Abstract
Clinical reports indicate that some infected individuals control HIV-1 replication through undefined mechanisms. Our group reported that human protein named X-DING-CD4 holds a potent antiviral activity blocking transcription of HIV-1 LTR through inhibition of NF-κB/DNA binding. Based on observations that transformed HIV-1 resistant CD4+T cells produce higher levels of soluble X-DING-CD4 protein upon their exposure to virus, we hypothesized that resistance to HIV-1 in these cells might be regulated through function of X-DING-CD4 gene. Real time PCR evaluations of X-DING-CD4 mRNA expression confirmed our hypothesis; HIV-1 exposure caused rapid up-regulation of X-DING-CD4 mRNA in resistant but not susceptible cells; and the burst of X-DING-CD4 mRNA expression correlated with restriction of HIV-1 transcription. Subsequently, we examined the activity of X-DING-CD4 gene in monocytes and macrophages from (n=13) HIV-negative donors. The assessment of HIV-1 gag mRNA showed that majority of cells were permissive to virus replication; however, macrophages from four donors were refractory to HIV-1 infection. In response to virus these cells up-regulated the X-DING-CD4 gene expression by 2 to 1000-fold. This data provide evidence that X-DING-CD4 gene contributes to early cellular protection from HIV infection in some individuals and this protection depends solely on the unique genetic regulation of the host.
The phenomenon of natural resistance to HIV challenged researchers for many years now. In time it became clear that the long time nonprogressors (LTNPs) and particularly the subset of this group – the elite controllers (ECs), effectively suppress virus replication through the undefined mechanism(s). Despite of being infected with the replication competent virus 1-3, the ECs maintain stable CD4+ T cell counts and frequently are naïve to the antiviral treatments 4, 5. The HIV target cells from ECs are susceptible to virus entry and productive infection 6 but gene expression signatures, although indistinguishable from the antiretroviral therapy (ART)-treated patients, are significantly different from those in HIV negative individuals 7. This indicates that the resistance to HIV in the ECs is not related to cell physiology or defects in the infecting virus, but to genetic regulation resulting in the intrinsic control of the pathogen.
We reported recently the isolation and identification of a novel human protein, the X-DING-CD4 (extracellular DING from CD4 T cells) that has potent anti-HIV-1 activity 8 and (GenBank [HQ586056]). The X-DING-CD4 is a soluble protein found in supernatant of transformed HIV-1 resistant CD4+ T cells in two isomeric forms distinguished by rare methylation of glutamic acid 8. The X-DING-CD4 protein exerts the anti-HIV-1 protective activity upon target cells through receptor independent pathway blocking LTR/NF-κB binding 8-11. The inducibility of X-DING-CD4 protein in response to the virus challenge observed in our CD4+ T cell - model of HIV-1 resistance 10 suggested that this intrinsic response to virus infection might be regulated through the activity of the X-DING-CD4 gene. As the current reports on virus restriction in ECs indicate that their resistance to virus might be a consequence of the unique genetic regulation, we hypothesized that association of the inducible X-DING-CD4 gene activity and virus restriction could be a key to understanding the HIV restriction in ECs.
To evaluate this proposition we employed a quantitative real time PCR (qRT-PCR) for analysis of the X-DING-CD4 gene activity in transformed CD4+ T cells and primary human monocytes and macrophages exposed or not to HIV-1. The goal of this study was to observe the correlation between the X-DING-CD4 gene activity and restriction of HIV-1 transcription. First we employed our HIV-1 resistant T cell model to measure kinetics of X-DING-CD4 mRNA expression and to establish experimental parameters for the subsequent analysis of this gene expression in primary human cells. Then we evaluated the expression of X-DING-CD4 gene in naïve monocytes and macrophages derived from thirteen HIV negative individuals; we established an interquartile range (IQR) for the X-DING-CD4 mRNA expression in our donor cells in the absence and presence of virus exposure; and finally we correlated the X-DING-CD4 activity in the virus exposed human macrophages to HIV-1 gag mRNA transcription rate at the early stage of infection.
Materials and methods
Cell cultures, viruses and reagents
HIV-1-resistant cells called here X-DING-CD4(+) have been described previously by name HRF(+) 9-15; these cells express and secrete the X-DING-CD4 protein upon their exposure to HIV-1 8. The control HIV-1 susceptible cells used for this research were CEM 16 obtained from the American Type Culture Collection (Rockville). CEM tested negative for the production of the soluble X-DING-CD4 protein (not shown) and based on this evidence we annotated this control cell system as X-DING-CD4(−). The continuous cultures of X-DING-CD4(+) or control X-DING-CD4(−) CEM cells were maintained in RPMI 1640 (Sigma) supplemented with 5%/vol fetal bovine serum, antibiotics and glutamine at 37°C in a 5% CO2 95% air-humidified incubator.
