Summary
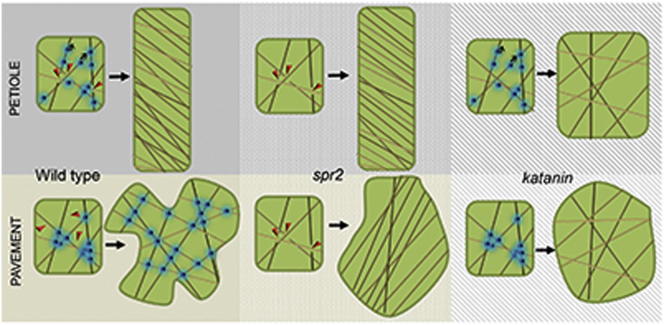
One of the defining characteristics of plant growth and morphology is the pivotal role of cell expansion. While the mechanical properties of the cell wall determine both the extent and direction of cell expansion, the cortical microtubule array plays a critical role in cell wall organization and, consequently, determining directional (anisotropic) cell expansion [1–6]. The microtubule-severing enzyme katanin is essential for plants to form aligned microtubule arrays [7–10]; however, increasing severing activity alone is not sufficient to drive microtubule alignment [11]. Here, we demonstrate that katanin activity depends upon the behavior of the microtubule-associated protein (MAP) SPIRAL2 (SPR2). Petiole cells in the cotyledon epidermis exhibit well-aligned microtubule arrays, whereas adjacent pavement cells exhibit unaligned arrays, even though SPR2 is found at similar levels in both cell types. In pavement cells, however, SPR2 accumulates at microtubule crossover sites, where it stabilizes these crossovers and prevents severing. In contrast, in the adjacent petiole cells, SPR2 is constantly moving along the microtubules, exposing crossover sites that become substrates for severing. Consequently, our study reveals a novel mechanism whereby microtubule organization is determined by dynamics and localization of a MAP that regulates where and when microtubule severing occurs.
Graphical Abstract
Highlights
-
•
SPR2 is a multifunctional MAP that is able to inhibit katanin-based MT severing
-
•
In cells with net-like microtubules, SPR2 prevents severing at microtubule crossovers
-
•
In cells with well-aligned microtubules, SPR2 mobility allows microtubule severing
-
•
SPR2 dynamics control microtubule organization by regulating microtubule severing
Results and Discussion
SPR2 Promotes the Formation of Unaligned Microtubule Arrays by Inhibiting Katanin Activity
Cotyledon development results in the differentiation of several distinctive cell types that make it an excellent system for studying microtubule (MT) organization (see Figure S1A available online). Pavement cells exhibit “net-like” arrays in which MTs exhibit no particular alignment, in contrast to adjacent petiole cells that exhibit anisotropic expansion and have clearly aligned arrays [10]. Several studies on Arabidopsis hypocotyls have demonstrated a role for katanin in releasing newly formed MTs from branch sites following their nucleation on an existing MT [12–15]. Such nucleation events are characterized by branched MTs. In the cotyledon, however, branched MTs indicative of nucleation are very rare, whereas new MTs frequently arise from the severing of existing MTs that occurs almost exclusively at sites where MTs cross one another. This provides a mechanism for removing unaligned MTs and drives MT alignment [10]. Consistent with this mechanism, petiole cells exhibit much higher rates of MT severing than pavement cells [10]. MT severing in plants is catalyzed by the enzyme katanin, which is composed of two subunits. In Arabidopsis, there is only a single gene for the p60 catalytic subunit (AtKatp60). Several AtKatp60 mutant alleles have been independently isolated, including fra2, lue1, erh3, and bot1-7 [7–9, 16]. Katanin mutants fail to form aligned MT arrays (Figure S1B) and exhibit cell expansion that is largely isotropic [7] (Figures S1B–S1D). A number of models have proposed to explain the basis of MT alignment [17–19], but to date, none explain the pivotal role of katanin in this process. Primordia formation in the shoot apical meristem has been shown to depend upon MT rearrangements that result from the perception of mechanical cues [20]. Katanin is essential for this process [21], confirming the idea that katanin is pivotal for most, if not all, MT rearrangements in plant cells. A recent report, based upon overexpression of both ROP6 small GTPase and its effector RIC1, suggests that activation of katanin by GTPase is a mechanism for generating local MT alignment in pavement cells [13]. The authors of that study, however, were unable to measure MT severing activity in pavement cells directly [13]. Furthermore, previous studies using inducible overexpression of katanin in pavement cells have demonstrated that increased MT severing activity resulted in shorter, more bundled MTs but no increase in MT alignment [11]. Consequently, katanin-dependent MT severing is necessary but not sufficient to drive MT alignment. In animal systems, katanin plays pivotal roles in processes such as neuronal growth and meiotic spindle organization [22–24]. In these systems, severing of MTs is frequently regulated by microtubule-associated proteins (MAPs) [22, 25]. To date, however, no such regulators of MT severing in plants have been reported. SPIRAL2 (SPR2) is a plant-specific MT binding protein that contains a series of HEAT repeats but no other homology to proteins of known function [26–28]. It has a complex distribution and localizes both at the growing ends of MTs and along their length [28] (Figure S2A). It has also been reported that SPR2 is enriched at sites of MT crossover [28]. Due to the pivotal importance of MT crossovers as sites for MT severing, these data prompted us to examine more closely the effect of SPR2 on MT organization.
