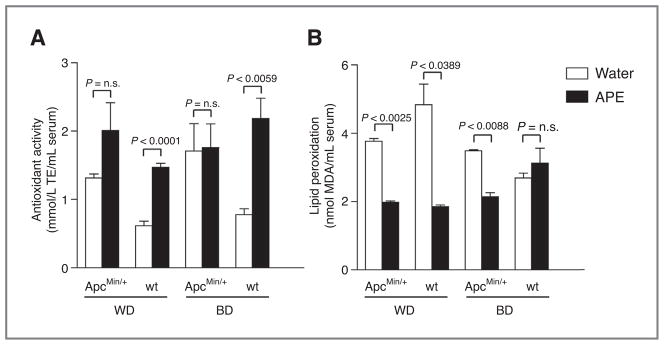
Antioxidant effects of APE. A, APE treatment increases serum FRAP in wt and ApcMin/+ animals. B, serum MDA concentrations were significantly lower in APE-treated ApcMin/+ mice fed with both WD and BD as well as in WD-fed wt animals. Abbreviation: n.s., not significant.
APE protects against DNA hypomethylation
In an attempt at clarifying the possible mechanisms exerted by APE and following up on our previous findings that APE could reactivate silenced tumor suppressor genes (TSG) in vitro (20), we analyzed the methylation status of TSGs commonly silenced in human polyps and colon cancers (Cdkn2a, Rassf1a, Mlh1, Mgmt, Timp3, and Tmeff2). Surprisingly, we found a very low level of methylation of these genes (average 6.6%), without differences between normal tissues and polyps irrespective of the dietary regimens or what they were drinking (Supplementary Fig. S5).
We also analyzed the methylation status of LINE-1 (Fig. 4A) and age-related hypomethylated genes Igf2-DMR1 and P2rx7 (Fig. 4B and C). A higher level of LINE-1 methylation was observed among animals receiving the BD and drinking APE in both normal tissues and polyps, while no differences were found for animals fed with the WD (Fig. 4A). For Igf2-DMR1, we found a higher level of methylation in normal tissues from animals fed with the BD and drinking APE (Fig. 4B), whereas for P2rx7, a higher level of methylation was observed in normal tissues from animals fed with the WD and drinking APE (Fig. 4C). Also, as expected, polyps showed lower levels of methylation (34%) compared with normal tissues (35.73%; P = 0.0163).
Figure 4.
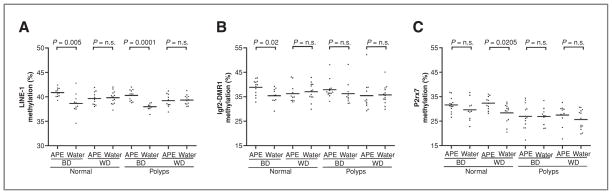
APE affects methylation of age-related hypomethylated genes. Methylation of (A) LINE-1, (B) Igf2-DMR1, and (C) P2rx7. Abbreviation: n.s., not significant.
Finally, we analyzed the methylation of age-related hypermethylated genes (Esr1, Nkx2–5, Gpr37, Prdm5, Myod1, Nptx2, and Igf2-DMR2) but the differences were not significant (Supplementary Fig. S6).
Discussion
In this study, we show that APE is a very effective chemo-preventive agent in the ApcMin/+ mouse, a model of familial adenomatous polyposis. APE supplementation to the drinking water decreased polyp numbers and sizes, improved mouse activity, and eliminated (in the BD group) or greatly reduced (in the WD group) rectal bleeding. Importantly, APE strongly reduced lipid peroxidation, increased antioxidant activity, and protected against increased hypomethylation, particularly in the normal tissue. In accordance with previous reports (28), our data suggest that the WD is associated with a larger number of polyps in animals genetically predisposed to polyp formation, leading to increased rectal bleeding, cachexia, and cancer.
We found that APE significantly reduced the polyp number when comparing groups matched by diet, with a reduction by 35% in animals fed with the BD and 42% in those fed with the WD; moreover, the polyp load was decreased by 52% to 60%. Importantly, APE is not only biologically active in polyp reduction in the BD-fed animals, but it also counteracts the tumorigenic effect of the WD on the ApcMin+ animals. In fact, in the WD group, APE significantly decreased the number of polyps to a degree less than that found in the BD water-drinking group. Therefore, although a WD significantly increases the number of polyps, a BD supplemented with APE was the most effective combination in the prevention of polyps in the ApcMin/+ mice.
