Abstract
The molecule serotonin (5-hydroxytryptamine or 5-HT) is involved in numerous biological processes both inside and outside of the central nervous system. 5-HT signals through 5-HT receptors and it is the diversity of these receptors and their subtypes that give rise to the varied physiological responses. It is clear that platelet derived serotonin is critical for normal wound healing in multiple organs including, liver, lung heart and skin. 5-HT stimulates both vasoconstriction and vasodilation, influences inflammatory responses and promotes formation of a temporary scar which acts as a scaffold for normal tissue to be restored. However, in situations of chronic injury or damage 5-HT signaling can have deleterious effects and promote aberrant wound healing resulting in tissue fibrosis and impaired organ regeneration. This review highlights the diverse actions of serotonin signaling in the pathogenesis of fibrotic disease and explores how modulating the activity of specific 5-HT receptors, in particular the 5-HT2 subclass could have the potential to limit fibrosis and restore tissue regeneration. This article is part of a Special Issue entitled: Fibrosis: Translation of basic research to human disease.
Keywords: Fibrosis, Serotonin
Highlights
► Introduction, overview of serotonin signaling and biology ► The role of serotonin in wound healing, regeneration and fibrosis ► Future perspectives
1. Introduction
Serotonin (5-hydroxytryptamine or 5-HT) is an ancient signaling molecule which is found in simple single cell eukaryotes such as Paramecium and Tetrahymena where it regulates growth and swim behavior [1]. 5-HT is also found in molds and plants as well as higher order multicellular organisms such as worms and insects where it controls diverse physiological functions such as swarming, ovulation and insulin secretion [2–4]. In humans 5-HT is perhaps best characterized for its role in the central nervous system (CNS) where it operates as a neurotransmitter at neuronal synapses influencing a broad range of neurophysiological functions including learning and memory, mood, pain and appetite. However, the majority of 5-HT is found outside of the CNS with the major site of synthesis in enterochromaffin cells of the gut where it helps to control smooth muscle contraction and digestion of food. 5-HT can be taken up from the serum by platelets and mast cells via the serotonin transporter (SERT). These cells transport 5-HT to a wide number of tissues and importantly will accumulate within injured tissues releasing 5-HT upon appropriate stimulation. Functions of 5-HT outside the CNS include vasoconstriction/vasodilation, cardiac development and function, respiratory drive, metabolic rate and temperature control, mammary gland development and milk release, uterine contraction, Oocyte maturation and in males the control of penile flaccidity and detumescence [5].
The ability of 5-HT to influence such a wide variety of CNS and systemic physiological functions can be attributed to its diverse receptor system. The 5-HT receptor family consists of 13 distinct genes encoding G-protein coupled seven-transmembrane receptors (GPCRs) and in addition 1 ligand-gated ion channel (the 5-HT3 receptor). The GPCRs are divided into three major groupings according to whether they signal by coupling to Gαq/11 (5-HT2A, 5-HT2B and 5-HT2c), Gαi/o (5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, 5-HT1F and 5-HT5A/B) or Gαs (5-HT4, 5-HT6 and 5-HT7) G proteins [6]. However, it should be noted that GPCRs including the 5-HT receptors are able to couple to more than one G-protein and to a variety of other types of intracellular signaling molecules. Focusing on the three G-proteins alone, each is able to trigger distinct downstream signaling events, which explains the diverse range of physiological responses that are attributed to 5-HT. Gαq coupled receptors stimulate the formation of diacylglycerol (DAG) and inositol phosphates leading to activation of protein kinase C (PKC) and elevation of intracellular calcium [7,8]. In addition Gαq-coupled 5-HT receptors activate Rho resulting in formation of stress fibers and focal adhesions which influences cell migration and adhesion [9]. Activation of the Gαi/o class of 5-HT receptor causes suppression of adenylyl cyclase and reduced levels of cAMP which in neurons results in suppression of neuronal firing [6]. Conversely the activation of Gαs-coupled 5-HT receptors stimulates adenylyl cyclases promoting the accumulation of cAMP and downstream activation of protein kinase A (PKA) and the cAMP response element binding protein (CREB). It is therefore critical to take into account the importance of cellular context and the expression profile of the 5-HT receptors when considering the physiological impact of 5-HT on normal and pathological tissue processes.
2. Serotonin in wound healing
Wound healing is a highly complex coordinated process that can be separated into four distinct phases beginning immediately after injury with (i) coagulation and homeostasis, which is rapidly followed by (ii) inflammation and later by (iii) a fibroproliferative process characterized by scar tissue formation which can eventually resolve over weeks/months by (iv) full restorative regeneration of functional tissue depending on the severity of the wound and the tissue. Platelets obviously play a central role in coagulation and the aggregation of platelets at sites of tissue damage leads to the release of 5-HT which then contributes to homeostasis through its powerful vasoactive properties. Serotonin can either stimulate constriction or dilation of microvasculature depending on the tissue in question. In the liver, 5-HT appears to mainly promote constriction of hepatic sinusoids, since mice lacking peripheral serotonin (Tph−/−) display enhanced sinusoidal perfusion under both normal and diseased states [10]. By contrast, platelet-derived 5-HT coordinates the formation of gaps between endothelial cells in the joint microvasculature, which in arthritic conditions may contribute to inflammation [11]. Precisely how these differential responses are regulated is not well defined but presumably involves differential signaling via specific 5-HT receptors expressed on vascular endothelial and smooth muscle cells.