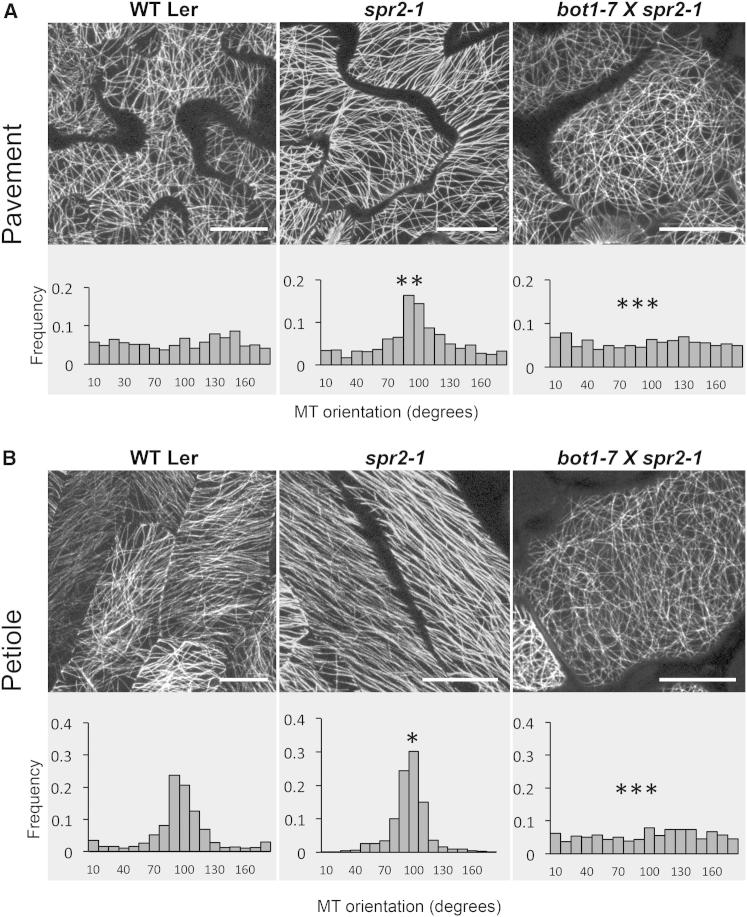
Examination of cortical MTs in pavement cells of spr2-1 clearly revealed well-aligned arrays that resembled the organization present in petiole cells, in contrast to the net-like organization in wild-type pavement cells (Figure 1). Furthermore, the petiole cells of spr2-1 appeared to exhibit “hyperaligned” MTs, even compared to the already well-aligned array in wild-type petiole cells (Figure 1). To determine whether katanin and SPIRAL2 regulate MT organization in the same pathway, we quantified rates of MT severing. Consistent with previous reports [10], wild-type (WT) pavement cells exhibited low severing rates; however, severing frequency in spr2-1 pavement cells was greatly increased and was comparable to that of petiole cells (Figure 2A; Table S1). Furthermore, the hyperaligned MT organization seen in spr2-1 is dependent upon a functional katanin, since double mutants exhibited solely unaligned arrays in all cell types (Figure 1), similar to those found in katanin single mutants (Figure S1B). We use the bot1-7 alleles to be consistent with previous studies; however, bot1-7 is in the Ws background, whereas spr2-1 is in the Landsberg background. Consequently, we also constructed a double mutant using the lue1 allele of katanin p60 and the spr2-2 allele, which are both from the Columbia background. We found that these plants exhibited patterns of MT organization very similar to bot1-7,spr2-1 (Figures 1, S1E, and S1F).
Figure 1.
Cortical Microtubule Organization and Alignment in Cotyledon Epidermal Cells
(A) Pavement cell.
(B) Petiole cells.
The top panels show a representative confocal image of MTs in each cell type, while the bottom panels show the distribution of MT orientations based on examining more than 1,000 MTs from each cell type. Scale bar represents 20 μm. Asterisks above graphs give the level of statistical significance of the difference in MT angle distribution with the corresponding WT. For the double mutant, statistical significance was obtained comparing with both the Ler and Ws backgrounds. See also Figure S1.