Human monocytes from (n=13) healthy, HIV-1 negative volunteers were obtained by elutriation from the whole blood; the detail culture conditions are described in the section below. The studies reported here using human peripheral blood monocytes were granted exempt status by Institutional Review Board under qualifications listed in section 45.101 (b) (4).
Testing the expression of X-DING-CD4 and HIV-1 mRNAs in transformed and primary cells
For X-DING-CD4 and HIV-1 vif and gag mRNA or infectivity studies the X-DING-CD4(+) and control X-DING-CD4(−) cells were exposed to NL4-3 HIV-1 isolate 17, while the primary human macrophages were infected with R5 HIV-1-ADA 18 or NL4-3 HIV-1, all at MOI of 0.01. After 1 hour incubation at 37°C, virus was removed by wash in ice cold PBS. Infected cells were cultured in the respective media as described above and below and at the designated time-points cells were harvested for RNA isolation and evaluations of viral proteins by immunofluorescence (IF) and Elisa. The IF staining of HIV-1 infected transformed cells was performed with pooled serum from AIDS patients (gift from Dr. Volsky) followed by goat anti-human FITC antibody (Santa Cruz Biotechnology, Inc.). The concentration of HIV-1 p24 core antigen in supernatants of HIV-1 infected cells was established by Elisa (Perkin Elmer).
For testing the expression of X-DING-CD4 mRNA in primary cells each 5×106 human monocytes were selected upon arrival to the laboratory, pelleted by centrifugation and suspended in Trizol reagent (Invitrogen) for RNA isolation. This sample represented the mitogen and HIV-1 naïve monocytes. The remaining monocytes were allowed to differentiate into macrophages as described before 13. Briefly: cells were seeded into 6-well plates (Corning Scientific) at the concentration of 5×106/well in the Dulbeco's modified Eagle's medium (DMEM) (Sigma) supplemented with 10%/vol endotoxin-free, heat inactivated human serum (Cambrex), 10%/vol giant cell tumor conditioned medium (BioVertis), 2mM glutamine and antibiotics. Cells were cultured for 5-7 days until fully adherent, thereafter they were cultured in DMEM with 10%/vol endotoxin free fetal bovine serum (FBS), glutamine and antibiotics. Based on previous examinations of gene expression profiles in human macrophages cultured under the same conditions 19 we are confident that cells were fully differentiated. After change of the medium cells were cultured for another 24 hours and then exposed or not to HIV-1. Samples for RNA analysis were collected in the 24-hour intervals; the uninfected macrophages cultured along the experimental time line were collected at the respective time points to observe the background X-DING-CD4 mRNA expression.
The total RNA was isolated using Trizol reagent (Invitrogen) according to the manufacturer's protocol. The cDNA was prepared from 2μg RNA sample pretreated with DNAse I. For cDNA synthesis we used the Transcriptor First Strand cDNA Synthesis kit (Roche) applying experimental conditions that we published before 20. The reverse primer was a mixture of 60μM random hexamers and 2.5μM X-DING-CD4 gene specific primer (Table 1).
Table 1.
Primers and probes used for qRT-PCR analysis and cDNA synthesis.
| Name | Primers | Probe |
|---|---|---|
|
| ||
| X-DING-CD4 | DING_F_Q: 5′-CACCGGCACCAAGAACGT-3′ DING_R_Q: 5′-GACAGTTCAGTCGAGGTCAGCTT-3′ |
DING_P_Q: 5′-6FAM-CGGGCAGCGACTC-MGBNFQ-3′ |
| VIFsp | VIF_F_Q: 5′-CGCGCACGGCAAGAG-3′ VIF_R_Q: 5′-CTTTGCTTTTCTTCTTGGCACTACT-3′ |
VIF_P_Q: 5′- 6FAM-AGTAGTAATACAAGATAATAGTGA-MGBNFQ-3′ |
| GAG | GAG_F_Q: 5′-GGACCAGGAGCGACACTAGAA-3′ GAG_R_Q: 5′- CAGCCAAAACTCTTGCTTTATGG-3′ |
GAG_P_Q: 6FAM-TGATGACAGCATGTCAGGGAGTGG-MGBNFQ-3′ |
| X-DING-CD4 primer for cDNA synthesis | TCGTTCAGCTTGGTCATCACGCCTTGGCTGGTCACGGC | |
Quantitative Real time PCR (qRT-PCR) analysis
The Q-PCR primers and probes were designed based on the X-DING-CD4 sequence (GenBank [HQ586056]) and HIV-1 NL4-3 gag and single spliced vif mRNA sequence; all primers were synthesized by Invitrogen; probes were synthesized by Applied Biosystems, and all sequences are listed in Table 1. The real-time detection of each tested mRNA expression was performed through the absolute quantification protocol using TaqMan Gene Expression Assay on 7300 realtime PCR System (Applied Biosystems). Briefly; triplicates of total reaction mixture of 25μl were composed of 2μl of cDNA, 12.5μl of 2×ABI TaqMan PCR Master Mix, and 0.25μl (90μM) of both sense and antisense primers and 0.5μl (10μM) of the probe. After the initial denaturation at 95°C (10min), target genes were amplified through 40 cycles of universal cycling conditions (95°C/10s, 60°C/1min). All PCR reactions were normalized to GAPDH or β-actin mRNA in transformed and primary cells, respectively. The GAPDH and β-actin target genes were amplified using pre-designed pair of primers and a probe in concentrations suggested by manufacturer (Applied Biosystems). Standard cell dilution curves were calibrated using serial dilutions of control pTA_X-DING-CD4, pTA_gag or pTA_vif plasmids representing 1×106, 1×105, 1×104, 1×103, 1×102 and 10 copies of X-DING-CD4 cDNA per reaction and all values are expressed as means ± standard deviation of the mean.