Beyond its homeostatic role which contributes to recruitment and retention of leukocytes at sites of injury, functions for 5-HT in the inflammatory response are not well defined; however it is clear that 5-HT can influence the behavior and function of many types of immune cell. 5-HT has chemotactic actions on mast cells and eosinophils [12,13] and mature dendritic cells (DCs) respond to 5-HT via 5-HT3, 5-HT4 and 5-HT7 receptors to increase their expression of IL-6 [14,15]. Human monocytes primed with LPS respond to 5-HT via 5-HT3, 5-HT4 and 5-HT7 receptor subtypes to increase their secretion of IL-1β, IL-6, IL-8, IL12p40 and TNF-α [16]. 5-HT also inhibits monocyte apoptosis via the 5-HT1 and 5-HT7 receptors allowing monocytes to persist in tissues and to promote inflammation [17]. Tph1−/− mice are less susceptible to experimental colitis, which is associated with depressed cytokine expression and macrophage infiltration to the bowel suggesting a key role for 5-HT in GI inflammation [18]. T cells display a concentration dependent response to 5-HT, with low levels stimulating proliferation and IL-2 expression [19], whereas high concentrations inhibit the mitogenic stimulation of T cell proliferation and IL-2 receptor expression [20]. Tph−/− mice are less susceptible to steatosis-induced hepatic inflammation, although in this case there was no influence of 5-HT on cytokine expression and instead the inflammatory properties of 5-HT were attributed to production of intracellular ROS in hepatocytes following 5-HT uptake and degradation. As IL-1β and TNF-α can induce the expression of the serotonin transporter SERT [21], uptake of 5-HT and production of ROS may be part of an inflammatory positive feedback mechanism.
3. Serotonin, a stimulator of tissue fibrosis
Mechanistic links between fibrosis and 5-HT were first reported in the 1960s for the condition called carcinoid syndrome, which is caused by neuroendocrine carcinoid tumors that secrete vast quantities of 5-HT [22]. The syndrome is characterized by tissue fibrosis that particularly affects cardiac valves but also impacts on other organs including the lung and skin [23,24]. In the 1980s it was determined that retroperitoneal fibrosis caused by the ergot methysergide is due to the metabolism of this compound into methylergonovine which converts it from a 5-HT2B receptor antagonist to agonist [25]. Subsequently agonism of 5-HT2B has been implicated in fibrosis caused by fenfluramine used in the treatment of obesity [26] and psychiatric disorders [27] and the hallucinogen MDMA [28], both of which trigger 5-HT2B signaling. Dopamine agonists with structural similarity to 5-HT such as pergolide and cabergoline that are used in the treatment of Parkinson's disease have also been associated with development of fibrosis in heart valves involving 5-HT2B agonism, thus limiting their clinical utility [29]. Studies with animal models have confirmed the necessity to screen serotonergic compounds for activation of 5-HT2B activation and promotion of valvulopathy [30].
4. Serotonin signaling in hepatic regeneration and fibrosis
The adult mammalian liver is a highly regenerative organ. It is capable of rapidly and effectively restoring lost liver mass and rebuilding complex tissue structures such as hepatic sinusoids and bile ducts that are vital for normal liver function. Recent investigations employing experimental rodent models of liver regeneration have led to the discovery of autocrine and paracrine hepatic 5-HT signaling pathways that help to regulate the growth and regeneration of parenchymal liver cells. Moreover there appears to be important cross-talk between these 5-HT-driven epithelial cell growth mechanisms and 5-HT signaling pathways that act on myofibroblasts to stimulate hepatic fibrosis. Since there is growing evidence of an inverse relationship between the efficiency of epithelial regeneration and development of tissue fibrosis, manipulating 5-HT pathways that cross-talk between these processes may offer therapeutic opportunities in chronic liver disease.
Seventy percent partial hepatectomy (PHx) provides a model of liver regeneration in which lost mass and tissue architecture is completely restored within 14 days [31]. Intestinal 5-HT is rapidly mobilized and accumulates in the remnant liver following PHx. Early studies demonstrated enhanced regeneration of hepatocyte proliferation following administration of 5-HT to PHx mice and indicated a requirement for 5-HT1A/1B and 5-HT2B receptors, although these early studies suffered from limited availability of receptor-specific agonists and antagonists. In 2006, Lesurtel et al. identified platelets as the primary source of 5-HT in the regenerating liver and demonstrated profound impairment of hepatocyte regeneration in Thp1−/− mice recovery from PHx [32]. Further experiments suggested a role for 5-HT2A, and to a lesser extent 5-HT2B receptors as mediators of 5-HT-driven hepatocyte regeneration. Subsequent studies by the same group of investigators have confirmed a pro-inflammatory and pro-regenerative role for 5-HT in post-ischemic liver repair [33], they discovered that serotonin agonism of 5-HT2B receptors improves animal survival in small liver graft transplantation [34] and age-associated impairments in regenerative capacity [35]. However, of note a different team of investigators working with SERT-deficient rats which lack platelet-derived 5-HT due to absence of uptake from the gut failed to demonstrate a role for this source of 5-HT in liver regeneration [36]. This latter finding may suggest that low levels of free 5-HT in the serum or possibly 5-HT produced by cells resident in the liver may be involved. In this regard a recent study by Omenetti and colleagues is of interest since it reported an unexpected source and role for 5-HT in the hepatic biliary tract [37]. Cholangiocytes are epithelial cells of the bile duct, which have a variety of roles in liver homeostasis and immunity. The study by Omenetti revealed that cholangiocytes express neural-restricted TPH2 and can produce 5-HT, which upon secretion represses cholangiocyte proliferation by a negative feedback mechanism. However, in response to biliary injury 5-HT triggers production of TGFβ1 by wound healing myofibroblasts and in turn soluble TGFβ1 acts on cholangiocytes to repress THP2 expression and enable their proliferation. It will be interesting to learn which 5-HT receptor subtypes transmit the growth suppressive effects of 5-HT on cholangiocytes given that hepatocytes predominantly utilize 5-HT2A to stimulate their proliferation. It will also be of interest to determine if cholangiocyte-derived 5-HT contributes to hepatocyte regeneration following liver damage.
In 2006 our group reported that sinusoidal hepatic stellate cells (HSC) strongly upregulate expression of 5-HT2A and 5-HT2B upon their transdifferentiation to a myofibroblast phenotype [38]. HSC are the major contributor to fibrogenesis in liver disease, producing vast quantities of extracellular matrix and the collagenase inhibitor TIMP-1, as such they are considered important targets for prevention and treatment of fibrosis in chronic liver disease. HSC also express SERT and are able to uptake, release and respond to 5-HT by this autocrine route as well as via paracrine routes such as platelet-derived and cholangiocyte-derived 5-HT [38]. 5-HT2 receptor-selective antagonists inhibit HSC proliferation and induce apoptosis which implicates 5-HT/5-HT2 signaling in the regulation of fibrosis, since the balance between myofibroblast proliferation and apoptosis is an important determinant of fibrosis progression. Taking this work further, we have more recently proposed that signaling through the 5-HT2B receptor on HSC-derived myofibroblasts is both pro-fibrogenic and anti-regenerative in the diseased liver [39]. Specific genetic (5-HT2B knockout) or pharmacological blockade of 5-HT2B stimulates hepatocyte proliferation and suppresses fibrosis in multiple distinct injury models. A common pathway may be responsible for these dual functions of 5-HT/5-HT2B signaling since it triggers ERK- and JunD-dependent activation of TGFβ1 expression, the latter being a potent suppressor of hepatocyte proliferation as well as being a powerful stimulator of fibrogenic gene expression [40,41].