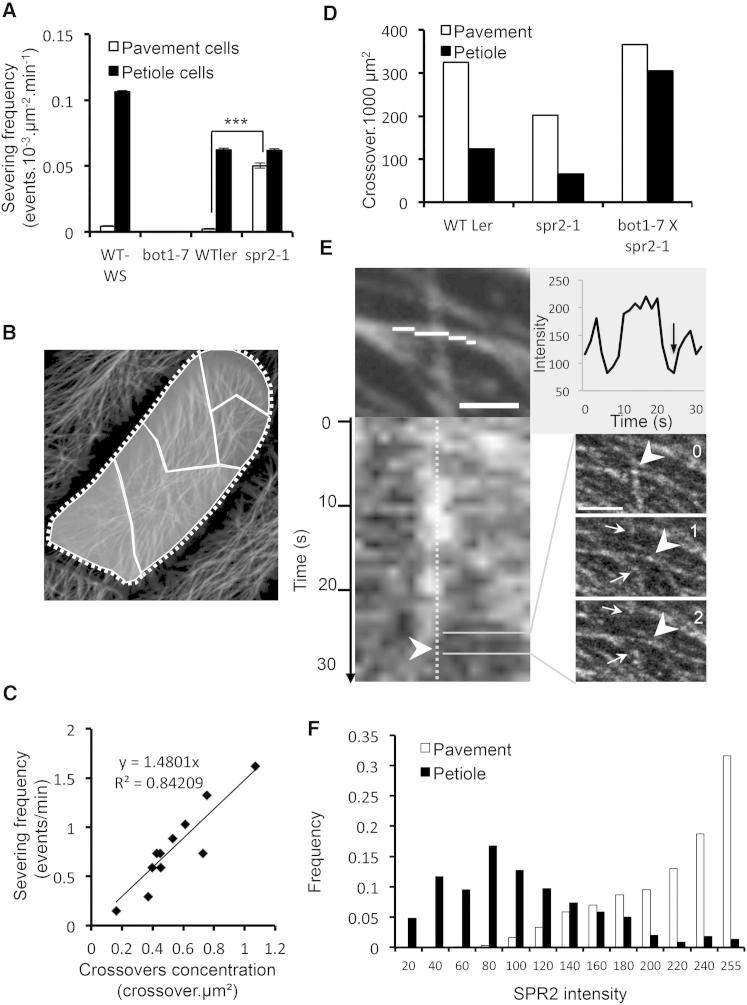
Figure 2.
Regulation of Microtubule Severing by SPR2
(A) MT severing frequency in different cell types. Absence of severing in bot1-7 represents a negative control. Error bars show the SD between squared zones representing the different cells used for the analysis.
(B) Example of a WT Ler petiole cell arbitrarily divided into different regions with differing degrees of organization.
(C) Severing frequency is linearly correlated with crossover concentration. Data are based on the delimitation of 11 MT subpopulations from three WT petiole cells, as shown in (B).
(D) MT crossover density in petiole and pavement cells from different genotypes.
(E) SPR2 dynamics and intensity at MT crossover. The middle panel shows a kymograph of part of the MT depicted by the white line in the top panel. The white dotted line (middle panel) represents the crossover position over time. A plot of the intensity of the kymograph (bottom panel) gives the intensity of SPR2 at the crossover over time. The arrowhead and arrow mark the point at which severing occurred. In the right panel, individual images taken 1 s apart from the movie sequenced show that the severing occurs following the clearance of the crossover by a SPR2 particle that moves rapidly away from the crossover site (arrowhead). Scale bars represent 1 μm (left panels) and 2 μm (right panels).
(F) Distribution of SPR2 intensity at MT crossovers monitored over time in pavement and petiole cells. This distribution graph is based on ten crossovers in pavement and in petiole cells monitored each second for 1 min (600 s per cell type). SPR2 intensity is plotted in increments of 20 grayscale units; there is a maximum of 255 grayscale units for this image type.
See also Figure S3.
In this study and in a previous report, we found that nearly all severing events occurred at MT crossovers, and as such, the number of MT crossovers is a reflection of the number of potential substrates for MT severing. Within a single petiole cell, we were able to identify regions of differing MT alignment with consequent variation in crossover density (Figure 2B). When we compared the crossover density with the severing frequency, we found a clear linear relationship (Figure 2C), consistent with MT crossovers being the substrate sites for MT severing. Furthermore, when we compared severing frequency in different cell types and different genotypes, it showed that severing was low in WT pavement cells, even though the number of potential substrates was very high (Figure 2D), and that high rates of severing occurred in spr2 pavement cells, even though the number of crossovers was low in these cells (Figures 2A and 2D).
Together, these data strongly suggest that the increased alignment of the MT array observed in spr2-1 is caused by a specific increase in severing at MT crossovers and reveal that both cell types in wild-type cotyledons possess MT-severing activity, but that in pavement cells, severing is inhibited by the presence of SPR2. The data also provide additional support for a mechanism by which increased katanin-dependent severing at MT crossover sites drives MT alignment [10], whereas in pavement cells, the presence of SPR2 is able to inhibit this katanin-based severing and explains why overexpression of katanin alone does not drive MT alignment in pavement cells [11].
Regulation of Microtubule Severing by Katanin Is Dependent upon SPR2 Dynamics and Localization
To address why severing occurs at much higher rates in wild-type petiole cells as compared to pavement cells, we performed live-cell imaging using a previously described SPR2:GFP fusion [28]. Average intensity of time-lapse series showed that SPR2 accumulated to similar levels in both pavement and petiole cells, but in pavement cells, SPR2 accumulated at sites where MTs cross over one another, whereas in petiole cells, the distribution was more even along the MT (Figure S2B). SPR2 has been reported to preferentially bind MT plus ends [28]. We further observed in both pavement and petiole cells that, shortly after the growing MT plus end crossed another MT, it rapidly deposited SPR2 to the newly formed MT crossovers (Figure 3A; Movie S1) and so contributes to a mechanism for distributing SPR2 around the MT array.
