Abstract
Background:
Long-acting opioids are a leading cause of accidental death in the United States, and methadone is associated with greater mortality rates. Whether this increase is related to the proarrhythmic properties of methadone is unclear.
Objective:
To describe methadone-associated arrhythmia events reported in the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS).
Design:
Description of national adverse event registry data before and after publication of a 2002 report describing an association between methadone and arrhythmia.
Setting:
FAERS, November 1997 and June 2011.
Patients:
Adults with QTc prolongation or torsade de pointes and ventricular arrhythmia or cardiac arrest.
Measurements:
FAERS reports before and after the 2002 report.
Results:
1646 cases of ventricular arrhythmia or cardiac arrest and 379 cases of QTc prolongation or torsade de pointes were associated with methadone. Monthly reports of QTc prolongation or torsade de pointes increased from a mean of 0.3 (95% CI, 0.1 to 0.5) before the 2002 publication to a mean of 3.5 (CI, 2.5 to 4.8) after it. After 2000, methadone was the second-most common primary suspect in cases of QTc prolongation or torsade de pointes after dofetilide (a known proarrhythmic drug) and was associated with disproportionate reporting similar to that of antiarrhythmic agents known to promote torsade de pointes. Antiretroviral drugs for HIV were the most common coadministered drugs.
Limitation:
Reports to FAERs are voluntary and selective, and incidence rates cannot be determined from spontaneously reported data.
Conclusion:
Since 2002, reports to FAERS of methadone-associated arrhythmia have increased substantially and are disproportionately represented relative to other events with the drug. Coadministration of methadone with antiretrovirals in patients with HIV may pose particular risk.
Primary Funding Source:
Colorado Clinical and Translational Sciences Institute, National Institutes of Health, and Agency for Healthcare Research and Quality.
Methadone is a long-acting synthetic opioid agonist prescribed in the United States for chronic pain and opioid dependency, and its use has increased by more than 500% in the past decade compared with the previous decade (1, 2). Methadone has been implicated in 30% to 40% of opioid-related deaths despite representing a minority of opioid prescriptions, and the mortality rate associated with it is substantially greater per morphine milligram equivalent than all other prescription opioids (1-5). Because drugs are now the second-leading cause of accidental death in U.S. adults and 40% are attributable to opioids (2), the U.S. Food and Drug Administration (FDA) convened a joint meeting of the Anesthetic and Life Support Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee in 2010 to discuss a risk evaluation and mitigation strategy (REMS) (1). Although opioid-related death is generally attributed to respiratory depression from overdose, methadone has been associated with QTc interval prolongation and torsade de pointes. Because of the disproportionate mortality rate of methadone compared with other opioids and the long elimination half-life (8 to 130 hours) and QTc-prolonging properties of methadone, a separate REMS for methadone was considered. However, the FDA ultimately adopted a single REMS for all long-acting opioids that did not outline strategies to reduce arrhythmia risk (1, 6).
An association between methadone and torsade de pointes was first described in 2002 (7) and subsequently found to be mediated by a blockade of the human Ether-à-go-go ion channel responsible for the delayed rectifier potassium current (8). A boxed warning was added to FDA labeling for methadone in 2006 about arrhythmia risk (9), followed by a clinical practice guideline in 2009 recommending QTc interval screening (10). Universal adoption of a cardiac safety program was recently recommended for all U.S. opioid treatment programs by the Substance Abuse and Mental Health Services Administration, but the need for QTc interval screening remained controversial (11). Debate about which strategies should be used to mitigate the increasing mortality rates associated with prescription opioids continues (12-14), and some have argued that QTc interval screening for methadone is unnecessary (15, 16). This lack of consensus is reflected in the limited use of opioid risk-reduction strategies, even among high-risk patients in primary care (17).
Given uncertainty about the clinical relevance of QTc prolongation and the frequency of arrhythmia events, we evaluated the FDA Adverse Event Reporting System (FAERS) database for a signal of disproportionate reporting of QTc prolongation or torsade de pointes associated with methadone relative to other opioids and antiarrhythmic drugs. We also examined whether methadone associated arrhythmia reports have increased after the initial publication of the association between methadone and torsade de pointes.
Methods
Data Extraction
After we received an exemption from the Colorado Multiple Institutional Review Board, publicly available data from FAERS between November 1997 and June 2011 were obtained (18). The FDA Adverse Event Reporting System is a relational database of spontaneous adverse event reports collected from patients, providers, and pharmaceutical companies through FDA-required postmarketing surveillance and the voluntary MedWatch submission system (19, 20). Reports submitted to FAERS are identified by an individual safety report number. When multiple individual safety reports refer to the same adverse event, they are linked using a unique case identifier. To minimize redundant counting, we quantified unique cases rather than reports because rare or life-threatening adverse events are often reported by several sources (21).