Depending on the experimental setting, the results of qRT-PCR analyses are presented as the X-DING-CD4 mRNA fold change, the X-DING-CD4 mRNA copy/ng total RNA or the interquartile range (IQR). The fold change was calculated as a fold of the X-DING-CD4 mRNA increase or decrease between the experimental sample and the uninfected control; the IQR for the X-DING-CD4 mRNA expression was calculated by Sigma Plot 11 (Systat Software, Inc) for all thirteen samples measured at the specific time points and represent copy of X-DING-CD4 mRNA / ng total RNA; the correlation between the expression of X-DING-CD4 and HIV-1 gag mRNAs was analyzed by GraphPad Prism5 (GraphPad Software, Inc).
Results
The X-DING-CD4 mRNA is rapidly up-regulated in HIV-1 resistant X-DING-CD4(+) cells and the enhanced expression of the X-DING-CD4 gene coincides with inhibition of HIV-1 transcription
We reported previously that X-DING-CD4 mediated restrictive activity in HIV-1 resistant X-DING-CD4(+) cells (previously called HRF(+)) 10 did not affect virus cell entry or reverse transcription but blocked HIV-1 at the stage of the LTR transcription 10, 11, 13. This data suggested that X-DING-CD4-mediated antiviral protection was induced shortly after cell exposure to HIV-1 and could be previewed by changes in the activity of the X-DING-CD4 gene. To test this hypothesis we analyzed levels of X-DING-CD4 transcripts in HIV-1 resistant and control HIV-1 susceptible cells exposed to a low virus dose.
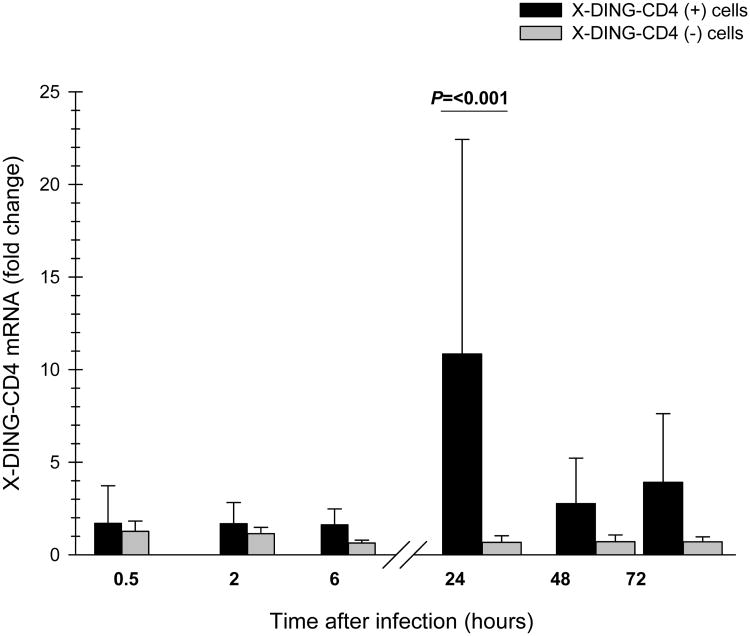
Cells were infected with HIV-1 at MOI of 0.01 and total RNA was harvested at the short time intervals. The activity of X-DING-CD4 mRNA was evaluated by the qRT-PCR and data from respective experimental systems were compared to those obtained from respective uninfected controls to gauge the variations in the gene's expression. As shown in Fig. 1, transcription of X-DING-CD4 mRNA in HIV-resistant cells increased by 1.8 to 4 fold within the first 30 minutes of the HIV-1 exposure; expression of this gene in the control HIV-1 susceptible cells increased by 1.3 fold at 30min time point and then was suppressed by 0.6-fold at the 6 hour time point. Twenty four hours after cell exposure to virus, the expression of X-DING-CD4 gene increased further by 11-23-fold in HIV-1 resistant cells and was suppressed by 0.7-fold in HIV susceptible cells. At 48 and 72 hour after infection, the expression of this gene was still induced by 2.5-5 and 4-7 fold in HIV-resistant cells and was unchanged in HIV susceptible cells.