Recently, synthetic and natural compounds have been tested as chemopreventive agents for CRC in the ApcMin/+ model. Synthetic drugs, such as coxibs, dramatically reduce polyp number (29, 30), but their use has been hampered by cardiovascular toxicity (31). Surprisingly, when tested in long-term animal studies (>3 months), the COX-2 inhibitor celecoxib has a protumorigenic effect in the intestine (32).
On the other hand, natural compounds have lower toxicity and fewer side effects than synthetic drugs, but they are often less effective for adenoma prevention. For example, resveratrol caused a 27% decrease in polyp formation (15), whereas curcumin caused a 40% reduction (33). EGCG, a polyphenol compound chemically similar to those in APE, administered to ApcMin/+ mice at doses of 0.01% (14), 0.08%, or 0.16% (22) in drinking fluids led to reductions in small intestinal tumors ranging from 37% to 47% (22). In our study, APE used at a dose of 60 μmol/L of catechin equivalents (~0.002%), a dosage 10- to 50-fold lower than the concentration of EGCG, had similar efficacy in reducing polyps, suggesting that the mixture of multiple polyphenols naturally present in the apple acted synergistically. We believe that the presence in APE of both monomeric polyphenols (catechin, epicatechin, chlorogenic acid, caffeic acid, and rutin) and polymeric polyphenols (procyanidins) may explain our striking reduction in number and size of polyps. Indeed, as also suggested by Gosse and colleagues (34), it is reasonable to speculate that the APE monomeric fraction, in the parent form or as metabolites, may act locally and systemically in the upper gastrointestinal tract, whereas the polymeric fraction may act as a dietary fiber in vivo, reaching the colon unmodified and playing a more important role in the lower intestine.
In this study, 2 important risk factors for polyp formation were investigated: genetic predisposition and diet. We found that APE strongly reduced lipid peroxidation in ApcMin/+ mice fed with either diet and wt mice fed with the WD. In WD groups, the effect was evident in both ApcMin/+ and wt arms, and this was probably associated with the high-fat diet which increases lipid peroxidation in the absence of APE supplementation (35–37). Our data suggest that APE prevents lipid peroxidation in high-risk subjects either because they have an “unbalanced” diet (ApcMin/+fed with the WD) or an excessive sensitivity to oxidative stress because of the underlying disease (ApcMin/+fed with the BD). Recently, in support of this interpretation, EGCG has been shown to reduce the adipose tissue mass and the levels of plasma triglyceride and liver lipids in high-fat diet-induced obese mice (38).
With regard to antioxidant activity, APE increased FRAP in both wt groups. Because of the higher standard error associated with the measurement of FRAP in the ApcMin/+ animals, we did not find significant differences between water- and APE-drinking animals. In the ApcMin/+ BD group treated with APE, FRAP increased minimally compared with water-drinking animals. This might be a consequence of the intrinsic antioxidant properties of the BD (higher vitamin C), which may have masked the antioxidant capacity of APE.
Several studies have shown that apple derivatives might modulate tumor formation or progression by antioxidant or anti-inflammatory properties, as well as by inhibiting cell proliferation and increasing cell death (39). We tested the hypothesis that the effect of APE on polyps is mediated by epigenetic modulation. It is known that methylation of promoters in CpG islands plays a critical role in colon carcinogenesis. Several TSGs are commonly hypermethylated in human CRC including, p16ARF/INK4a, TIMP3, and hMLH1 (for review see ref. 40). Interestingly, we found a low level of methylation (average 6.6%) among all samples and no changes based on diet or APE. These findings suggest that contrarily to what happens in humans and what had been previously reported in the same strain of mice (41), methylation of TSGs does not contribute to polyp growth in the ApcMin/+ mouse model.
Besides the role of CpG island hypermethylation in tumorigenesis, genome-wide DNA hypomethylation can cause genomic instability, predisposing to DNA strand breakage and recombination (42). Global genomic hypomethylation seems to be associated with age, explaining the higher incidence of cancer with aging. Furthermore, it is known that chronic inflammation can increase the level of global hypomethylation, thus increasing susceptibility to cancer (43). Because APE is known to have anti-inflammatory properties, we tested changes in methylation in repetitive sequences such as LINE-1, and age-related hypomethylated genes such as Igf2 and P2rx7. As expected, we found an increased level of hypomethylation in polyps compared with normal tissues. Importantly, we found that APE protected against hypomethylation, particularly in normal tissue as shown by an increase in methylation in LINE-1 and Igf2-DMR1 for BD-fed animals drinking APE. The regulation of LINE-1 methylation is poorly understood. It is possible that there could be a synergic effect between APE and BD in normal tissues, whereas the smaller effects found in polyps (limited to LINE-1 for animals fed with the BD) may be driven by tumor-related hypomethylation which cannot be overcome by APE. Our data suggest that, even with short-term treatment, APE protects against hypomethylation. However, the methylation differences need to be confirmed in other studies because the use of multiple comparisons could have led to false positive findings.