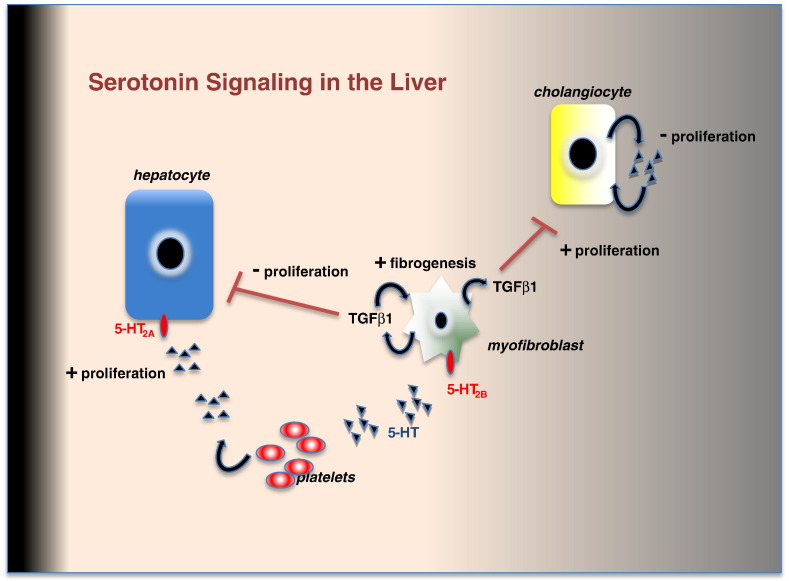
As illustrated in Fig. 1, based on current available knowledge there is substantial complexity in the mechanisms by which 5-HT influences liver injury and repair, with multiple effects on different cell types and the activation of cell-cell signaling pathways that link epithelial cell regeneration and fibrosis. At present, the available data suggest that 5-HT/5-HT2A signaling in hepatocytes is pro-regenerative whereas 5-HT/5-HT2B signaling via HSC in a fibrogenic microenvironment is anti-regenerative. However, this current model may be refined by the future availability of more specific 5-HT receptor agonists and antagonist, and conditional genetic targeting systems that allow 5-HT receptor genes to be deleted in each of the relevant parenchymal and non-parenchymal liver cells.
Fig. 1.
The fibrogenic and proliferative actions of serotonin in liver homeostasis and disease. Platelet derived 5-HT stimulates hepatocyte proliferation via 5-HT2A receptors whilst in hepatic myofibroblasts 5-HT signaling via 5-HT2B receptors enhances TGFβ1 production which promotes fibrosis and limits liver regeneration. Local production of 5-HT by cholangiocytes inhibits their proliferation; however this autocrine negative feedback loop is suppressed by TGFβ1.
5. Serotonin signaling in the lung
5-HT has been linked to pulmonary fibrosis since the 1960s when it was documented that the headache medicine methysergide, which has close structural similarities with 5-HT, caused pleuro-pulmonary fibrosis that in some patients was reversible upon cessation of methysergide treatment [42]. Fibrosis is a feature of many different types of chronic respiratory diseases including idiopathic pulmonary fibrosis (IPF), pulmonary arterial hypertension (PAH), chronic obstructive pulmonary disease (COPD), post-transplant bronchial obliterative syndrome (BOS) and asthma [43]. In addition, lung fibrosis is a serious pathological component of systemic sclerosis [44] and can also be caused by a variety of medications (e.g. methotrexate) [45] as well as radiation therapy to the chest [46].
The role of 5-HT in these different respiratory pathologies has not been fully investigated; however evidence is emerging that it can be secreted locally by many different cell types and in addition to its powerful vasoactive effects on pulmonary arteries, 5-HT can stimulate the proliferation and fibrogenic actions of lung (myo)fibroblasts [47,48]. Importantly, platelets and mast cells are not the only source of 5-HT in the respiratory system. In addition, to recruited platelets and mast cells resident pulmonary neuroendocrine cells synthesize and secrete 5-HT [49]. These neuroendocrine cells become proliferative in PAH and this correlates with proliferation of myofibroblasts in the pulmonary arteries. In some conditions, such as acute post-operative PAH in children it is suggested that proliferating pulmonary neuroendocrine cells may be the major source of 5-HT. Expression of Tph1 is found in pulmonary epithelial cells and levels are increased in patients with idiopathic PAH [50]. Noteworthy is that SERT is particularly highly expressed in the lung and has been postulated to be involved in promoting the proliferation of pulmonary arterial fibroblasts and pulmonary arterial smooth muscle cells via the activation of ERK and ROS following the internalization of 5-HT [51].
The effects of 5-HT in the respiratory systems are pulmonary arterial constriction, bronchoconstriction and stimulation of hyperplastic and hypertrophic alterations in smooth muscle cells and myofibroblasts [52]. This stimulates sclerotic remodeling of the pulmonary vasculature and/or airways, with the end result being increased pulmonary vascular resistance and/or lung fibrosis. There remains uncertainty regarding the precise contributions to specific 5-HT receptors in PAH and lung fibrosis. Prior to 1993 it was assumed that 5-HT2A mediated vasoconstriction of pulmonary arteries and its antagonist ketanserin were found to have some clinical utility, particularly in the elderly [53]. However, more recent work has suggested contributions of 5-HT1B and 5-HT2B receptors [54–56]. The Eickelberg group recently reported increased expression of 5-HT1A/B and 5-HT2B in the lungs of patients with IPF and in sufferers of non-specific interstitial pneumonia [57]. 5-HT2A was also increased selectively in IPF lungs and was localized to fibroblasts, whereas 5-HT2B was chiefly found at the lung epithelium. The authors also demonstrated that the 5-HT2A/B antagonist terugide is a potent repressor of fibroblast TGFβ1 expression and when administered in vivo the drug improved lung function and decreased fibrosis when applied in a therapeutic regimen to mice with established bleomycin-induced lung disease. This latter finding confirmed earlier observations by Fabre et al. that bleomycin-induced fibrosis is associated with increased serotonin in the lung and can be attenuated by blockade of either 5-HT2A (by ketanserin) or 5-HT2B (by SB215505) [58]. Although Pferidone has recently been approved for the treatment of IPF it remains a deadly disease with rising incidence and as such clinical studies in IPF with 5-HT2A and 5-HT2B antagonists are certainly warranted.