Fig. 1. The expression of X-DING-CD4 mRNA is induced in HIV-1 resistant cells.
The expression of X-DING-CD4 gene was tested by qRT-PCR using X-DING-CD4 gene specific primers. Different samples of cell cultures were exposed to HIV-1 as described in Materials and Methods throughout a period of one year. The fold change was calculated as a fold increase or decrease of the X-DING-CD4 mRNA absolute copy number between the experimental sample and the uninfected control. The X-DING-CD4(+) and X-DING-CD4(−) denote the HIV-1 resistant CD4+T cell line previously known as HRF(+) and HIV-1 susceptible CEM cells. The P value for 24hr time point was derived from the Mann-Whitney rank sum test; the alpha level was set at 0.050
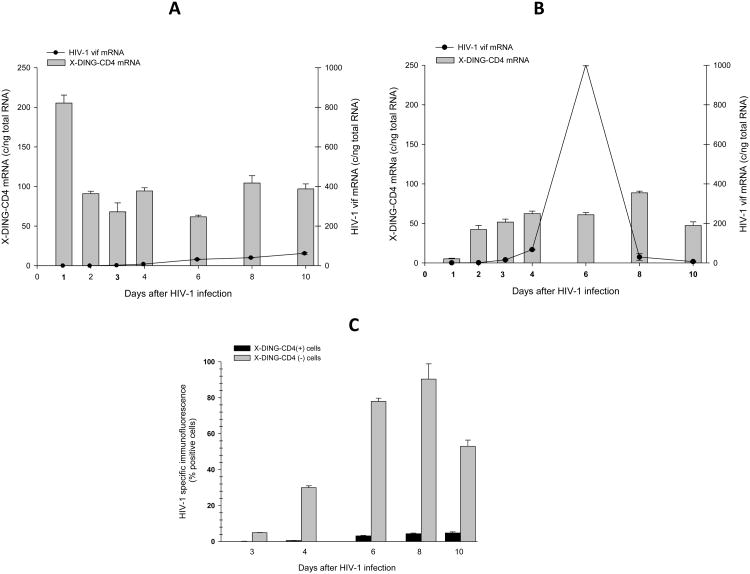
Subsequently, we used the same experimental setting and evaluated the expression of X-DING-CD4 and single spliced HIV-1 vif transcripts during ten days infection. As observed before, twenty four hours after exposure to virus, the expression of X-DING-CD4 mRNA was induced in HIV-1 resistant cells (Fig. 2A, bars, left axis) and suppressed in HIV-1 susceptible cells (Fig.2B, bars, left axis). At the subsequent time points the expression of X-DING-CD4 gene normalized to range between 60-80 copies/ng total RNA in HIV-1 resistant cells and 50-80 copies/ng total RNA in HIV susceptible cells (Fig. 2B, bars, left axis).
Fig. 2. The elevated expression of X-DING-CD4 mRNA associates with inhibition of HIV-1 vif gene transcription.
X-DING-CD4 and HIV-1 vif mRNA expression was tested by qRT-PCR in (A) HIV-1-resistant (X-DING-CD4(+)); and (B) HIV-1-susceptible cell lines. Levels ofX-DING-CD4 mRNA (bars) are calculated on the left y axis; levels of HIV-1 vif mRNA (lines)are calculated on the right y axis. Expression of HIV-1 proteins was tested by immunofluorescence (IF). This figure summarizes results of three independent experiments.
The vif mRNA was undetectable in HIV-1 resistant cells for up to three days after infection and at the six day time point was still blocked by 97% (Fig. 2A, line, right axis). Opposite to this the control HIV-1 susceptible cells supported virus replication; the vif transcripts could be detected as early as one day after HIV-1 exposure and transcription of this gene reached a peak at day six (Fig. 2B, line, right axis); The qRT-PCR data were confirmed by evaluation of HIV-1 proteins by IF (Fig. 2C) and Elisa (not shown). The viral proteins could be detected as early as three days after infection in the control HIV-1 susceptible cells and the maximum of viral infectivity was observed at eight days after infection. Concurrent with the qRT-PCR data, the X-DING-CD4(+) HIV-1 - resistant cells did not support virus replication. The viral proteins were not detected until six days after infection, when the IF value reached 3%. At the following 8 and 10-day time points the IF was measured at 5%.