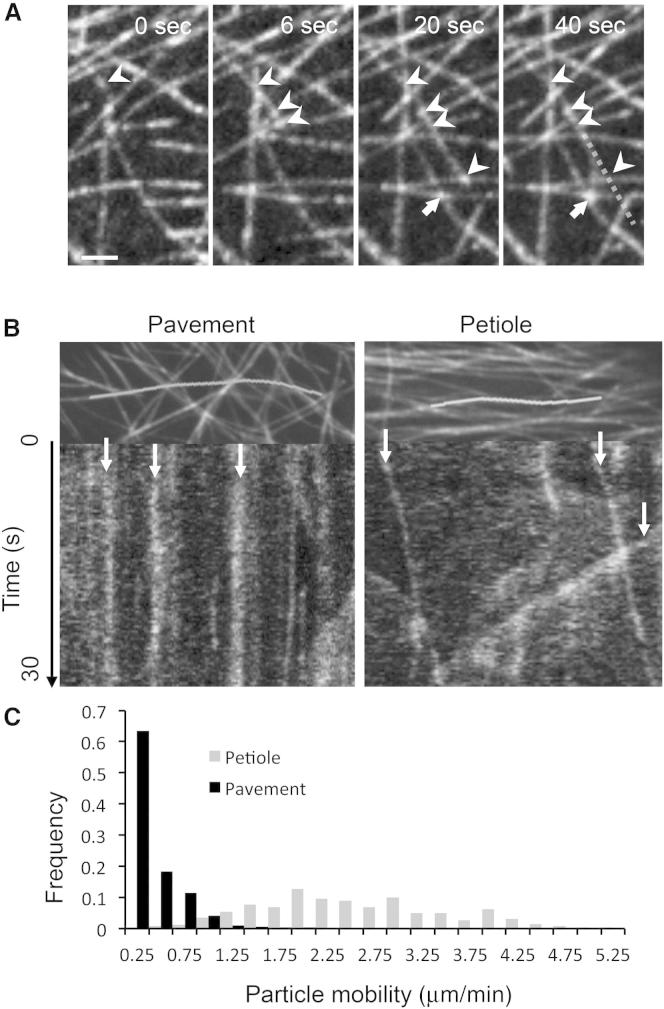
Figure 3.
Variation in SPR2 Dynamics in Different Cell Types
(A) SPR2 is deposited at newly formed MT crossovers. The sequence shown was taken in WT Ler pavement cells. SPR2 is localized at the MT plus end and moves to the crossover as the plus end crosses another MT. Arrowheads point to the MT plus end and the SPR2 particles it deposits at crossovers. Arrow points at a complex MT crossover, which the growing MT will join following a 32.3° reorientation from the initial trajectory (dotted line). Scale bar represents 2 μm.
(B) Kymograph analysis of SPR2 behavior in SPR2-GFP pavement and petiole cells. The top panels show a projection of a 100 s movie sequence. The white line indicates the MT that was selected for the corresponding kymograph analysis shown at the bottom (9.4 μm for petiole cells; 14.4 μm for pavement cells). Arrows indicate particles that are stationary during the course of the movie in pavement cells and mobile particles in petiole cells.
(C) Quantitative analysis of SPR2-GFP particle movement in pavement cells (n = 253 particles; n = 18 cells) and petiole cells (n = 258 particles; n = 16 cells). The average particle velocity is 0.24 μm/min in pavement cells versus 2.4 μm/min in petiole cells.
It has previously been shown that SPR2 particles move along the MT lattice [28]. In order to understand how the difference in SPR2 distribution between pavement and petiole is generated, we analyzed the dynamics of SPR2-GFP particles in pavement and petiole cells. To investigate whether there were differences in SPR2 dynamics, we monitored the behavior of individual particles along single MTs in a time-lapse image series using kymographs. A representative kymograph from pavement cells shows a series of straight lines indicating that the SPR2-GFP particles remained stationary during the course of the time-lapse series (100 s; Figure 3B). Conversely, diagonal lines in kymographs from petiole cells indicate that SPR2 particles were constantly moving. SPR2 particles move by alternating pauses and dynamic moments [28] (Figure S2D; Movie S2). To quantify the dynamics of SPR2-GFP particles in pavement versus petiole cells, we measured the velocities of single particles (n = 253 in pavement cells; n = 258 in petiole cells). Most dynamic moments were short (1–45 s; Figure S2D). As reported earlier [28], fusion and fission of SPR2 particles were observed. Fission and fusion mechanisms typically occurred in chains; thus, the movement of SPR2 particles often happened in relay (Figure S2D; Movie S2). Whereas we often observed this kind of complex dynamics in petiole cells, it appeared to be much rarer in pavement cells. To quantify these differences, we measured the movement of SPR2 particles. The mean velocity of SPR2 particles was 10-fold higher in petiole cells (0.24 ± 0.02 versus 2.4 ± 0.06 μm/min). In pavement cells, 64% of the total particles had a velocity lower than 0.25 μm/min, whereas in petiole cells, 61% of the particles had a velocity higher than 2 μm/min (Figure 3C). The distribution of SPR2-GFP particle velocity in petiole cells was significantly higher than in pavement cells (Kolmogorov-Smirnov test; p < 0.001). Altogether, the data suggest that the differences in the ability of SPR2 to prevent severing in different cell types are correlated with large differences in SPR2 dynamics in the two cell types. Yao et al. [28] previously suggested a possible mechanism of SPR2 mobility including binding to growing or shrinking ends of MTs. Our previous studies, however, have shown broadly similar rates of MT growth and shrinkage in petiole and pavement cells [10]. Consequently, the difference in SPR2 mobility is unlikely to be explained by differences in MT dynamics, and given the complex nature of SPR2 movement, it seems likely that a MT-based motor is involved.