6. Serotonin signaling in the heart
In addition to the impact of 5-HT-like drugs and carcinoid tumors on valvular fibrosis, 5-HT receptors and transporters may also contribute to cardiac hypertrophy which is characterized by the loss of cardiac myocytes, accumulation of interstitial fibroblasts and collagen deposition [59]. The importance of 5-HT in the regulation of heart structure was demonstrated in mice lacking the 5-HT2B receptor, which are susceptible to embryonic and neonatal death due to lack of trabeculae in the heart [60]. Furthermore, targeted over-expression of 5-HT2B in cardiomyocytes induces cardiac hypertrophy [61]. Collectively these observations suggest that 5-HT/5-HT2B signaling may act either directly on cardiomyocytes or indirectly through other cell types via the release of paracrine regulators.
As cardiac hypertrophy can be initiated by processes resulting from ligands of a variety of other G protein-coupled receptors such as the AT1 angiotensin II receptor, ETA endothelin 1 receptor and adrenergic receptors, there is potential for 5-HT/5-HT2B signaling to cross-talk with other hypertrophic pathways. Jaffré and colleagues have discovered direct effects of 5-HT on ventricular fibroblasts including production of IL-6, IL-1β and TNF-α in a 5-HT2B-dependent manner. Adrenergic receptor stimulation has also been shown to induce these hypertrophic cytokines from cardiac fibroblasts, which are found at elevated levels in the diseased heart [62]. The study by Jaffré modeled β-adrenergic stimulation of cardiac hypertrophy by perfusion with isoproterenol (ISO) [63]. As expected ISO perfusion elevated plasma levels of IL-6, IL-1β and TNF-α, which was not seen in 5-HT2B knockout mice or wild type mice co-administered the 5-HT2B antagonist SB206553. Moreover, SB206553 treatment or absence of the 5-HT2B gene protected mice from ISO-induced hypertrophic remodeling of the heart muscle. Whether these effects result from signaling cross-talk between adrenergic ligand/receptor and 5-HT/5-HT2B pathways in fibroblasts or may instead be due to direct signaling emerging from heterodimer complexes formed between adrenergic and 5-HT2B receptors is still to be determined. A potential mechanism by which 5-HT2B receptors may cross-talk with other G protein-linked receptors is through pathways leading to ROS generation. 5-HT2B is coupled to NAD(P)H oxidase [64] which also plays a pivotal role in physiological responses to angiotensin II/AT1 signaling. Monassier et al. recently reported that selective antagonism of 5-HT2B with SB215505 prevented cardiac superoxide generation and hypertrophy caused by infusion of either angiotensin II or ISO [65]. Hence, these studies support the emerging role of the 5-HT2B receptor in the control of (myo)fibroblast function and add to the growing evidence that 5-HT2B antagonists may be useful in the treatment or prevention of pathological cardiac remodeling.
7. Serotonin signaling in systemic sclerosis (scleroderma)
Systemic scleroderma (SSc) is a rare autoimmune disease of unknown cause mainly affecting females with an onset between the ages of 30–50. The disease is characterized by deposition of fibril-forming collagen in the skin, lungs, stomach, heart and the kidneys, with the latter being a poor prognostic factor [44]. Most SSc patients also have vascular disease and Raynaud's phenomenon [66]. Experimental studies in the 1950s revealed that 5-HT can stimulate the proliferation of skin fibroblasts and that when injected subcutaneously in rodents causes remodeling of skin in a manner that resembles skin pathology in SSc [67]. Roddie et al. also reported in the mid-1950s that infusion of 5-HT into brachial arteries of man induces features of Raynaud's [68]. Scleroderma is reported in patients with carcinoid tumors [23] and was observed following the treatment of intention myoclorius with L-5-hydroxytryptophan and carbidopa, which was associated with high serotonin levels [69].
The pathophysiological basis for involvement of 5-HT in SSc is unclear; however patients suffer progressive endothelial cell damage and this is evident before fibrosis is observed. It has been proposed that loss of anticoagulant properties of the endothelium may trigger platelet activation and release of 5-HT; this idea being supported by elevated plasma levels of 5-HT in SSc [70], although others failed to reproduce this finding [71]. A recent elegant study by Dees and colleagues provided stronger experimental evidence for a causative role of platelet-derived 5-HT in scleroderma [72]. They showed that cultured dermal fibroblasts from SSc patients and healthy individuals respond to 5-HT by increasing their expression of collagen Ia1, collagen 1a2 and fibronectin. These effects of 5-HT on matrix synthesis were blocked by the 5-HT2B antagonist SB 204741 or by transfected 5-HT2B siRNAs, whereas the 5-HT2A antagonist ketanserin was without effect. The authors also showed that broad specificity 5-HT2 inhibitors as well as the more selective 5-HT2B antagonist SB 204741 prevent bleomycin-induced dermal fibrosis, this being associated with reduced numbers of myofibroblasts. Furthermore, SB 204741 also reduced fibrosis and myofibroblast transdifferentiation in the genetic tight skin1 (Tsk-1) model and similar effects were observed when Tsk-1 mice were crossed with either 5-HT2B or Tph1 knockout mice. Intriguingly, 5-HT/5-HT2B stimulation of matrix synthesis by dermal fibroblasts was shown to be dependent on activation of TGFβ1 gene transcription and subsequent TGFβ1 signaling. The same group later reported that the AP-1 transcription factor JunD, which is over-expressed in SSc skin and cultured fibroblasts, mediates TGFβ1-induced fibroblast activation and bleomycin-induced fibrosis [73]. These data resemble our findings in the diseased liver and indicate the 5-HT/5-HT2B-ERK-JunD-TGFβ pathway in fibroblastic wound healing cells may be a core fibrogenic signaling route in multiple organs.