Drug names were matched to corresponding entries in the Drugs@FDA database using whole-word string matching (22). Once a drug name was matched, all active ingredients listed for the drug were linked to the corresponding adverse event report. For example, the drug names “methadone HCl,” “dolophine HCl,” and “methadone dispersible (orange flavored)” were all mapped to “methadone hydrochloride.” Comparative data sets were assembled for amiodarone hydrochloride, sotalol hydrochloride, and dofetilide, which are associated with torsade de pointes because of the same delayed rectifier potassium current blockade mechanism as methadone (23). Propoxyphene, a synthetic opioid recently withdrawn from the market due to QTc prolongation and dysrhythmia (24), was also analyzed. Morphine, oxycodone, and hydrocodone were assessed as opioid comparators not known to be associated with cardiac arrhythmia.
Adverse drug reactions are categorized in FAERS using the Medical Dictionary for Regulatory Activities, a standardized medical terminology developed by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. The MedDRA also aggregates related adverse events into greater levels of abstraction that have been previously shown to improve identification of signals of disproportionate reporting in FAERS (25). Terms of interest were QTc prolongation, torsade de pointes, ventricular arrhythmias, and cardiac arrest. Clinical indications for methadone were classified as “pain” or “opioid dependency” and role of methadone as “primary suspect,” “secondary suspect,” “interacting,” or “concomitant,” as reported to FAERS. Data integration was done using MySQL Server, version 5.5.18 (Oracle Corporation, Redwood Shores, California).
Statistical Analysis
Because QTc prolongation is the requisite substrate for developing torsade de pointes (26), QTc prolongation or torsade de pointes was combined for analysis. Similarly, ventricular arrhythmias or cardiac arrest was combined using the MedDRA hierarchy. Where data were unambiguously reported, summary statistics for age and dose were generated and frequencies for sex, indication, outcome, and drug role in the adverse event were calculated. Reporting frequency and monthly reporting rates were aggregated before and after the 2002 publication describing the association. Because analysis of raw counts from spontaneous reporting databases may be biased by many factors, we also calculated the proportional reporting ratio (PRR) to identify disproportionate reporting of specific adverse drug reactions. The PRR is calculated by dividing the fraction of reports involving the reaction of interest for a given drug by the fraction of reports involving the reaction of interest for all other drugs (27, 28). Greater PRR values suggest that the adverse event of interest is reported disproportionately compared with all other reactions for that drug. The PRR, including the 95% CI, was calculated for ventricular arrhythmias or cardiac arrest and QTc prolongation or torsade de pointes for methadone, other opioids, and the antiarrhythmic agents. Counts for all comparator drugs were included in the denominator for each calculation to provide the most conservative PRR estimate. In accordance with previously derived and validated empirical criteria (28-30), a PRR was considered indicative of disproportionate reporting if it was 2 or greater, its chi-square value was 4 or greater, and at least 3 unequivocal reports were documented. Statistical analyses were done using SAS, version 9.3 (SAS Institute, Cary, North Carolina).
Role of the Funding Source
This study was funded by the Colorado Clinical and Translational Sciences Institute, National Institutes of Health, and Agency for Healthcare Research and Quality. The funding source was not involved in the design, conduct, or reporting of the study or in the decision to submit the manuscript for publication.
Results
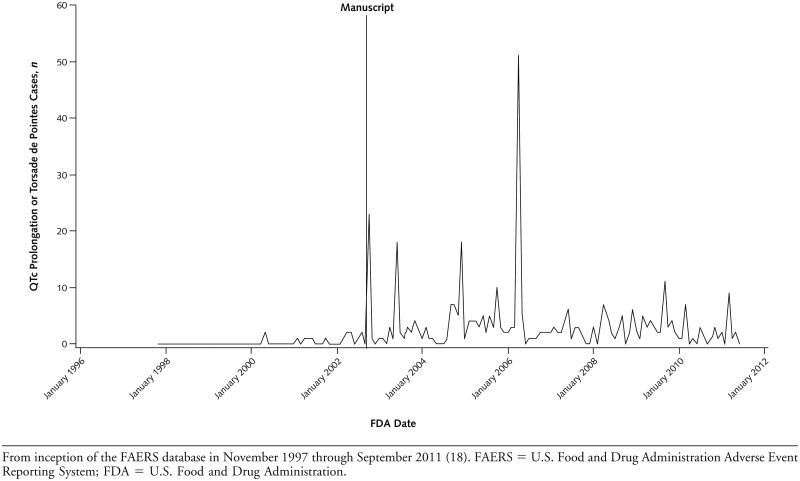
A total of 3 331 270 FAERS cases, 11 015 of which involved methadone hydrochloride, were available from 1997 to 2011. Of methadone-associated adverse events, 1646 (14.9%) involved ventricular arrhythmia or cardiac arrest and 379 (3.4%) involved QTc prolongation or torsade de pointes. After 2002, ventricular arrhythmia or cardiac arrest was the most common category of adverse event associated with methadone. The mean number of monthly reports of QTc prolongation or torsade de pointes between November 1997 and September 2002 was 0.3 (95% CI, 0.1 to 0.5), which increased to 3.5 (CI, 2.5 to 4.8) from October 2002 to June 2011 (Figure).