To further address the relationship between SPR2 and severing in petiole cells, we used kymograph analysis to monitor the level of SPR2-GFP at MT crossovers in petiole cells during a 30 s period when severing occurred. The intensity of SPR2-GFP fluctuated depending on whether a SPR2-GFP particle occupied a MT crossover site. As expected, severing events occurred at minima of SPR2 level, following the clearance of the crossover by a SPR2 particle (Figures 2E and S3). To quantify this analysis, we monitored ten MT severing events from WT Ler petiole cells and compared the mean intensity of the SPR2-GFP signal at crossover point in the 30 s prior to severing (134 ± 8.56) with the intensity at the time of severing and found it to be over twice that found at the point of severing (48.09 ± 2.37), a very significant difference (paired t test; p < 0.001). Furthermore, a quantitative analysis of SPR2 intensity over time at MT crossover sites (Figure 2F) demonstrated that the distribution of SPR2-GFP intensity was significantly lower (Kolmogorov-Smirnov test; p < 0.001) in petiole cells as compared to pavement cells. Consequently, even in petiole cells, SPR2 particles appear able to prevent severing, but its constant movement means that crossover sites without SPR2 are frequently exposed, and these represent suitable substrates for katanin-mediated MT severing. Given that regardless of the type of MT array, MT severing does not occur when SPR2 accumulates at MT crossovers, it is likely that SPR2 has a role in physically preventing access of katanin to crossover sites. The observation that katanin transiently localizes to MTs crossovers [15] is consistent with this hypothesis. Similarly, it is currently unknown what regulates the behavior of SPR2 in different cell types, but publicly available data suggest that SPR2 is phosphorylated on at least three different sites. Whether phosphorylation regulates SPR2 dynamics activity is the subject of further research.
SPR2 Promotes Unaligned Arrays in Pavement Cells by Promoting Formation of Complex Microtubule Crossovers
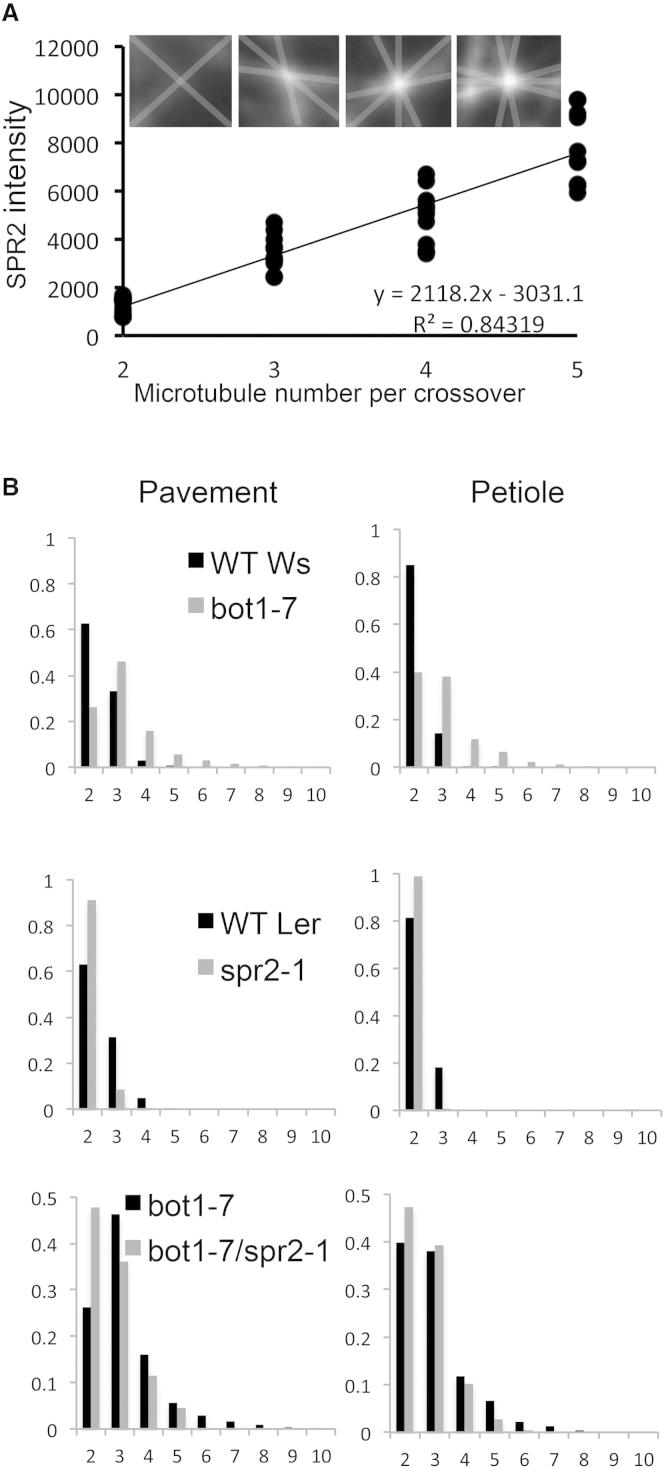
The relationship between SPR2 and MT crossover points was further exemplified when we examined what we describe as complex crossover sites, where more than two MTs appeared to cross at the same point. In WT pavement cells, there is a linear relationship between the intensity of SPR2-GFP at crossover sites and the number of MTs per crossover (Figure 4A). While monitoring SPR2 dynamics, we frequently observed events in which complex crossovers involving three or more MTs were favored over simple crossovers that only involved two MTs. Examples of this behavior are shown in Figure 3A and Movies S3 and S4, where a growing MT changes trajectory following a simple crossover formation to establish a complex MT crossover. To examine whether SPR2 itself might contribute to the formation and/or stability of complex MT crossovers, we analyzed the number of crossovers in spr2-1 mutants and found significantly fewer MTs per crossover in the mutant as compared to the wild-type (Figure 4B). The opposite is true in katanin mutants, where there is a large shift toward complex MT crossover (Figure 4B). To ensure that decreases in formation of complex crossovers