Summary
Biobrane® is commonly used in paediatric burns to cover partial thickness burns and donor sites of split thickness skin (SSG). The purpose of this study is to evaluate the use of Biobrane® in dressing SSG adjacent to skin graft donor sites or partial thickness burns. A retrospective review was undertaken to determine the use of Biobrane® in dressing SSG, where the grafted areas were adjacent to donor sites or partial thickness burns. Between 2009 and 2012, we reported five cases of using Biobrane® to dress SSG, where the grafted areas were adjacent to partial thickness burns and two cases where the grafted areas were adjacent to donor sites. Biobrane® promoted adherence of the SSG to the wound, prevented shearing, and allowed fluid drainage. At the same time, Biobrane® also facilitated healing of the adjacent donor sites or partial thickness burns.
Keywords: Biobrane®, SSG, skin graft donor sites, paediatric burns
Abstract
Biobrane® est couramment utilisé dans les brûlures pédiatriques pour couvrir les brûlures d’épaisseur partielle et les sites donneurs de greffe de peau mince. Le but de cette étude est d’évaluer l’utilisation de Biobrane® sur les greffes de peau mince adjacentes aux sites donneurs des greffes de peau ou des brûlures d’épaisseur partielle. Une étude rétrospective a été menée pour éxaminer l’utilité de Biobrane® sur les greffes de peau mince où les zones greffées étaient adjacentes aux sites donneurs ou aux brûlures d’épaisseur partielle. Entre 2009 et 2012, nous avons enregistré cinq cas de l’utilisation de Biobrane® pour ce type de greffe, où les zones greffées étaient adjacentes à des brûlures d’épaisseur partielle, et deux cas où les zones greffées étaient à côté de sites donneurs. Biobrane® a promu l’adérence de la greffe à la plaie, a empêché de cisaillement, et a permis le drainage du fluide. Dans le même temps, Biobrane® a également facilité la guérison des sites donneurs adjacentes ou les brûlures d’épaisseur partielle.
Introduction
Biobrane® is a flexible biosynthetic wound dressing constructed of silicone bonded to woven nylon containing peptides derived from type I porcine collagen.1 The use of Biobrane ® has been shown to significantly reduce hospital stay, wound healing time, and pain in comparison to other dressings. For these reasons it is widely used in paediatric partial- thickness scald burns.2-4 Biobrane® has also been used in burn units for temporary coverage of excised full thickness burns and to dress donor sites of split skin grafts (SSG).5,6 The purpose of this study is to assess the effectiveness of using Biobrane® to dress and secure SSG within areas of partial thickness burns or adjacent to skin graft donor sites.
Method
A retrospective review of cases from March 2009 to March 2012, in which Biobrane® (Smith & Nephew Wound Management, PO Box 81, 101 Hessle Road, HULL, HU3 2BN, UK) was used to dress SSG in children, where the grafted areas were adjacent to donor sites or partial thickness burns.
Results
We reported seven cases of using Biobrane® dressing in children to cover the SSGs and nearby skin graft donor sites or partial thickness burns. The mechanisms of burn injury in these paediatric cases were identified as a contact burn injury in case I, and scald injuries in cases IIVII. The burn depth was variable in nature in all the cases of scald injuries, and all involved more than one anatomical body surface area. At time of presentation, the burnt areas were cleaned and dressed with a primary non adherent or paraffin based dressing, with secondary layers of dry gauze and bandage. Laser Doppler imaging was used to assess mixed depth burns.
In two cases, Biobrane® was used to dress grafted areas and adjacent donor sites in the scalp (Table I). In both cases, Vicryl rapide® was used to fix the SSGs to the recipient beds, (Fig. 1). Biobrane® was applied with slight tension, but not overstretched, over the donor site and SSG, then fixed with staples. The outer dressing consisted of a few layers of plain gauze. On day 2 the outer dressings were removed and the Biobrane® left exposed. On day 5 staples were removed and the Biobrane® came off the sheet grafts. On day 14 the Biobrane® came off the meshed graft area and donor site. Parents reported that the children were comfortable with regular paediatric pain scores being undertaken.
Table I. Biobrane® used to dress SSG and adjacent skin graft donor sites.
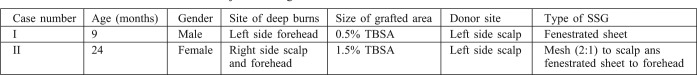
Fig. 1 . A 24 month old female presented with scald burns. a. SSG was fixed to the recipient beds. b. Biobrane® was applied with slight tension over the donor site and SSG. c. On day 5 the Biobrane® came off the sheet graft. d. On day 14 the Biobrane® came off the meshed graft area and donor site.