The overexpression of X-DING-CD4 gene in HIV infected macrophages correlates with the inhibition of HIV-1 transcription
Based on the evidence presented in Fig. 2, we hypothesized that the X-DING-CD4 gene mediated antiviral activity might be effective in human population and lend an explanation as to why some individuals are capable to control HIV replication despite of being infected with the replication competent virus. To evaluate this proposition we tested the activity of X-DING-CD4 gene and transcription of HIV-1 gag mRNA in macrophages from thirteen HIV-1 negative individuals.
First we collected the mitogen and HIV-1 naïve monocytes to obtain the X-DING-CD4 mRNA expression background; then we tested the expression of the X-DING-CD4 gene in respective macrophages exposed or not to HIV-1. We measured the expression of HIV-1 gag and X-DING-CD4 mRNAs in 24-hour intervals for the period of the first three days after infection.
To exclude the natural resistance to HIV infection through a genetic defect in CCR5 receptor, we used the X4 NL4-3 HIV-1 strain. Prior evaluations on a smaller randomly selected sample showed that infections with R5 ADA and X4 NL4-3 HIV-1 induced similar levels of X-DING-CD4 mRNA in human macrophages and produced a similar quantity of HIV-1 gag mRNA transcripts during the first three days after infection (not shown).
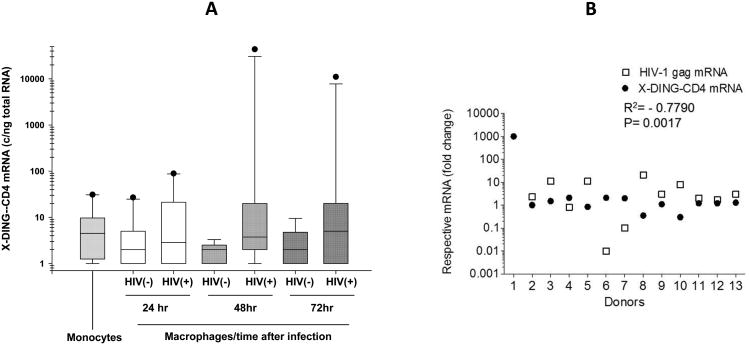
To observe variations in X-DING-CD4 mRNA, the qRT-PCR data were subjected to the interquartile range (IQR) analysis (Fig. 3A). The half the sample readings for mitogen naïve monocytes were between 1.3 and 9.76 X-DING-CD4 mRNA copies/ng total RNA; the outlier sample reached the expression of this gene at 27 copies/ ng total RNA and the median value for all monocytes was 4.5 X-DING-CD4 mRNA copy/ng total RNA (Fig. 3 A – monocytes).
Fig. 3. Evaluation of the X-DING-CD4 and HIV-1 gag mRNA expression in human monocytes and monocyte - derived macrophages.
(A) The X-DING-CD4 mRNA levels were tested in monocytes and macrophages from n=13 healthy HIV-1 negative donors. The IQR for each experimental system was calculated by SigmaPlot. Each data point for individual donor is derived from three amplifications of the sample. Monocytes denote the mitogen- and HIV-1-naïve cells. (B) – correlation between changes in the expression of X-DING-CD4 and HIV-1 gag mRNAs for each sample was calculated by GrapPad Prism 5 software. The HIV-1 gag mRNA was undetectable for macrophages from Donor 1. This figure (A and B) summarizes results of two independent real time PCR evaluations.
The exposure of monocytes to mitogens and their differentiation to macrophages resulted in the modest 1.9 fold decrease of X-DING-CD4 expression; the half the sample readings ranged from 1 to 4.9 copies/ng total RNA; however, similar as for the monocyte group the outlier samples reached 27copies/ng total RNA (Fig. 3A – HIV(−) macrophages 24 hours).
HIV-1 exposure induced the expression of X-DING-CD4 mRNA at all time-points tested (Fig. 3A HIV(+)). At the 24 hour time point the half sample readings placed in the range of 1-21 copies / ng total RNA and the outlier samples reached 87 copies/ng total RNA. At the 48 hour time point the expression of this gene ranged from 2-19.4 copies/ng total RNA in the infected macrophages, and 1-3.3 copies/ng total RNA in the control cells tested along. Interestingly, the expression of X-DING-CD4 in the outlier samples, reached 45,143 copies / ng total RNA in cells from donor 1. At the 72 hour time point the range of X-DING-CD4 expression in the uninfected sample was comparable to this tested at 24-hour time point expanding from 1-4.7 copies/ng total RNA; the expression of the gene in the 50% infected sample ranged between 1-20 copies /ng total RNA and the expression in the outlier sample from donor 1 was reduced to 11,545 copies /ng total RNA.