Because of the stability and lack of toxicity, mice received APE ad libitum and drank approximately 5 mL/d. Thus, the effects in this study were attributable to a daily APE dosage of approximately 8 μmol/L catechin equivalents/kg body weight (corresponding to ~0.9 mg chlorogenic acid/kg). Considering that Annurca is a small apple (mean ~140 g) and contains approximately 9 mg of chlorogenic acid per 100 g fresh weight (21), the daily dosage of APE used in this study corresponds to a daily consumption of about 5 Annurca apples by a 70-kg person.
In conclusion, our data suggest that APE is a candidate chemopreventive agent for CRC, particularly for high-risk populations (such as FAP) eating a WD. Human studies are warranted.
Supplementary Material
Acknowledgments
Grant Support
The work was supported by NIH Grant R01 CA72851 and funds from the Sammons Cancer Center and Baylor Research Institute, Baylor University Medical Center, Dallas, TX.
Footnotes
Note: Supplementary data for this article are available at Cancer Prevention Research Online (http://cancerprevres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
L. Ricciardiello, C.R. Boland, M. Romano, and V. Fogliano filed a patent application for Annurca Apple Polyphenol extract, US patent Publication No. 2009/0076131.
Authors’ contributions
L. Fini participated in acquisition of the data, analysis and interpretation of the data. G. Piazzi participated in analysis and interpretation of the data, and drafting of the manuscript. Y. Daoud participated in statistical analysis, analysis and interpretation of the data. M. Selgrad participated in analysis and interpretation of the data, and technical support. S. Maegawa participated in analysis and interpretation of pyrosequencing data. M. Garcia participated in technical and material support. V. Fogliano participated in acquisition and interpretation of MDA and FRAP analysis, and critical revision of the manuscript. M. Romano participated in interpretation of the data and critical revision of the manuscript. G. Graziani participated in acquisition of MDA and FRAP data. P. Vitaglione participated in acquisition of MDA and FRAP data. S.W. Carmack participated in pathological analysis. A. Gasbarrini participated in analysis and interpretation of the data, and critical revision of the manuscript. R.M. Genta participated in pathological analysis. J-P. Issa participated in analysis and interpretation of pyrosequencing data and critical revision of the manuscript for important intellectual content. C.R. Boland participated in analysis and interpretation of the data, and critical revision of the manuscript for important intellectual content, and L. Ricciardiello participated in study concept and design, analysis and interpretation of data, drafting of the manuscript, and study supervision.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Colomer R, Menendez JA. Mediterranean diet, olive oil and cancer. Clin Transl Oncol. 2006;8:15–21. doi: 10.1007/s12094-006-0090-0. [DOI] [PubMed] [Google Scholar]
- 3.Giacosa A. The Mediterranean diet and its protective role against cancer. Eur J Cancer Prev. 2004;13:155–7. doi: 10.1097/01.cej.0000130009.53407.a3. [DOI] [PubMed] [Google Scholar]
- 4.Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, et al. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113(Suppl 9B):71S–88S. doi: 10.1016/s0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 5.Haenszel W, Berg JW, Segi M, Kurihara M, Locke FB. Large-bowel cancer in Hawaiian Japanese. J Natl Cancer Inst. 1973;51:1765–79. doi: 10.1093/jnci/51.6.1765. [DOI] [PubMed] [Google Scholar]
- 6.Le ML, Wilkens LR, Hankin JH, Kolonel LN, Lyu LC. A case-control study of diet and colorectal cancer in a multiethnic population in Hawaii (United States): lipids and foods of animal origin. Cancer Causes Control. 1997;8:637–48. doi: 10.1023/a:1018406716115. [DOI] [PubMed] [Google Scholar]
- 7.Le ML, Wilkens LR, Hankin JH, Kolonel LN, Lyu LC. Independent and joint effects of family history and lifestyle on colorectal cancer risk: implications for prevention. Cancer Epidemiol Biomarkers Prev. 1999;8:45–51. [PubMed] [Google Scholar]