Figure.
Trends in monthly adverse event reporting of methadone-associated QTc prolongation or torsade de pointes.
The demographic and clinical characteristics of persons with ventricular arrhythmia or cardiac arrest and QTc prolongation or torsade de pointes are summarized in Table 1. The median methadone dose among 208 QTc prolongation or torsade de pointes cases with available data was 150 mg/d (interquartile range [IQR], 90 to 360 mg/ d), and QTc prolongation or torsade de pointes was more commonly reported among persons receiving methadone for opioid dependency (28.0%) than among those treated for pain (10.8%). Concomitant medications most frequently cited in methadone-associated cases of QTc prolongation or torsade de pointes were antiretroviral drugs for HIV and benzodiazepines (Table 2).
Table 1.
Patient Characteristics*
| Characteristic | Ventricular Arrhythmia or Cardiac Arrest (n = 1646) |
QTc Prolongation or Torsade de Pointes† (n = 379) |
|---|---|---|
| Mean age (SD), y‡ | 37.0 (14.0) | 44.0 (12.0) |
| Sex | ||
| Male | 574 (34.9) | 202 (53.3) |
| Female | 407 (24.7) | 155 (40.9) |
| Unspecified | 665 (40.4) | 22 (5.8) |
| Methadone as primary suspect§ | 678 (41.2) | 217 (57.3) |
| Median methadone dose (IQR), mg/d|| |
112.5 (45–270) | 150 (90–360) |
| Methadone indication | ||
| Pain | 52 (3.2) | 41 (10.8) |
| Opioid dependency | 107 (6.5) | 106 (28.0) |
| Outcome | ||
| Death | 1333 (80.9) | 42 (11.1) |
| Life-threatening outcome | 143 (8.6) | 118 (31.1) |
| Hospitalization | 254 (15.4) | 189 (49.9) |
| Disability | 4 (0.2) | 4 (1.1) |
| Required intervention | 40 (2.4) | 36 (9.5) |
IQR = interquartile range.
Values are numbers (percentages) unless otherwise indicated. Percentages may not sum to 100 due to rounding.
Term preferred in the Medical Dictionary for Regulatory Activities.
Available data for 1546 patients with ventricular arrhythmia or cardiac arrest and 330 patients with QTc prolongation or torsade de pointes.
The U.S. Food and Drug Administration Adverse Event Reporting System defines "primary suspect" as the first drug that the initial reporter deemed most likely to be associated with the reactions.
Available data for 240 patients with ventricular arrhythmia or cardiac arrest and 208 patients with QTc prolongation or torsade de pointes.
Table 2.