Next we set to evaluate the correlation between the expression of X-DING-CD4 and HIV-1 gag mRNAs in the infected macrophages. Based on the X-DING-CD4 mRNA expression kinetics in transformed and primary cells (Fig. 2 and 3A) and assumption that the activation of X-DING-CD4 gene should precede the blockage of HIV-1 LTR we selected the qRT-PCR data from the 24 and 48 hour time-points for the X-DING-CD4 gene and 48 and 72 hour time points for the HIV-1 gag mRNA. We established the numerical value representing a fold change in X-DING-CD4 and HIV-1 gag mRNAs transcripts for the selected time points and calculated correlation between the expressions of both tested genes.
This analysis showed a significant negative correlation between the expression of X-DING-CD4 and HIV-1 gag mRNAs (R2= − 0.7790); the induction of X-DING-CD4 gene coincided with the blockage of HIV-1 LTR transcription (Fig. 3B). For example macrophages that did not alter expression of X-DING-CD4 gene by more than two-fold, supported replication of HIV-1. Donors 1, 4, 6 and 7, whose cells had increased levels of X-DING-CD4 mRNA restricted expression of HIV-1 gag mRNA. Particularly cells from donor 1, who had very high X-DING-CD4 mRNA activity, restricted replication of virus from 9 copies at 48hr time point to the undetectable level at three days after infection (Fig. 3B donor 1). Macrophages from donors 4, 6 and 7 had a modest 2-2.5-fold increase of X-DING-CD4 mRNA activity and also restricted replication of HIV-1; however with a dissimilar efficiency. For example cells from donor 4 blocked replication of HIV-1 gag gene by 1.3 fold, while cells from donors 6 and 7 blocked transcription of this viral gene by 122- and 12 – fold, respectively (Fig. 3B).
Discussion
The identity of eukaryotic DING genes is controversial 21 and although fragments of cDNA were found in diverse organisms, lack of their chromosomal assignment puzzled scientists for years. It has been suggested that DING proteins arise from bacterial species and do not exist in eukaryotes 22. We and others provided evidence that the eukaryotic DING proteins are coded by plant or animal genomes and exert specific biological functions in the host cells 20, 23, 24.
The antiviral activity of the X-DING-CD4 protein has been reported before 8, 9, 11 and in this study we tested hypothesis that the activity of X-DING-CD4 gene might be linked to the cellular restriction of HIV-1 transcription in human cells. In these studies we showed that exposure of cells to HIV-1 altered expression of X-DING-CD4 gene. It was expected in our X-DING-CD4(+) cells which are refractory to HIV-1 infection; we observed previously the enhanced activity of X-DING-CD4 protein upon their exposure to HIV-1 13. Interestingly, we found a similar restriction of HIV-1 promoter activity in primary macrophages of four donors whose cells also reacted to virus exposure with an enhanced transcription of X-DING-CD4 gene.
The induction of X-DING-CD4 gene always correlated to the inhibition of HIV transcripts in transformed cells; but it was not so obvious in the primary macrophages. Although four donors had elevated levels of X-DING-CD4 gene; only one restricted transcription of HIV-1 to the undetectable level. The other three donors had a modest 2-2.5-fold increase in expression of the X-DING-CD4 mRNA and although all had diminished levels of gag mRNA; there was a notable unevenness in the LTR restriction level and study in a larger sample and future evaluations will clarify current observations. However, based on data in hand we believe that the protective status of X-DING-CD4 can be achieved in cells that produced a very high concentration of protein preceded by high activation of its gene. The sensitivity of X-DING-CD4 gene to cellular exposure to HIV-1 might be a part of the unique host mechanism regulating cellular resistance to virus; and we showed that some individuals who have the inducible X-DING-CD4 gene responses have also higher capacity to restrict HIV-1.
Our data showed that all tested T cells had transcriptionally active X-DING-CD4 gene. In this sense all cells should have the capacity to restrict virus replication; this is not, however, what we observed. We consider that X-DING-CD4 has a dual function in human cells: physiological – as a phosphatase and antiviral – as HIV-1 LTR transcription inhibitor. Based on our previous reports we hypothesize that the antiviral function of X-DING-CD4 involves a rare methylation of the glutamic acid 69 present in about 10% of its molecules 8; we postulate that target cells from some individuals, in the event of virus infection induce the expression of X-DING-CD4 mRNA followed by methylation of X-DING-CD4 protein; this modification facilitates a “switch” into X-DING-CD4 protective mode and stimulates further the induction of X-DING-CD4 gene. Considering that methylation of the glutamic acid 69 is a leading key for a switch of X-DING-CD4 activity from physiological to protective, we hypothesize that a larger concentration of a substrate for this modification warrants more successful outcome.