8. Summary
There is now a clear pattern emerging from independent studies in distinct organ systems that the 5-HT system activated during the earliest phases of wound repair has a major influence on fibrogenesis. In the context of acute injury the pro-fibrogenic and pro-regenerative influences of 5-HT will combine to ensure optimal repair and restoration of tissue architecture and function. However, in the context of chronic disease it may be desirable to tone down the fibrogenic actions of 5-HT. The latter may be achieved by modulating the activities of specific 5-HT receptors that trigger the activation of fibrogenic signal transduction. While there is still much more to be learned about the way in which the different 5-HT receptors combine to regulate tissue repair there is already sufficient pre-clinical data to warrant clinical investigations with antagonists of the 5-HT2 receptors. As discussed, 5-HT2 receptors have been strongly implicated as drivers of fibrosis in heart valves, lung, skin and liver, plus they are stimulators of the expression of TGFβ. 5-HT2 receptor antagonists are safe in man, are already in clinical trials for PAH [74] and should now be advanced into trials for fibrosis.
Acknowledgments
Work in the laboratories of D.A.M. and F.O. relevant to this review is funded by grants from the UK Medical Research Council, the Wellcome Trust, The European Council and Newcastle University. The authors additionally wish to acknowledge the work of many contributors who for reasons of brevity may not have been cited in this review.
Footnotes
This article is part of a Special Issue entitled: Fibrosis: Translation of basic research to human disease.
Declarations: DAM and FO are consultants for and in receipt of research funding from GlaxoSmithKline.
References
- 1.Csaba G. Presence in and effects of pineal indoleamines at very low level of phylogeny. Experientia. 1993;49:627–634. doi: 10.1007/BF01923943. [DOI] [PubMed] [Google Scholar]
- 2.Anstey M.L., Rogers S.M., Ott S.R., Burrows M., Simpson S.J. Serotonin mediates behavioral gregarization underlying swarm formation in desert locusts. Science. 2009;323:627–630. doi: 10.1126/science.1165939. [DOI] [PubMed] [Google Scholar]
- 3.Srinivasan S., Sadegh L., Elle I.C., Christensen A.G., Faergeman N.J., Ashrafi K. Serotonin regulates C. elegans fat and feeding through independent molecular mechanisms. Cell Metab. 2008;7:533–544. doi: 10.1016/j.cmet.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruaud A.F., Thummel C.S. Serotonin and insulin signaling team up to control growth in Drosophila. Genes Dev. 2008;22:1851–1855. doi: 10.1101/gad.1700708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger M., Gray J.A., Roth B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nichols D.E., Nichols C.D. Serotonin receptors. Chem. Rev. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- 7.Bockaert J., Roussignol G., Becamel C., Gavarini S., Joubert L., Dumuis A., Fagni L., Marin P. GPCR-interacting proteins (GIPs): nature and functions. Biochem. Soc. Trans. 2004;32:851–855. doi: 10.1042/BST0320851. [DOI] [PubMed] [Google Scholar]
- 8.Millan M.J., Marin P., Bockaert J. Mannoury la Cour C: signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharmacol. Sci. 2008;29:454–464. doi: 10.1016/j.tips.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Matsusaka S., Wakabayashi I. 5-Hydroxytryptamine as a potent migration enhancer of human aortic endothelial cells. FEBS Lett. 2005;579:6721–6725. doi: 10.1016/j.febslet.2005.10.068. [DOI] [PubMed] [Google Scholar]
- 10.Lang P.A., Contaldo C., Georgiev P., El-Badry A.M., Recher M., Kurrer M., Cervantes-Barragan L., Ludewig B., Calzascia T., Bolinger B., Merkler D., Odermatt B., Bader M., Graf R., Clavien P.A., Hegazy A.N., Lohning M., Harris N.L., Ohashi P.S., Hengartner H., Zinkernagel R.M., Lang K.S. Aggravation of viral hepatitis by platelet-derived serotonin. Nat. Med. 2008;14:756–761. doi: 10.1038/nm1780. [DOI] [PubMed] [Google Scholar]
- 11.Cloutier N., Pare A., Farndale R.W., Schumacher H.R., Nigrovic P.A., Lacroix S., Boilard E. Platelets can enhance vascular permeability. Blood. 2012;120:334–1343. doi: 10.1182/blood-2012-02-413047. [DOI] [PubMed] [Google Scholar]
- 12.Kushnir-Sukhov N.M., Gilfillan A.M., Coleman J.W., Brown J.M., Bruening S., Toth M., Metcalfe D.D. 5-Hydroxytryptamine induces mast cell adhesion and migration. J. Immunol. 2006;177:6422–6432. doi: 10.4049/jimmunol.177.9.6422. [DOI] [PubMed] [Google Scholar]
- 13.Boehme S.A., Lio F.M., Sikora L., Pandit T.S., Lavrador K., Rao S.P., Sriramarao P. Cutting edge: serotonin is a chemotactic factor for eosinophils and functions additively with eotaxin. J. Immunol. 2004;173:3599–3603. doi: 10.4049/jimmunol.173.6.3599. [DOI] [PubMed] [Google Scholar]
- 14.Idzko M., Panther E., Stratz C., Muller T., Bayer H., Zissel G., Durk T., Sorichter S., Di Virgilio F., Geissler M., Fiebich B., Herouy Y., Elsner P., Norgauer J., Ferrari D. The serotoninergic receptors of human dendritic cells: identification and coupling to cytokine release. J. Immunol. 2004;172:6011–6019. doi: 10.4049/jimmunol.172.10.6011. [DOI] [PubMed] [Google Scholar]