The Top 10 Most Frequently Reported Concomitant Medications for QTc Prolongation or Torsade de Pointes
| Medication | QTc Prolongation or Torsade de Pointes, n (%) |
|---|---|
| Lamivudine | 41 (10.8) |
| Ritonavir | 39 (10.3) |
| Zidovudine | 37 (9.8) |
| Lorazepam | 27 (7.1) |
| Morphine sulfate | 27 (7.1) |
| Apomorphine hydrochloride | 25 (6.6) |
| Tenofovir disoproxil fumarate | 22 (5.8) |
| Lopinavir | 21 (5.5) |
| Trimethoprim | 21 (5.5) |
| Ceftriaxone sodium | 20 (5.3) |
| Oxazepam | 20 (5.3) |
Death was reported in 41.9% of all methadone-related adverse events. Death occurred in 80.9% of ventricular arrhythmia or cardiac arrest cases and 11.1% of cases involving QTc prolongation or torsade de pointes. The most common primary suspects among all reports of QTc prolongation or torsade de pointes were dofetilide (n = 359), methadone (n = 211), and sotalol (n = 119) between the years 2000 and 2011 when all 3 drugs were available (dofetilide came on the market in 2000). The PRR for methadone and QTc prolongation or torsade de pointes from 1997 to 2011 was well above the significance threshold conventionally assigned to the measure (PRR, 11.2 [CI, 10.2 to 12.4]; events of interest/total events, 379/ 11 015) and similar to that for amiodarone (PRR, 10.5 [CI, 9.7 to 11.4]; 639/20 239), sotalol (PRR, 18.7 [CI, 16.7 to 21.0]; 294/5088), and dofetilide (PRR, 36.6 [CI, 33.4 to 40.2]; 415/3720). No signal of disproportionate reporting of QTc prolongation or torsade de pointes was observed for morphine (PRR, 1.8 [CI, 1.6 to 2.1]; 195/33 758), oxycodone (PRR, 0.95 [CI, 0.8 to 1.1]; 165/ 54 663), hydrocodone (PRR, 1.1 [CI, 0.9 to 1.3]; 164/ 47 596), or propoxyphene (PRR, 1.7 [CI, 1.4 to 2.1]; 87/16 118) during the study.
DISCUSSION
The number of methadone-associated arrhythmia reports has increased over the past decade after publication of a paper describing the association between high-dose methadone and torsade de pointes. Ventricular arrhythmia and cardiac arrest are currently the most frequent methadone-attributed adverse events reported to the FDA and have increased in parallel with the more specific reports of QTc prolongation and torsade de pointes. This increased reporting frequency may reflect heightened clinician awareness after the initial publication in 2002 of the association between methadone and arrhythmia, but we speculate that it may also reflect additional factors, such as increased prescribing, greater arrhythmia incidence, and clinicians attributing methadone-related deaths to a cardiac rather than a respiratory cause. Although the precise contribution of reporting factors cannot be determined from this analysis, we believe that these results suggest that arrhythmia represents an important but underappreciated contributor to methadone-related morbidity and mortality.
Methadone was also associated with a disproportionate signal of QTc prolongation or torsade de pointes reporting similar to that of drugs with established proarrhythmic properties, whereas the PRR for propoxyphene, which was recently withdrawn from the market due to QTc prolongation and dysrhythmia, was not. Although most opioids are not associated with QTc prolongation, a prospective, randomized trial demonstrated a 34-millisecond mean increase from baseline in QTc interval with methadone maintenance therapy (31). Recent simulation and modeling analysis of 5 clinical trials by FDA investigators determined that methadone may increase the mean QTc interval by as much as 20 milliseconds at doses exceeding 120 mg per day in patients who are opioid-dependent (32). These effects of methadone on cardiac repolarization approximate the expected 10- to 40-millisecond increase in mean QTc interval with sotalol (33).
In the present study, arrhythmia was more commonly reported among persons receiving methadone for opioid dependency. This may be attributable to the greater doses in this population, which magnifies the degree of QTc prolongation (10, 32). In addition, patients who are opioid-dependent are frequently exposed to illicit substances, including alcohol and cocaine, which also increase the QTc interval (34, 35). Finally, many patients who are opioid-dependent also have HIV, and we saw that reports of QTc prolongation or torsade de pointes with methadone most often occurred in association with antiretroviral therapy. This finding is corroborated by emerging evidence associating HIV with both QTc prolongation and sudden cardiac death (36-38). In addition, nearly all reported cases of torsade de pointes in patients with HIV involve methadone, antiretroviral therapy, or both (39-41).
This study has many limitations. Methadone-associated adverse events reported to FAERS are voluntary, and actual incidence cannot be inferred from these data. Furthermore, comparisons of adverse event rates using either traditional report counts or measures of disproportionate reporting, such as the PRR, can be artificially inflated or deflated by many factors, including reporting bias, absolute report number, and the presence of other adverse events associated with the same drug. Although disproportionate reporting measures are generally less biased than comparisons of report counts (42), measures based on reporting ratios, such as the PRR, can be particularly vulnerable to confounding factors and are, therefore, less reliable when the number of reports is low (<3 reports) (43). At the minimum volume of reports in the present analysis (87 for propoxyphene), the PRR performs similarly to other pharmacovigilance algorithms (29). However, because increases in adverse event reporting do not necessarily indicate increases in incidence, signals identified by any pharmacovigilance algorithm are suggestive of association only, and causality cannot be established without systematic review of clinical cases.