seen in spr2-1 were not a result of alteration in MT organization, we compared MTs in katanin (bot1-7) mutants with those of bot1-7,spr2-1 double mutants, since both lines exhibit the same distribution of MT orientation (Figures 1 and S1B). Consistent with the idea that mutation in spr2-1 results in fewer complex MT crossovers, the proportion of simple crossovers involving two MTs in bot1-7,spr2-1 double mutants (0.48) was approximately twice that found in bot1-7 single mutants (0.26; Figure 4B), although these differences were not statistically significant. These data support an additional role for SPR2 in promoting the formation of complex MT crossovers in a manner that is independent of its role in MT alignment and support a role of SPR2 in the formation of a robust crosslinked network of MTs in pavement cells.
Figure 4.
SPR2 Binding Prevents Severing and Promotes Microtubule Crossover Formation
(A) SPR2 intensity increases with the number of MTs per crossover in WT pavement cells. SPR2 intensity is measured as the difference of intensity at crossover minus the MT basal intensity. Ten crossovers were measured for each class of MT crossover. Insets show representatives of each class; gray lines indicate the position of MT crossovers added for clarity.
(B) Proportion of different classes of MT crossover in different cell types and mutant backgrounds. Measurements are based upon between 520 and 833 crossovers for each distribution.
Conclusion
In neurons, the MT-associated protein Tau protects axon MTs from katanin-mediated severing [22], indicating that a convergent mechanism for regulating katanin has likely evolved in both plants and animals. However, the mechanism defined in this study, in which binding and dynamics of a MAP regulate katanin activity, is unique. SPR2 is clearly a multifunctional MAP. In cells that exhibit unaligned arrays, such as pavement cells, it binds to and promotes MT crossover formation and remains relatively stationary at these sites, preventing katanin-based MT severing. Binding of SPR2 to growing ends may also be a means of distributing SPR2 to sites of MT crossover in these cells. In contrast, in cells with well-aligned MT arrays, SPR2 is highly mobile along the MT and consequently exposes MT crossover sites to MT severing. Such a mechanism allows tissue-specific katanin regulation while maintaining the function of SPR2 in promoting uninterrupted MT growth [28] in all tissues. Hence, SPR2 dynamics provides an efficient means of cytoskeletal regulation by directly modulating the accessibility of MT crossovers to katanin.
During plant development, the cytoskeleton is rearranged in response to a number of external cues, including mechanical forces that have recently been shown to be essential signals in the shoot apical meristem [20]. How these mechanical forces are perceived and transduced into cytoskeletal rearrangements is unclear, but in common with most, if not all, cytoskeletal rearrangements, it is dependent upon katanin [21]. Given that we have demonstrated the pivotal role of SPR2 in regulating katanin-based MT severing, it is likely that interactions between katanin and SPR2 provide the basis for a rapid and flexible system for transducing the signals that drive cytoskeletal rearrangements.