We observed two opposite trends in the expression of X-DING-CD4 mRNA when transformed CD4+ T cells were exposed to HIV-1: (i) rapid induction or (ii) rapid suppression. The induction of X-DING-CD4 activity occurred in cells that resisted HIV-1 infection, while reduced activity of this gene was seen in cells susceptible to virus. Response of X-DING-CD4 gene was more diverse in human macrophages. This observation brings forth a question if one of the HIV-1 evasive techniques include also a mechanism that down-regulates the expression of X-DING-CD4. This concept is true for other intrinsic proteins such APOBEC3g - deactivated by HIV-1 Vif protein 25 and Daxx neutralized by CMV pp71 protein 26.
The mobilization of X-DING-CD4 gene upon the virus exposure was rapid in primary and transformed cells, which is characteristic trait of the intrinsic response to pathogen invasion; however the X-DING-CD4 activity peaked at 24 hours in infected CD4+ transformed cells; while in primary macrophages – at 48 hours after infection. This difference in the responsiveness of X-DING-CD4 mRNA to virus invasion in these two cell systems could be related to the clonal selection of transformed X-DING-CD4 (+) cells.
The phenomenon of restricting the HIV-1 infection by host target cells is an exciting avenue for novel therapeutic interventions. The introduction of native X-DING-CD4 protein into HIV-1 susceptible infected CD4+ T cell culture blocks infection of HIV-1 as effectively as the endogenous protein expressed in the X-DING-CD4(+) cells system 8, 11. Moreover, another DING homologue, p27SJ isolated from Hyperforicum perforatum was found to have an anti-HIV activity 24 and a phosphatase activity 23. The phosphatase activity of the p27SJ plant DING protein associated with its antiviral activity. Future research on the X-DING-CD4 mechanism will show if its phosphatase activity is also indispensable for blocking the HIV-1 LTR transcription. This highly conserved anti-viral activity in two distinct members of DING protein family makes a human X-DING-CD4 an attractive target for development of a novel biotherapeutic.
It is reasonable to believe that the overall inhibition of HIV-1 in cells of the LTNPs and particularly in ECs might be regulated by a complex cellular immunity involving multiple proteins. Our results show that the X-DING-CD4 mechanism is a part of the complex cellular activities against the pathogen invasion. Some of the intrinsic molecules with an anti-viral activity have been already identified 25, 27, and it will be interesting to gauge their expression levels in cells of LTNPs, AIDS patients and healthy HIV-1 negative population.
Acknowledgments
We thank Dr. Potash for providing human monocytes for these studies. This work was supported by NS062649 grant from the National Institutes of Health.
Literature
- 1.Blankson JN, Bailey JR, Thayil S, et al. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J Virol. 2007;81:2508–18. doi: 10.1128/JVI.02165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Julg B, Pereyra F, Buzon MJ, et al. Infrequent recovery of HIV from but robust exogenous infection of activated CD4(+) T cells in HIV elite controllers. Clin Infect Dis. 2010;51:233–8. doi: 10.1086/653677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamine A, Caumont-Sarcos A, Chaix ML, et al. Replication-competent HIV strains infect HIV controllers despite undetectable viremia (ANRS EP36 study) Aids. 2007;21:1043–5. doi: 10.1097/QAD.0b013e3280d5a7ac. [DOI] [PubMed] [Google Scholar]
- 4.Migueles SA, Connors M. Long-term nonprogressive disease among untreated HIV-infected individuals: clinical implications of understanding immune control of HIV. Jama. 304:194–201. doi: 10.1001/jama.2010.925. [DOI] [PubMed] [Google Scholar]
- 5.Shacklett BL. Understanding the “lucky few”: the conundrum of HIV-exposed, seronegative individuals. Current HIV/AIDS reports. 2006;3:26–31. doi: 10.1007/s11904-006-0005-2. [DOI] [PubMed] [Google Scholar]
- 6.Rabi SA, O'Connell KA, Nikolaeva D, et al. Unstimulated primary CD4+ T cells from HIV-1-positive elite suppressors are fully susceptible to HIV-1 entry and productive infection. J Virol. 85:979–86. doi: 10.1128/JVI.01721-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vigneault F, Woods M, Buzon MJ, et al. Transcriptional Profiling of CD4 T Cells Identifies Distinct Subgroups of HIV-1 Elite Controllers. J Virol. 2011;85:3015–9. doi: 10.1128/JVI.01846-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lesner A, Shilpi R, Ivanova A, et al. Identification of X-DING-CD4, a new member of human DING protein family that is secreted by HIV-1 resistant CD4(+) T cells and has anti-viral activity. Biochemical and biophysical research communications. 2009;389:284–9. doi: 10.1016/j.bbrc.2009.08.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li G, Aaron S, Kazmierczak K, et al. Inhibition of HIV-1 or bacterial activation of macrophages by products of HIV-1-resistant human cells. Immunol Cell Biol. 2007;85:603–9. doi: 10.1038/sj.icb.7100092. [DOI] [PubMed] [Google Scholar]