The FAERS database has many limitations. First, it relies on spontaneous clinician reports without independent adjudication. In particular, QTc prolongation could have been inaccurately assessed (44), and retrieval of primary clinical data from the FAERS for validation was not possible. In addition, several adverse events may be reported in each case, leading to potential overlap. Drug names may be brand or generic and contain formatting errors that limit the identification of all cases. To address this, whole-word string matching between FAERS names and all brand and generic designations in the Drugs@FDA database was used (21), and cases were aggregated by active ingredient. However, we made no attempt to address misspellings, and partial words were excluded to maximize specificity. This conservative approach to data extraction may have resulted in an underestimated reporting rate for adverse events associated with methadone and comparator medications.
In general, clinicians underreport medication-related adverse events to the FDA (45), which is perhaps the most important limitation of pharmacovigilance studies using spontaneous report databases. In addition, the high mortality rate among methadone-associated adverse events leads to inherent diagnostic uncertainty. Since cause cannot be accurately categorized after death, observational studies may characterize only a fraction of methadone-associated sudden cardiac deaths. In support of this, 1 postmortem study suggested that 40% of methadone-related deaths could be arrhythmic in origin given therapeutic methadone levels and the absence of structural heart disease (46). Finally, although provider awareness is improving as evidenced herein by increased reporting since 2002, persistent knowledge gaps about the proarrhythmic properties of methadone (47) probably further contributes to underreporting.
In summary, ventricular arrhythmia and cardiac arrest are now the most frequent FDA-reported class of methadone-associated adverse events, and reports of QTc prolongation and torsade de pointes have increased sharply in the past decade. Because methadone is now considered an essential medication by the World Health Organization (48), use is likely to increase, creating broader exposure to the proarrhythmic properties. Because cardiac arrhythmia is not associated with other opioids currently marketed in the United States, we propose that a REMS specific to methadone and designed to reduce arrhythmia may be appropriate, acknowledging the 9 July 2012 FDA announcement that it would not require universal physician training on long-acting opioid safety (6) that overrode advice from the 2010 Drug Safety and Risk Management Advisory Committee (1). Therefore, for the time being, the potential to improve the cardiac safety of methadone among current prescribing physicians remains limited.
Context.
Methadone is associated with QTc interval prolongation and torsade de pointes.
Contribution
This review of U.S. Food and Drug Administration (FDA) adverse event reporting data between 1997 and 2011 found that the number of reports of QTc prolongation and torsade de pointes associated with methadone was similar to that for other highly proarrhythmic drugs, such as dofetilide. The reports were especially common for patients taking antiretroviral drugs for HIV.
Caution
Reporting to the FDA is voluntary and selective.
Implication
Reports to the FDA suggest that methadone-associated arrhythmia is more common than recognized and may pose special risk for patients with HIV receiving highly active antiretroviral therapy.
—The Editors
Acknowledgments
Grant Support: Data management and statistical analysis was supported by the Colorado Clinical and Translational Sciences Institute. Dr. Kao is supported by the National Institutes of Health (grant 2 T32 HL007822-12), a grant from the Leaffer family, and the University of Colorado Center for Women’s Health Research. Dr. Krantz is partially supported by the Agency for Healthcare Research and Quality (grant 1 P01 HS021138-01).
Footnotes
Current Author Addresses: Drs. Kao and Katz: 12700 East 19th Avenue, Box B-139, Division of Cardiology, University of Colorado School of Medicine, Aurora, CO 80045. Drs. Bucher Bartelson and Dart and Ms. Khatri: Rocky Mountain Poison and Drug Center Unit 29, 990 Bannock Street, Denver, CO 80204. Dr. Mehler: Denver Health Medical Center, 660 Bannock Street, MC 0960, Denver, CO 80204. Dr. Krantz: Denver Health Medical Center, 777 Bannock Street, MC 0960, Denver, CO 80204.
Potential Conflicts of Interest: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum =M12-2647.
Reproducible Research Statement: Study protocol: Not available. Statistical code: Available from Dr. Krantz (mori.krantz@dhha.org). Data set: All FAERS data are publically available.