- 8.Sung JJ, Lau JY, Goh KL, Leung WK. Increasing incidence of color-ectal cancer in Asia: implications for screening. Lancet Oncol. 2005;6:871–6. doi: 10.1016/S1470-2045(05)70422-8. [DOI] [PubMed] [Google Scholar]
- 9.Gallus S, Bosetti C, La VC. Mediterranean diet and cancer risk. Eur J Cancer Prev. 2004;13:447–52. doi: 10.1097/00008469-200410000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 11.Lanza E, Hartman TJ, Albert PS, Shields R, Slattery M, Caan B, et al. High dry bean intake and reduced risk of advanced colorectal adenoma recurrence among participants in the Polyp Prevention Trial. J Nutr. 2006;136:1896–903. doi: 10.1093/jn/136.7.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Duijnhoven FJ, Bueno-de-Mesquita HB, Ferrari P, Jenab M, Boshuizen HC, Ros MM, et al. Fruit, vegetables, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2009;89:1441–52. doi: 10.3945/ajcn.2008.27120. [DOI] [PubMed] [Google Scholar]
- 13.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–39. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sukhthankar M, Yamaguchi K, Lee SH, McEntee MF, Eling TE, Hara Y, et al. A green tea component suppresses posttranslational expression of basic fibroblast growth factor in colorectal cancer. Gastroenterology. 2008;134:1972–80. doi: 10.1053/j.gastro.2008.02.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sale S, Tunstall RG, Ruparelia KC, Potter GA, Steward WP, Gescher AJ. Comparison of the effects of the chemopreventive agent resveratrol and its synthetic analog trans 3,4,5,4′-tetramethoxystilbene (DMU-212) on adenoma development in the Apc(Min+) mouse and cyclooxygenase-2 in human-derived colon cancer cells. Int J Cancer. 2005;115:194–201. doi: 10.1002/ijc.20884. [DOI] [PubMed] [Google Scholar]
- 16.La Vecchia C. Association between Mediterranean dietary patterns and cancer risk. Nutr Rev. 2009;67 (Suppl 1):S126–9. doi: 10.1111/j.1753-4887.2009.00174.x. [DOI] [PubMed] [Google Scholar]
- 17.Visioli F, Bogani P, Grande S, Galli C. Mediterranean food and health: building human evidence. J Physiol Pharmacol. 2005;56 (Suppl 1):37–49. [PubMed] [Google Scholar]
- 18.Tulp M, Bruhn JG, Bohlin L. Food for thought. Drug Discov Today. 2006;11:1115–21. doi: 10.1016/j.drudis.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Napolitano A, Cascone A, Graziani G, Ferracane R, Scalfi L, Di VC, et al. Influence of variety and storage on the polyphenol composition of apple flesh. J Agric Food Chem. 2004;52:6526–31. doi: 10.1021/jf049822w. [DOI] [PubMed] [Google Scholar]
- 20.Fini L, Selgrad M, Fogliano V, Graziani G, Romano M, Hotchkiss E, et al. Annurca apple polyphenols have potent demethylating activity and can reactivate silenced tumor suppressor genes in colorectal cancer cells. J Nutr. 2007;137:2622–8. doi: 10.1093/jn/137.12.2622. [DOI] [PubMed] [Google Scholar]
- 21.Graziani G, D’Argenio G, Tuccillo C, Loguercio C, Ritieni A, Morisco F, et al. Apple polyphenol extracts prevent damage to human gastric epithelial cells in vitro and to rat gastric mucosa in vivo. Gut. 2005;54:193–200. doi: 10.1136/gut.2004.046292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ju J, Hong J, Zhou JN, Pan Z, Bose M, Liao J, et al. Inhibition of intestinal tumorigenesis in Apcmin/+mice by (−)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 2005;65:10623–31. doi: 10.1158/0008-5472.CAN-05-1949. [DOI] [PubMed] [Google Scholar]
- 23.Goecke JC, Awad H, Lawson JC, Boivin GP. Evaluating postoperative analgesics in mice using telemetry. Comp Med. 2005;55:37–44. [PubMed] [Google Scholar]
- 24.Vitaglione P, Donnarumma G, Napolitano A, Galvano F, Gallo A, Scalfi L, et al. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J Nutr. 2007;137:2043–8. doi: 10.1093/jn/137.9.2043. [DOI] [PubMed] [Google Scholar]
- 25.D’Argenio G, Mazzone G, Tuccillo C, Grandone I, Gravina AG, Graziani G, et al. Apple polyphenol extracts prevent aspirin-induced damage to the rat gastric mucosa. Br J Nutr. 2008;100:1228–36. doi: 10.1017/S0007114508988747. [DOI] [PubMed] [Google Scholar]