- 15.Muller T., Durk T., Blumenthal B., Grimm M., Cicko S., Panther E., Sorichter S., Herouy Y., Di Virgilio F., Ferrari D., Norgauer J., Idzko M. 5-Hydroxytryptamine modulates migration, cytokine and chemokine release and T-cell priming capacity of dendritic cells in vitro and in vivo. PLoS One. 2009;4:e6453. doi: 10.1371/journal.pone.0006453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durk T., Panther E., Muller T., Sorichter S., Ferrari D., Pizzirani C., Di Virgilio F., Myrtek D., Norgauer J., Idzko M. 5-Hydroxytryptamine modulates cytokine and chemokine production in LPS-primed human monocytes via stimulation of different 5-HTR subtypes. Int. Immunol. 2005;17:599–606. doi: 10.1093/intimm/dxh242. [DOI] [PubMed] [Google Scholar]
- 17.Soga F., Katoh N., Inoue T., Kishimoto S. Serotonin activates human monocytes and prevents apoptosis. J. Invest. Dermatol. 2007;127:1947–1955. doi: 10.1038/sj.jid.5700824. [DOI] [PubMed] [Google Scholar]
- 18.Ghia J.E., Li N., Wang H., Collins M., Deng Y., El-Sharkawy R.T., Cote F., Mallet J., Khan W.I. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649–1660. doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 19.Young M.R., Kut J.L., Coogan M.P., Wright M.A., Young M.E., Matthews J. Stimulation of splenic T-lymphocyte function by endogenous serotonin and by low-dose exogenous serotonin. Immunology. 1993;80:395–400. [PMC free article] [PubMed] [Google Scholar]
- 20.Slauson D.O., Walker C., Kristensen F., Wang Y., de Weck A.L. Mechanisms of serotonin-induced lymphocyte proliferation inhibition. Cell. Immunol. 1984;84:240–252. doi: 10.1016/0008-8749(84)90096-0. [DOI] [PubMed] [Google Scholar]
- 21.Zhu C.B., Blakely R.D., Hewlett W.A. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- 22.Oates J.A., Melmon K., Sjoerdsma A., Gillespie L., Mason D.T. Release of a kinin peptide in the carcinoid syndrome. Lancet. 1964;1:514–517. doi: 10.1016/s0140-6736(64)92907-1. [DOI] [PubMed] [Google Scholar]
- 23.Fries J.F., Lindgren J.A., Bull J.M. Scleroderma-like lesions and the carcinoid syndrome. Arch. Intern. Med. 1973;131:550–553. doi: 10.1001/archinte.1973.00320100078010. [DOI] [PubMed] [Google Scholar]
- 24.Pavlovic M., Saiag P., Lotz J.P., Marinho E., Clerici T., Izrael V. Regression of sclerodermatous skin lesions in a patient with carcinoid syndrome treated by octreotide. Arch. Dermatol. 1995;131:1207–1209. doi: 10.1001/archderm.131.10.1207. [DOI] [PubMed] [Google Scholar]
- 25.Reimund E. Methysergide and retroperitoneal fibrosis. Lancet. 1987;1:443. doi: 10.1016/s0140-6736(87)90140-1. [DOI] [PubMed] [Google Scholar]
- 26.Fowles R.E., Cloward T.V., Yowell R.L. Endocardial fibrosis associated with fenfluramine-phentermine. N. Engl. J. Med. 1998;338:1316. doi: 10.1056/NEJM199804303381816. [DOI] [PubMed] [Google Scholar]
- 27.Rothman R.B., Baumann M.H., Savage J.E., Rauser L., McBride A., Hufeisen S.J., Roth B.L. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102:2836–2841. doi: 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]
- 28.Setola V., Hufeisen S.J., Grande-Allen K.J., Vesely I., Glennon R.A., Blough B., Rothman R.B., Roth B.L. 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol. Pharmacol. 2003;63:1223–1229. doi: 10.1124/mol.63.6.1223. [DOI] [PubMed] [Google Scholar]
- 29.Antonini A., Poewe W. Fibrotic heart-valve reactions to dopamine-agonist treatment in Parkinson's disease. Lancet Neurol. 2007;6:826–829. doi: 10.1016/S1474-4422(07)70218-1. [DOI] [PubMed] [Google Scholar]
- 30.Rothman R.B., Baumann M.H. Serotonergic drugs and valvular heart disease. Expert Opin. Drug Saf. 2009;8:317–329. doi: 10.1517/14740330902931524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yokoyama H.O., Wilson M.E., Tsuboi K.K., Stowell R.E. Regeneration of mouse liver after partial hepatectomy. Cancer Res. 1953;13:80–85. [PubMed] [Google Scholar]
- 32.Lesurtel M., Graf R., Aleil B., Walther D.J., Tian Y., Jochum W., Gachet C., Bader M., Clavien P.A. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–107. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 33.Nocito A., Georgiev P., Dahm F., Jochum W., Bader M., Graf R., Clavien P.A. Platelets and platelet-derived serotonin promote tissue repair after normothermic hepatic ischemia in mice. Hepatology. 2007;45:369–376. doi: 10.1002/hep.21516. [DOI] [PubMed] [Google Scholar]
- 34.Tian Y., Graf R., El-Badry A.M., Lesurtel M., Furrer K., Moritz W., Clavien P.A. Activation of serotonin receptor-2B rescues small-for-size liver graft failure in mice. Hepatology. 2011;53:253–262. doi: 10.1002/hep.23960. [DOI] [PubMed] [Google Scholar]
- 35.Furrer K., Rickenbacher A., Tian Y., Jochum W., Bittermann A.G., Kach A., Humar B., Graf R., Moritz W., Clavien P.A. Serotonin reverts age-related capillarization and failure of regeneration in the liver through a VEGF-dependent pathway. Proc. Natl. Acad. Sci. U. S. A. 2011;108:2945–2950. doi: 10.1073/pnas.1012531108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matondo R.B., Punt C., Homberg J., Toussaint M.J., Kisjes R., Korporaal S.J., Akkerman J.W., Cuppen E., de Bruin A. Deletion of the serotonin transporter in rats disturbs serotonin homeostasis without impairing liver regeneration. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G963–G968. doi: 10.1152/ajpgi.90709.2008. [DOI] [PubMed] [Google Scholar]