References
- 1.Risk Evaluation and Mitigation Strategies (REMS) for Extended-Release and Long-Acting Opioid Analgesics. Joint Meeting of the Anesthetic and Life Support Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee; Adelphi, Maryland: Jul 22-23, 2010. Accessed at www.fda .gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ AnestheticAndLifeSupportDrugsAdvisoryCommittee/UCM217510.pdf on 15 April 2012. [Google Scholar]
- 2.Warner M, Chen LH, Makuc DM, Anderson RN, Mininño AM. Drug poisoning deaths in the United States, 1980-2008. NCHS Data Brief. 2011:1–8. [PMID: 22617462] [PubMed] [Google Scholar]
- 3.Paulozzi LJ, Logan JE, Hall AJ, McKinstry E, Kaplan JA, Crosby AE. A comparison of drug overdose deaths involving methadone and other opioid analgesics in West Virginia. Addiction. 2009;104:1541–8. doi: 10.1111/j.1360-0443.2009.02650.x. [PMID: 19686524] [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Vital signs: risk for overdose from methadone used for pain relief—United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2012;61:493–7. [PMID: 22763888] [PubMed] [Google Scholar]
- 5.Hall AJ, Logan JE, Toblin RL, Kaplan JA, Kraner JC, Bixler D, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613–20. doi: 10.1001/jama.2008.802. [PMID: 19066381] [DOI] [PubMed] [Google Scholar]
- 6.U.S. Food and Drug Administration FDA introduces new safety measures for extended-release and long-acting opioid medications [news release] 2012 Jul 9; Accessed at www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm310870.htm on 9 July 2012.
- 7.Krantz MJ, Lewkowiez L, Hays H, Woodroffe MA, Robertson AD, Mehler PS. Torsade de pointes associated with very-high-dose methadone. Ann Intern Med. 2002;137:501–4. doi: 10.7326/0003-4819-137-6-200209170-00010. [PMID: 12230351] [DOI] [PubMed] [Google Scholar]
- 8.Katchman AN, McGroary KA, Kilborn MJ, Kornick CA, Manfredi PL, Woosley RL, et al. Influence of opioid agonists on cardiac human ether-a-go-gorelated gene K(+) currents. J Pharmacol Exp Ther. 2002;303:688–94. doi: 10.1124/jpet.102.038240. [PMID: 12388652] [DOI] [PubMed] [Google Scholar]
- 9.Roxane Laboratories . Dolophine hydrochloride [package insert] Roxane Laboratories; Columbus, OH: 2006. Accessed at www.accessdata.fda.gov/drugsatfda_docs/label/2006/006134s028lbl.pdf on 15 April 2012. [Google Scholar]
- 10.Krantz MJ, Martin J, Stimmel B, Mehta D, Haigney MC. QTc interval screening in methadone treatment. Ann Intern Med. 2009;150:387–95. doi: 10.7326/0003-4819-150-6-200903170-00103. [PMID: 19153406] [DOI] [PubMed] [Google Scholar]
- 11.Martin JA, Campbell A, Killip T, Kotz M, Krantz MJ, Kreek MJ, et al. Substance Abuse and Mental Health Services Administration QT interval screening in methadone maintenance treatment: report of a SAMHSA expert panel. J Addict Dis. 2011;30:283–306. doi: 10.1080/10550887.2011.610710. [PMID: 22026519] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Office of National Drug Control Policy Epidemic: Responding to America’s Prescription Drug Abuse Crisis. 2011 Accessed at www.whitehouse.gov/sites/default/files/ondcp/issues-content/prescription-drugs/rx_abuse_plan.pdf on 15 April 2012.
- 13.Bohnert AS, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315–21. doi: 10.1001/jama.2011.370. [PMID: 21467284] [DOI] [PubMed] [Google Scholar]
- 14.Von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann Intern Med. 2011;155:325–8. doi: 10.1059/0003-4819-155-5-201109060-00011. [PMID: 21893626] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cruciani RA. Methadone: to ECG or not to ECG ... That is still the question. J Pain Symptom Manage. 2008;36:545–52. doi: 10.1016/j.jpainsymman.2007.11.003. [PMID: 18440771] [DOI] [PubMed] [Google Scholar]
- 16.Gourevitch MN. First do no harm ... Reduction? [Editorial] Ann Intern Med. 2009;150:417–8. doi: 10.7326/0003-4819-150-6-200903170-00111. [PMID: 19225161] [DOI] [PubMed] [Google Scholar]
- 17.Starrels JL, Becker WC, Weiner MG, Li X, Heo M, Turner BJ. Low use of opioid risk reduction strategies in primary care even for high risk patients with chronic pain. J Gen Intern Med. 2011;26:958–64. doi: 10.1007/s11606-011-1648-2. [PMID: 21347877] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Food and Drug Administration, Center for Drug Evaluation and Research The FDA Adverse Event Reporting System (AERS): Latest Quarterly Data Files. 2012 Accessed at www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm082193.htm on 15 April 2012.