- 26.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 27.Maegawa S, Hinkal G, Kim HS, Shen L, Zhang L, Zhang J, et al. Widespread and tissue specific age-related DNA methylation changes in mice. Genome Res. 2010;20:332–40. doi: 10.1101/gr.096826.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wasan HS, Novelli M, Bee J, Bodmer WF. Dietary fat influences on polyp phenotype in multiple intestinal neoplasia mice. Proc Natl Acad Sci U S A. 1997;94:3308–13. doi: 10.1073/pnas.94.7.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchanan FG, Holla V, Katkuri S, Matta P, DuBois RN. Targeting cyclooxygenase-2 and the epidermal growth factor receptor for the prevention and treatment of intestinal cancer. Cancer Res. 2007;67:9380–8. doi: 10.1158/0008-5472.CAN-07-0710. [DOI] [PubMed] [Google Scholar]
- 30.Swamy MV, Patlolla JM, Steele VE, Kopelovich L, Reddy BS, Rao CV. Chemoprevention of familial adenomatous polyposis by low doses of atorvastatin and celecoxib given individually and in combination to APCMin mice. Cancer Res. 2006;66:7370–7. doi: 10.1158/0008-5472.CAN-05-4619. [DOI] [PubMed] [Google Scholar]
- 31.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Breazna A, Kim K, et al. Five-year efficacy and safety analysis of the Adenoma Prevention with Celecoxib Trial. Cancer Prev Res. 2009;2:310–21. doi: 10.1158/1940-6207.CAPR-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carothers AM, Moran AE, Cho NL, Redston M, Bertagnolli MM. Changes in antitumor response in C57BL/6J-Min/+mice during long-term administration of a selective cyclooxygenase-2 inhibitor. Cancer Res. 2006;66:6432–8. doi: 10.1158/0008-5472.CAN-06-0992. [DOI] [PubMed] [Google Scholar]
- 33.Perkins S, Verschoyle RD, Hill K, Parveen I, Threadgill MD, Sharma RA, et al. Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev. 2002;11:535–40. [PubMed] [Google Scholar]
- 34.Gosse F, Guyot S, Roussi S, Lobstein A, Fischer B, Seiler N, et al. Chemopreventive properties of apple procyanidins on human colon cancer-derived metastatic SW620 cells and in a rat model of colon carcinogenesis. Carcinogenesis. 2005;26:1291–5. doi: 10.1093/carcin/bgi074. [DOI] [PubMed] [Google Scholar]
- 35.Berquin IM, Edwards IJ, Chen YQ. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008;269:363–77. doi: 10.1016/j.canlet.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diniz YS, Cicogna AC, Padovani CR, Santana LS, Faine LA, Novelli EL. Diets rich in saturated and polyunsaturated fatty acids: metabolic shifting and cardiac health. Nutrition. 2004;20:230–4. doi: 10.1016/j.nut.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Milagro FI, Campion J, Martinez JA. Weight gain induced by high-fat feeding involves increased liver oxidative stress. Obesity (Silver Spring) 2006;14:1118–23. doi: 10.1038/oby.2006.128. [DOI] [PubMed] [Google Scholar]
- 38.Lee MS, Kim CT, Kim Y. Green tea (−)-epigallocatechin-3-gallate reduces body weight with regulation of multiple genes expression in adipose tissue of diet-induced obese mice. Ann Nutr Metab. 2009;54:151–7. doi: 10.1159/000214834. [DOI] [PubMed] [Google Scholar]
- 39.Gerhauser C. Cancer chemopreventive potential of apples, apple juice, and apple components. Planta Med. 2008;74:1608–24. doi: 10.1055/s-0028-1088300. [DOI] [PubMed] [Google Scholar]
- 40.Kondo Y, Issa JP. Epigenetic changes in colorectal cancer. Cancer Metastasis Rev. 2004;23:29–39. doi: 10.1023/a:1025806911782. [DOI] [PubMed] [Google Scholar]
- 41.Eads CA, Nickel AE, Laird PW. Complete genetic suppression of polyp formation and reduction of CpG-island hypermethylation in Apc(Min/+) Dnmt1-hypomorphic Mice. Cancer Res. 2002;62:1296–9. [PubMed] [Google Scholar]
- 42.Ehrlich M. DNA methylation in cancer: too much, but also too little. Oncogene. 2002;21:5400–13. doi: 10.1038/sj.onc.1205651. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto E, Toyota M, Suzuki H, Kondo Y, Sanomura T, Murayama Y, et al. LINE-1 hypomethylation is associated with increased CpG island methylation in Helicobacter pylori-related enlarged-fold gastritis. Cancer Epidemiol Biomarkers Prev. 2008;17:2555–64. doi: 10.1158/1055-9965.EPI-08-0112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.