- 37.Omenetti A., Yang L., Gainetdinov R.R., Guy C.D., Choi S.S., Chen W., Caron M.G., Diehl A.M. Paracrine modulation of cholangiocyte serotonin synthesis orchestrates biliary remodeling in adults. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;300:G303–G315. doi: 10.1152/ajpgi.00368.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruddell R.G., Oakley F., Hussain Z., Yeung I., Bryan-Lluka L.J., Ramm G.A., Mann D.A. A role for serotonin (5-HT) in hepatic stellate cell function and liver fibrosis. Am. J. Pathol. 2006;169:861–876. doi: 10.2353/ajpath.2006.050767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ebrahimkhani M.R., Oakley F., Murphy L.B., Mann J., Moles A., Perugorria M.J., Ellis E., Lakey A.F., Burt A.D., Douglass A., Wright M.C., White S.A., Jaffre F., Maroteaux L., Mann D.A. Stimulating healthy tissue regeneration by targeting the 5-HT(2)B receptor in chronic liver disease. Nat. Med. 2011;17:1668–1673. doi: 10.1038/nm.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fausto N., Campbell J.S., Riehle K.J. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 41.Gressner A.M., Weiskirchen R., Breitkopf K., Dooley S. Roles of TGF-beta in hepatic fibrosis. Front. Biosci. 2002;7:d793–d807. doi: 10.2741/A812. [DOI] [PubMed] [Google Scholar]
- 42.Graham J.R. Cardiac and pulmonary fibrosis during methysergide therapy for headache. Trans. Am. Clin. Climatol. Assoc. 1967;78:79–92. [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson M.S., Wynn T.A. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2:103–121. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Almeida I., Faria R., Vita P., Vasconcelos C. Systemic sclerosis refractory disease: from the skin to the heart. Autoimmun. Rev. 2011;10:693–701. doi: 10.1016/j.autrev.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 45.van der Veen M.J., Dekker J.J., Dinant H.J., van Soesbergen R.M., Bijlsma J.W. Fatal pulmonary fibrosis complicating low dose methotrexate therapy for rheumatoid arthritis. J. Rheumatol. 1995;22:1766–1768. [PubMed] [Google Scholar]
- 46.Denham J.W., Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound’. Radiother. Oncol. 2002;63:129–145. doi: 10.1016/s0167-8140(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 47.Lee S.L., Wang W.W., Lanzillo J.J., Fanburg B.L. Regulation of serotonin-induced DNA synthesis of bovine pulmonary artery smooth muscle cells. Am. J. Physiol. 1994;266:L53–L60. doi: 10.1152/ajplung.1994.266.1.L53. [DOI] [PubMed] [Google Scholar]
- 48.Welsh D.J., Harnett M., MacLean M., Peacock A.J. Proliferation and signaling in fibroblasts: role of 5-hydroxytryptamine2A receptor and transporter. Am. J. Respir. Crit. Care Med. 2004;170:252–259. doi: 10.1164/rccm.200302-264OC. [DOI] [PubMed] [Google Scholar]
- 49.Johnson D.E., Georgieff M.K. Pulmonary neuroendocrine cells. Their secretory products and their potential roles in health and chronic lung disease in infancy. Am. Rev. Respir. Dis. 1989;140:1807–1812. doi: 10.1164/ajrccm/140.6.1807. [DOI] [PubMed] [Google Scholar]
- 50.Eddahibi S., Guignabert C., Barlier-Mur A.M., Dewachter L., Fadel E., Dartevelle P., Humbert M., Simonneau G., Hanoun N., Saurini F., Hamon M., Adnot S. Cross talk between endothelial and smooth muscle cells in pulmonary hypertension: critical role for serotonin-induced smooth muscle hyperplasia. Circulation. 2006;113:1857–1864. doi: 10.1161/CIRCULATIONAHA.105.591321. [DOI] [PubMed] [Google Scholar]
- 51.Maclean M.R., Dempsie Y. The serotonin hypothesis of pulmonary hypertension revisited. Adv. Exp. Med. Biol. 2010;661:309–322. doi: 10.1007/978-1-60761-500-2_20. [DOI] [PubMed] [Google Scholar]
- 52.MacLean M.R., Herve P., Eddahibi S., Adnot S. 5-Hydroxytryptamine and the pulmonary circulation: receptors, transporters and relevance to pulmonary arterial hypertension. Br. J. Pharmacol. 2000;131:161–168. doi: 10.1038/sj.bjp.0703570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Demoulin J.C., Bertholet M., Soumagne D., David J.L., Kulbertus H.E. 5-HT2-receptor blockade in the treatment of heart failure. A preliminary study. Lancet. 1981;1:1186–1188. doi: 10.1016/s0140-6736(81)92352-7. [DOI] [PubMed] [Google Scholar]
- 54.Dumitrascu R., Kulcke C., Konigshoff M., Kouri F., Yang X., Morrell N., Ghofrani H.A., Weissmann N., Reiter R., Seeger W., Grimminger F., Eickelberg O., Schermuly R.T., Pullamsetti S.S. Terguride ameliorates monocrotaline-induced pulmonary hypertension in rats. Eur. Respir. J. 2011;37:1104–1118. doi: 10.1183/09031936.00126010. [DOI] [PubMed] [Google Scholar]
- 55.Launay J.M., Herve P., Peoc'h K., Tournois C., Callebert J., Nebigil C.G., Etienne N., Drouet L., Humbert M., Simonneau G., Maroteaux L. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat. Med. 2002;8:1129–1135. doi: 10.1038/nm764. [DOI] [PubMed] [Google Scholar]
- 56.Morecroft I., Heeley R.P., Prentice H.M., Kirk A., MacLean M.R. 5-Hydroxytryptamine receptors mediating contraction in human small muscular pulmonary arteries: importance of the 5-HT1B receptor. Br. J. Pharmacol. 1999;128:730–734. doi: 10.1038/sj.bjp.0702841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konigshoff M., Dumitrascu R., Udalov S., Amarie O.V., Reiter R., Grimminger F., Seeger W., Schermuly R.T., Eickelberg O. Increased expression of 5-hydroxytryptamine2A/B receptors in idiopathic pulmonary fibrosis: a rationale for therapeutic intervention. Thorax. 2010;65:949–955. doi: 10.1136/thx.2009.134353. [DOI] [PubMed] [Google Scholar]