- 19.U.S. Food and Drug Administration MedWatch Online Voluntary Reporting Form (3500) 2012 Accessed at www.accessdata.fda.gov/scripts/medwatch/medwatch-online.htm on 17 December 2012.
- 20.U.S. Food and Drug Administration Postmarketing reporting of adverse drug experiences. 21 CFR §314.80. Revised 1 April 2012. Accessed at www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=314.80 on 18 September 2012.
- 21.Kao DP, Hiatt WR, Krantz MJ. Proarrhythmic potential of dronedarone: emerging evidence from spontaneous adverse event reporting. Pharmacotherapy. 2012;32:767–71. doi: 10.1002/j.1875-9114.2012.01118.x. [PMID: 22744806] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S. Food and Drug Administration Drugs@FDA Data files. Accessed at www.fda.gov/Drugs/InformationOnDrugs/ucm079750.htm on 15 April 2012.
- 23.Riera AR, Uchida AH, Ferreira C, Ferreira Filho C, Schapachnik E, Dubner S, et al. Relationship among amiodarone, new class III antiarrhythmics, miscellaneous agents and acquired long QT syndrome. Cardiol J. 2008;15:209–19. [PMID: 18651412] [PubMed] [Google Scholar]
- 24.Food and Drug Administration Public Health Service, U.S. Department of Health and Human Services Food and Drug Administration recommends against the continued use of propoxyphene. J Pain Palliat Care Pharmacother. 2011;25:80–2. doi: 10.3109/15360288.2010.549553. [PMID: 21426228] [DOI] [PubMed] [Google Scholar]
- 25.Pearson RK, Hauben M, Goldsmith DI, Gould AL, Madigan D, O’Hara DJ, et al. Influence of the MedDRA hierarchy on pharmacovigilance data mining results. Int J Med Inform. 2009;78:e97–e103. doi: 10.1016/j.ijmedinf.2009.01.001. [PMID: 19230751] [DOI] [PubMed] [Google Scholar]
- 26.Roden DM, Woosley RL, Primm RK. Incidence and clinical features of the quinidine-associated long QT syndrome: implications for patient care. Am Heart J. 1986;111:1088–93. doi: 10.1016/0002-8703(86)90010-4. [PMID: 3716982] [DOI] [PubMed] [Google Scholar]
- 27.Alvarez Y, Hidalgo A, Maignen F, Slattery J. Validation of statistical signal detection procedures in eudravigilance post-authorization data: a retrospective evaluation of the potential for earlier signalling. Drug Saf. 2010;33:475–87. doi: 10.2165/11534410-000000000-00000. [PMID: 20486730] [DOI] [PubMed] [Google Scholar]
- 28.Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001;10:483–6. doi: 10.1002/pds.677. [PMID: 11828828] [DOI] [PubMed] [Google Scholar]
- 29.Johnson K, Guo C, Gosink M, Wang V, Hauben M. Multinomial modeling and an evaluation of common data-mining algorithms for identifying signals of disproportionate reporting in pharmacovigilance databases. Bioinformatics. 2012;28:3123–30. doi: 10.1093/bioinformatics/bts576. [PMID: 23064001] [DOI] [PubMed] [Google Scholar]
- 30.Hauben M, Horn S, Reich L. Potential use of data-mining algorithms for the detection of ‘surprise’ adverse drug reactions. Drug Saf. 2007;30:143–55. doi: 10.2165/00002018-200730020-00004. [PMID: 17253879] [DOI] [PubMed] [Google Scholar]
- 31.Wedam EF, Bigelow GE, Johnson RE, Nuzzo PA, Haigney MC. QT-interval effects of methadone, levomethadyl, and buprenorphine in a randomized trial. Arch Intern Med. 2007;167:2469–75. doi: 10.1001/archinte.167.22.2469. [PMID: 18071169] [DOI] [PubMed] [Google Scholar]
- 32.Florian J, Garnett CE, Nallani SC, Rappaport BA, Throckmorton DC. A modeling and simulation approach to characterize methadone QT prolongation using pooled data from five clinical trials in MMT patients. Clin Pharmacol Ther. 2012;91:666–72. doi: 10.1038/clpt.2011.273. [PMID: 22378153] [DOI] [PubMed] [Google Scholar]
- 33.Betapace (sotalol HCl) [package insert] Bayer HealthCare Pharmaceuticals; Wayne, NJ: 2010. Accessed at www.accessdata.fda.gov/drugsatfda_docs/label/2011/019865s019lbl.pdf on 29 June 2012. [Google Scholar]