Acknowledgments
We are grateful for the assistance of Patrick Hill with scanning electron microscopy images and Peter March and the University of Manchester Bioimaging Facility with fluorescent imaging. Use of the Zeiss confocal microscope was kindly provided by the Sainsbury Laboratory at the University of Cambridge. The authors would like to thank Ottoline Leyser, Elliot Meyerowitz, Patrick Gallois, Stephen High, and Martin Pool for their comments on the manuscript. This work was begun by R.W. when he was supported by the Biotechnology and Biological Sciences Research Council (BBSRC), grant 34/C1928. M.K. and P.C. were supported by BBSRC grant BB/H012923/1.
Published: September 19, 2013
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplemental Information includes three figures, one table, Supplemental Experimental Procedures, and four movies and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2013.07.061.
Supplemental Information
Arrows indicate SPR2-GFP particles that are successively deposited as new crossovers are formed.
The movie was taken from a petiole cell. The original image sequence is shown in the left panel. The right panel has been processed to aid visualization. Arrows illustrate two dynamic particles (white arrows) that undergo first a fusion event (black arrow) and then fission (white arrows).
SPR2-GFP is deposited at sites of crossover formation (red arrows), followed by a reorientation of the plus end (yellow arrow) to join a complex crossover, consisting of three MTs.
A growing plus end of a MT (yellow arrow) visualized using the YFP:MAP4 reporter undergoes a rapid reorientation and joins a complex crossover.
References
- 1.Baskin T.I. On the alignment of cellulose microfibrils by cortical microtubules: a review and a model. Protoplasma. 2001;215:150–171. doi: 10.1007/BF01280311. [DOI] [PubMed] [Google Scholar]
- 2.Buschmann H., Lloyd C.W. Arabidopsis mutants and the network of microtubule-associated functions. Mol. Plant. 2008;1:888–898. doi: 10.1093/mp/ssn060. [DOI] [PubMed] [Google Scholar]
- 3.Ehrhardt D.W., Shaw S.L. Microtubule dynamics and organization in the plant cortical array. Annu. Rev. Plant Biol. 2006;57:859–875. doi: 10.1146/annurev.arplant.57.032905.105329. [DOI] [PubMed] [Google Scholar]
- 4.Green P.B. Mechanism for plant cellular morphogenesis. Science. 1962;138:1404–1405. doi: 10.1126/science.138.3548.1404. [DOI] [PubMed] [Google Scholar]
- 5.Wasteneys G.O., Ambrose J.C. Spatial organization of plant cortical microtubules: close encounters of the 2D kind. Trends Cell Biol. 2009;19:62–71. doi: 10.1016/j.tcb.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Wasteneys G.O., Fujita M. Establishing and maintaining axial growth: wall mechanical properties and the cytoskeleton. J. Plant Res. 2006;119:5–10. doi: 10.1007/s10265-005-0233-3. [DOI] [PubMed] [Google Scholar]
- 7.Bichet A., Desnos T., Turner S., Grandjean O., Höfte H. BOTERO1 is required for normal orientation of cortical microtubules and anisotropic cell expansion in Arabidopsis. Plant J. 2001;25:137–148. doi: 10.1046/j.1365-313x.2001.00946.x. [DOI] [PubMed] [Google Scholar]
- 8.Bouquin T., Mattsson O., Naested H., Foster R., Mundy J. The Arabidopsis lue1 mutant defines a katanin p60 ortholog involved in hormonal control of microtubule orientation during cell growth. J. Cell Sci. 2003;116:791–801. doi: 10.1242/jcs.00274. [DOI] [PubMed] [Google Scholar]
- 9.Burk D.H., Liu B., Zhong R.Q., Morrison W.H., Ye Z.H. A katanin-like protein regulates normal cell wall biosynthesis and cell elongation. Plant Cell. 2001;13:807–827. [PMC free article] [PubMed] [Google Scholar]
- 10.Wightman R., Turner S.R. Severing at sites of microtubule crossover contributes to microtubule alignment in cortical arrays. Plant J. 2007;52:742–751. doi: 10.1111/j.1365-313X.2007.03271.x. [DOI] [PubMed] [Google Scholar]
- 11.Stoppin-Mellet V., Gaillard J., Vantard M. Katanin’s severing activity favors bundling of cortical microtubules in plants. Plant J. 2006;46:1009–1017. doi: 10.1111/j.1365-313X.2006.02761.x. [DOI] [PubMed] [Google Scholar]
- 12.Chan J., Sambade A., Calder G., Lloyd C. Arabidopsis cortical microtubules are initiated along, as well as branching from, existing microtubules. Plant Cell. 2009;21:2298–2306. doi: 10.1105/tpc.109.069716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin D., Cao L., Zhou Z., Zhu L.Z., Ehrhardt D., Yang Z., Fu Y. Rho GTPase signaling activates microtubule severing to promote microtubule ordering in Arabidopsis. Curr. Biol. 2013;23:290–297. doi: 10.1016/j.cub.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Murata T., Sonobe S., Baskin T.I., Hyodo S., Hasezawa S., Nagata T., Horio T., Hasebe M. Microtubule-dependent microtubule nucleation based on recruitment of gamma-tubulin in higher plants. Nat. Cell Biol. 2005;7:961–968. doi: 10.1038/ncb1306. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura M., Ehrhardt D.W., Hashimoto T. Microtubule and katanin-dependent dynamics of microtubule nucleation complexes in the acentrosomal Arabidopsis cortical array. Nat. Cell Biol. 2010;12:1064–1070. doi: 10.1038/ncb2110. [DOI] [PubMed] [Google Scholar]