- 10.Simm M, Miller LS, Durkin HG, et al. Induction of secreted human immunodeficiency virus type 1 (HIV-1) resistance factors in CD4-positive T lymphocytes by attenuated HIV-1 infection. Virology. 2002;294:1–12. doi: 10.1006/viro.2001.1300. [DOI] [PubMed] [Google Scholar]
- 11.Lesner A, Li Y, Nitkiewicz J, et al. A soluble factor secreted by an HIV-1-resistant cell line blocks transcription through inactivating the DNA-binding capacity of the NF-kappa B p65/p50 dimer. J Immunol. 2005;175:2548–54. doi: 10.4049/jimmunol.175.4.2548. [DOI] [PubMed] [Google Scholar]
- 12.Lesner A, Kartvelishvili A, Lesniak J, et al. Monoubiquitinated histone H1B is required for antiviral protection in CD4(+)T cells resistant to HIV-1. Biochemistry. 2004;43:16203–11. doi: 10.1021/bi0492758. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Li G, Ivanova A, Aaron S, Simm M. The critical role of human transcriptional repressor CTCF mRNA up-regulation in the induction of anti-HIV-1 responses in CD4(+) T cells. Immunol Lett. 2008;117:35–44. doi: 10.1016/j.imlet.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simm M, Su Z, Huang EY, et al. Cloning of differentially expressed genes in an HIV-1 resistant T cell clone by rapid subtraction hybridization, RaSH. Gene. 2001;269:93–101. doi: 10.1016/s0378-1119(01)00456-5. [DOI] [PubMed] [Google Scholar]
- 15.Su ZZ, Chen Y, Kang DC, et al. Customized rapid subtraction hybridization (RaSH) gene microarrays identify overlapping expression changes in human fetal astrocytes resulting from human immunodeficiency virus-1 infection or tumor necrosis factor-alpha treatment. Gene. 2003;306:67–78. doi: 10.1016/s0378-1119(03)00404-9. [DOI] [PubMed] [Google Scholar]
- 16.Foley GE, Lazarus H, Farber S, Uzman BG, Boone BA, McCarthy RE. Continuous Culture of Human Lymphoblasts from Peripheral Blood of a Child with Acute Leukemia. Cancer. 1965;18:522–9. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Adachi A, Gendelman HE, Koenig S, et al. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–91. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gendelman HE, Orenstein JM, Martin MA, et al. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1428–41. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kazmierczak K, Potash MJ. Host and virus strain dependence in activation of human macrophages by human immunodeficiency virus type 1. J Neurovirol. 2007;13:452–61. doi: 10.1080/13550280701510104. [DOI] [PubMed] [Google Scholar]
- 20.Sachdeva R, Simm, M The application of linear polyacrylamide co-precipitation of denatured templates for the PCR amplification of ultra-rapidly reannealing DNA. BioTechniques. 2011;50:217–9. doi: 10.2144/000113654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berna A, Scott K, Chabriere E, Bernier F. The DING family of proteins: ubiquitous in eukaryotes, but where are the genes? Bioessays. 2009;31:570–80. doi: 10.1002/bies.200800174. [DOI] [PubMed] [Google Scholar]
- 22.Lewis AP, Crowther D. DING proteins are from Pseudomonas. FEMS Microbiol Lett. 2005;252:215–22. doi: 10.1016/j.femsle.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 23.Darbinian N, Gomberg R, Mullen L, et al. Suppression of HIV-1 transcriptional elongation by a DING phosphatase. Journal of cellular biochemistry. 2011;112:225–32. doi: 10.1002/jcb.22915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darbinian-Sarkissian N, Darbinyan A, Otte J, et al. p27(SJ), a novel protein in St John's Wort, that suppresses expression of HIV-1 genome. Gene therapy. 2006;13:288–95. doi: 10.1038/sj.gt.3302649. [DOI] [PubMed] [Google Scholar]
- 25.Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–50. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 26.Saffert RT, Kalejta RF. Inactivating a cellular intrinsic immune defense mediated by Daxx is the mechanism through which the human cytomegalovirus pp71 protein stimulates viral immediate-early gene expression. J Virol. 2006;80:3863–71. doi: 10.1128/JVI.80.8.3863-3871.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bieniasz PD. Intrinsic immunity: a front-line defense against viral attack. Nature immunology. 2004;5:1109–15. doi: 10.1038/ni1125. [DOI] [PubMed] [Google Scholar]