- 34.Hillebrand J, Marsden J, Finch E, Strang J. Excessive alcohol consumption and drinking expectations among clients in methadone maintenance. J Subst Abuse Treat. 2001;21:155–60. doi: 10.1016/s0740-5472(01)00198-2. [PMID: 11728789] [DOI] [PubMed] [Google Scholar]
- 35.Krantz MJ, Rowan SB, Mehler PS. Cocaine-related torsade de pointes in a methadone maintenance patient. J Addict Dis. 2005;24:53–60. doi: 10.1300/J069v24n01_05. [PMID: 15774410] [DOI] [PubMed] [Google Scholar]
- 36.Reinsch N, Buhr C, Krings P, Kaelsch H, Neuhaus K, Wieneke H, et al. German Heart Failure Network. Prevalence and risk factors of prolonged QTc interval in HIV-infected patients: results of the HIV-HEART study. HIV Clin Trials. 2009;10:261–8. doi: 10.1310/hct1004-261. [PMID: 19723613] [DOI] [PubMed] [Google Scholar]
- 37.Sani MU, Okeahialam BN. QTc interval prolongation in patients with HIV and AIDS. J Natl Med Assoc. 2005;97:1657–61. [PMID: 16396057] [PMC free article] [PubMed] [Google Scholar]
- 38.Tseng ZH, Secemsky EA, Dowdy D, Vittinghoff E, Moyers B, Wong JK, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59:1891–6. doi: 10.1016/j.jacc.2012.02.024. [PMID: 22595409] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castillo R, Pedalino RP, El-Sherif N, Turitto G. Efavirenz-associated QT prolongation and torsade de pointes arrhythmia. Ann Pharmacother. 2002;36:1006–8. doi: 10.1345/aph.1A454. [PMID: 12022902] [DOI] [PubMed] [Google Scholar]
- 40.Falconer M, Molloy D, Ingerhaug J, Barry M. Methadone induced torsade de pointes in a patient receiving antiretroviral therapy. Ir Med J. 2007;100:631–2. [PMID: 18277734] [PubMed] [Google Scholar]
- 41.Kocheril AG, Bokhari SA, Batsford WP, Sinusas AJ. Long QTc and torsades de pointes in human immunodeficiency virus disease. Pacing Clin Electro-physiol. 1997;20:2810–6. doi: 10.1111/j.1540-8159.1997.tb05439.x. [PMID: 9392812] [DOI] [PubMed] [Google Scholar]
- 42.Waller P. Dealing with uncertainty in drug safety: lessons for the future from sertindole. Pharmacoepidemiol Drug Saf. 2003;12:283–7. doi: 10.1002/pds.849. [PMID: 12812007] [DOI] [PubMed] [Google Scholar]
- 43.Moore N, Hall G, Sturkenboom M, Mann R, Lagnaoui R, Begaud B. Biases affecting the proportional reporting ratio (PPR) in spontaneous reports pharmacovigilance databases: the example of sertindole. Pharmacoepidemiol Drug Saf. 2003;12:271–81. doi: 10.1002/pds.848. [PMID: 12812006] [DOI] [PubMed] [Google Scholar]
- 44.Viskin S, Rosovski U, Sands AJ, Chen E, Kistler PM, Kalman JM, et al. Inaccurate electrocardiographic interpretation of long QT: the majority of physicians cannot recognize a long QT when they see one. Heart Rhythm. 2005;2:569–74. doi: 10.1016/j.hrthm.2005.02.011. [PMID: 15922261] [DOI] [PubMed] [Google Scholar]
- 45.McAdams M, Staffa J, Dal Pan G. Estimating the extent of reporting to FDA: a case study of statin-associated rhabdomyolysis. Pharmacoepidemiol Drug Saf. 2008;17:229–39. doi: 10.1002/pds.1535. [PMID: 18175291] [DOI] [PubMed] [Google Scholar]
- 46.Chugh SS, Socoteanu C, Reinier K, Waltz J, Jui J, Gunson K. A community-based evaluation of sudden death associated with therapeutic levels of methadone. Am J Med. 2008;121:66–71. doi: 10.1016/j.amjmed.2007.10.009. [PMID: 18187075] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krantz MJ, Rowan SB, Schmittner J, Bucher Bartelson B. Physician awareness of the cardiac effects of methadone: results of a national survey. J Addict Dis. 2007;26:79–85. doi: 10.1300/J069v26n04_10. [PMID: 18032235] [DOI] [PubMed] [Google Scholar]
- 48.World Health Organization WHO Model Lists of Essential Medicines. Accessed at www.who.int/medicines/publications/essentialmedicines/en/index.html on 21 June 2012.