- 16.Webb M., Jouannic S., Foreman J., Linstead P., Dolan L. Cell specification in the Arabidopsis root epidermis requires the activity of ECTOPIC ROOT HAIR 3—a katanin-p60 protein. Development. 2002;129:123–131. doi: 10.1242/dev.129.1.123. [DOI] [PubMed] [Google Scholar]
- 17.Allard J.F., Wasteneys G.O., Cytrynbaum E.N. Mechanisms of self-organization of cortical microtubules in plants revealed by computational simulations. Mol. Biol. Cell. 2010;21:278–286. doi: 10.1091/mbc.E09-07-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eren E.C., Gautam N., Dixit R. Computer simulation and mathematical models of the noncentrosomal plant cortical microtubule cytoskeleton. Cytoskeleton (Hoboken) 2012;69:144–154. doi: 10.1002/cm.21009. [DOI] [PubMed] [Google Scholar]
- 19.Hawkins R.J., Tindemans S.H., Mulder B.M. Model for the orientational ordering of the plant microtubule cortical array. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2010;82:011911. doi: 10.1103/PhysRevE.82.011911. [DOI] [PubMed] [Google Scholar]
- 20.Hamant O., Heisler M.G., Jönsson H., Krupinski P., Uyttewaal M., Bokov P., Corson F., Sahlin P., Boudaoud A., Meyerowitz E.M. Developmental patterning by mechanical signals in Arabidopsis. Science. 2008;322:1650–1655. doi: 10.1126/science.1165594. [DOI] [PubMed] [Google Scholar]
- 21.Uyttewaal M., Burian A., Alim K., Landrein B.T., Borowska-Wykręt D., Dedieu A., Peaucelle A., Ludynia M., Traas J., Boudaoud A. Mechanical stress acts via katanin to amplify differences in growth rate between adjacent cells in Arabidopsis. Cell. 2012;149:439–451. doi: 10.1016/j.cell.2012.02.048. [DOI] [PubMed] [Google Scholar]
- 22.Qiang L., Yu W.Q., Andreadis A., Luo M.H., Baas P.W. Tau protects microtubules in the axon from severing by katanin. J. Neurosci. 2006;26:3120–3129. doi: 10.1523/JNEUROSCI.5392-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharp D.J., Ross J.L. Microtubule-severing enzymes at the cutting edge. J. Cell Sci. 2012;125:2561–2569. doi: 10.1242/jcs.101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srayko M., Buster D.W., Bazirgan O.A., McNally F.J., Mains P.E. MEI-1/MEI-2 katanin-like microtubule severing activity is required for Caenorhabditis elegans meiosis. Genes Dev. 2000;14:1072–1084. [PMC free article] [PubMed] [Google Scholar]
- 25.McNally K.P., Buster D., McNally F.J. Katanin-mediated microtubule severing can be regulated by multiple mechanisms. Cell Motil. Cytoskeleton. 2002;53:337–349. doi: 10.1002/cm.10080. [DOI] [PubMed] [Google Scholar]
- 26.Buschmann H., Fabri C.O., Hauptmann M., Hutzler P., Laux T., Lloyd C.W., Schäffner A.R. Helical growth of the Arabidopsis mutant tortifolia1 reveals a plant-specific microtubule-associated protein. Curr. Biol. 2004;14:1515–1521. doi: 10.1016/j.cub.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 27.Shoji T., Narita N.N., Hayashi K., Asada J., Hamada T., Sonobe S., Nakajima K., Hashimoto T. Plant-specific microtubule-associated protein SPIRAL2 is required for anisotropic growth in Arabidopsis. Plant Physiol. 2004;136:3933–3944. doi: 10.1104/pp.104.051748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao M., Wakamatsu Y., Itoh T.J., Shoji T., Hashimoto T. Arabidopsis SPIRAL2 promotes uninterrupted microtubule growth by suppressing the pause state of microtubule dynamics. J. Cell Sci. 2008;121:2372–2381. doi: 10.1242/jcs.030221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Arrows indicate SPR2-GFP particles that are successively deposited as new crossovers are formed.
The movie was taken from a petiole cell. The original image sequence is shown in the left panel. The right panel has been processed to aid visualization. Arrows illustrate two dynamic particles (white arrows) that undergo first a fusion event (black arrow) and then fission (white arrows).
SPR2-GFP is deposited at sites of crossover formation (red arrows), followed by a reorientation of the plus end (yellow arrow) to join a complex crossover, consisting of three MTs.
A growing plus end of a MT (yellow arrow) visualized using the YFP:MAP4 reporter undergoes a rapid reorientation and joins a complex crossover.