- 58.Fabre A., Marchal-Somme J., Marchand-Adam S., Quesnel C., Borie R., Dehoux M., Ruffie C., Callebert J., Launay J.M., Henin D., Soler P., Crestani B. Modulation of bleomycin-induced lung fibrosis by serotonin receptor antagonists in mice. Eur. Respir. J. 2008;32:426–436. doi: 10.1183/09031936.00126907. [DOI] [PubMed] [Google Scholar]
- 59.Hutcheson J.D., Setola V., Roth B.L., Merryman W.D. Serotonin receptors and heart valve disease—it was meant 2B. Pharmacol. Ther. 2011;132:146–157. doi: 10.1016/j.pharmthera.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nebigil C.G., Choi D.S., Dierich A., Hickel P., Le Meur M., Messaddeq N., Launay J.M., Maroteaux L. Serotonin 2B receptor is required for heart development. Proc. Natl. Acad. Sci. U. S. A. 2000;97:9508–9513. doi: 10.1073/pnas.97.17.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nebigil C.G., Jaffre F., Messaddeq N., Hickel P., Monassier L., Launay J.M., Maroteaux L. Overexpression of the serotonin 5-HT2B receptor in heart leads to abnormal mitochondrial function and cardiac hypertrophy. Circulation. 2003;107:3223–3229. doi: 10.1161/01.CIR.0000074224.57016.01. [DOI] [PubMed] [Google Scholar]
- 62.Jaffre F., Callebert J., Sarre A., Etienne N., Nebigil C.G., Launay J.M., Maroteaux L., Monassier L. Involvement of the serotonin 5-HT2B receptor in cardiac hypertrophy linked to sympathetic stimulation: control of interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha cytokine production by ventricular fibroblasts. Circulation. 2004;110:969–974. doi: 10.1161/01.CIR.0000139856.20505.57. [DOI] [PubMed] [Google Scholar]
- 63.Jaffre F., Bonnin P., Callebert J., Debbabi H., Setola V., Doly S., Monassier L., Mettauer B., Blaxall B.C., Launay J.M., Maroteaux L. Serotonin and angiotensin receptors in cardiac fibroblasts coregulate adrenergic-dependent cardiac hypertrophy. Circ. Res. 2009;104:113–123. doi: 10.1161/CIRCRESAHA.108.180976. [DOI] [PubMed] [Google Scholar]
- 64.Pietri M., Schneider B., Mouillet-Richard S., Ermonval M., Mutel V., Launay J.M., Kellermann O. Reactive oxygen species-dependent TNF-alpha converting enzyme activation through stimulation of 5-HT2B and alpha1D autoreceptors in neuronal cells. FASEB J. 2005;19:1078–1087. doi: 10.1096/fj.04-3631com. [DOI] [PubMed] [Google Scholar]
- 65.Monassier L., Laplante M.A., Jaffre F., Bousquet P., Maroteaux L., de Champlain J. Serotonin 5-HT(2B) receptor blockade prevents reactive oxygen species-induced cardiac hypertrophy in mice. Hypertension. 2008;52:301–307. doi: 10.1161/HYPERTENSIONAHA.107.105551. [DOI] [PubMed] [Google Scholar]
- 66.Generini S., Matucci Cerinic M. Raynaud's phenomenon and vascular disease in systemic sclerosis. Adv. Exp. Med. Biol. 1999;455:93–100. doi: 10.1007/978-1-4615-4857-7_13. [DOI] [PubMed] [Google Scholar]
- 67.Macdonald R.A., Robbins S.L., Mallory G.K. Dermal fibrosis following subcutaneous injections of serotonin creatinine sulphate. Proc. Soc. Exp. Biol. Med. 1958;97:334–337. doi: 10.3181/00379727-97-23734. [DOI] [PubMed] [Google Scholar]
- 68.Roddie I.C., Shepherd J.T., Whelan R.F. The action of 5-hydroxytryptamine on the blood vessels of the human hand and forearm. Br. J. Pharmacol. Chemother. 1955;10:445–450. doi: 10.1111/j.1476-5381.1955.tb00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sternberg E.M., Van Woert M.H., Young S.N., Magnussen I., Baker H., Gauthier S., Osterland C.K. Development of a scleroderma-like illness during therapy with L-5-hydroxytryptophan and carbidopa. N. Engl. J. Med. 1980;303:782–787. doi: 10.1056/NEJM198010023031403. [DOI] [PubMed] [Google Scholar]
- 70.Biondi M.L., Marasini B., Bianchi E., Agostoni A. Plasma free and intraplatelet serotonin in patients with Raynaud's phenomenon. Int. J. Cardiol. 1988;19:335–339. doi: 10.1016/0167-5273(88)90238-0. [DOI] [PubMed] [Google Scholar]
- 71.Klimiuk P.S., Grennan A., Weinkove C., Jayson M.I. Platelet serotonin in systemic sclerosis. Ann. Rheum. Dis. 1989;48:586–589. doi: 10.1136/ard.48.7.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dees C., Akhmetshina A., Zerr P., Reich N., Palumbo K., Horn A., Jungel A., Beyer C., Kronke G., Zwerina J., Reiter R., Alenina N., Maroteaux L., Gay S., Schett G., Distler O., Distler J.H. Platelet-derived serotonin links vascular disease and tissue fibrosis. J. Exp. Med. 2011;208:961–972. doi: 10.1084/jem.20101629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palumbo K., Zerr P., Tomcik M., Vollath S., Dees C., Akhmetshina A., Avouac J., Yaniv M., Distler O., Schett G., Distler J.H. The transcription factor JunD mediates transforming growth factor {beta}-induced fibroblast activation and fibrosis in systemic sclerosis. Ann. Rheum. Dis. 2011;70:1320–1326. doi: 10.1136/ard.2010.148296. [DOI] [PubMed] [Google Scholar]
- 74.McLaughlin V.V. Looking to the future: a new decade of pulmonary arterial hypertension therapy. Eur. Respir. Rev. 2011;20:262–269. doi: 10.1183/09059180.00006411. [DOI] [PMC free article] [PubMed] [Google Scholar]