Biobrane® was used to dress grafted areas and adjacent partial thickness burns in five cases (Table II). The full thickness burns were adjacent or surrounded by the partial thickness burns (Fig. 2). Debridement of the superficial partial thickness burns was with Versajet® (Smith & Nephew Wound Management, PO Box 81, and 101 Hessle Road, HULL, HU3 2BN, UK). Tangential excision of the full thickness burns and SSG was applied as fenestrated sheet graft to the excised deep burn areas. In cases III-VII (Table II), the SSG was harvested from thighs and dressed with paraffin gauze, mutiple layers of dry gauze, and bandage. Then Biobrane® was used to dress the grafted areas and adjacent partial thickness burns. In cases III and IV, the graft was fixed to the recipient bed with Vicryl rapide® and, therefore, Biobrane® was left to peel off spontaneously on days 10 and 6 respectively. In cases V, VI and VII, staples were used to fix the grafts and, therefore, the Biobrane® over the SSG was trimmed and the staples were removed on day 5 or 6. In all reported cases, the patients were comfortable and there were no reports of short term complications.
Table II. Biobrane® used to dress SSG and adjacent partial thickness burns.
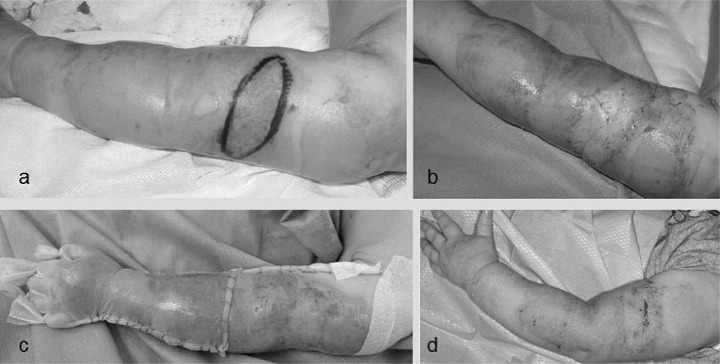
Fig. 2 . A 12 month old female presented with scald burns. a. Full thickness burn surrounded by partial thickness burns. b. SSG was applied to the excised deep burn area. c. Biobrane® was used to dress the whole left upper limb. d. On day 10 the Biobrane® came off.
Discussion
Accidental scald burns in children are commonly mixed depth burns and often involve more than one anatomical area, as in cases II – VII.7 Due to the nature of scald injuries, areas with initial or longer contact develop deeper burn injuries, surrounded by zones where the cooling liquid leads to more superficial injuries, thus creating a mixed depth pattern. Unless the burns are very extensive, leaving very limited donor sites, it is not recommended to harvest SSG from exposed areas (distal to knees and elbows), because it will result in unacceptably noticeable scarring.
In this case series, the maximum size of burns was 7% TBSA (total body surface area) and, therefore, SSG was harvested from hidden donor sites only. To gain the best aesthetic outcome for the face, the scalp was used as the donor site in cases I-II. This gave the most appropriate colour match of the SSG to the forehead wounds in case I, and forehead and scalp in case II.8,9 Another advantage of choosing the scalp is that the residual donor site scar or pigment changes would be hidden by hair. In cases III-VII (Table II), Biobrane® was used only to dress the grafted areas and adjacent superficial burns. The donor sites in these cases were away from the burnt areas, with SSG being harvested from thighs and dressed with paraffin gauze.
Optimal dressings of SSG promote adherence of the graft to the wound allowing fibrin adherence, serum imbibition, decreased shearing, and drainage of haematoma and seroma.10 The Biobrane® applied with slight tension over SSG on flat and convex body surfaces such as scalp, forehead, arm, leg and trunk, provided immobilisation of the SSG on its recipient beds. This is possible because of RÉSUMÉ. Biobrane® est couramment utilisé dans les brûlures pédiatriques pour couvrir les brûlures d’épaisseur partielle et les sites donneurs de greffe de peau mince. Le but de cette étude est d’évaluer l’utilisation de Biobrane® sur les greffes de peau mince adjacentes aux sites donneurs des greffes de peau ou des brûlures d’épaisseur partielle. Une étude rétrospective a été menée pour éxaminer l’utilité de Biobrane® sur les greffes de peau mince où les zones greffées étaient adjacentes aux sites donneurs ou aux brûlures d’épaisseur partielle. Entre 2009 et 2012, nous avons enregistré cinq cas de l’utilisation de Biobrane® pour ce type de greffe, où les zones greffées étaient adjacentes à des brûlures d’épaisseur partielle, et deux cas où les zones greffées étaient à côté de sites donneurs. Biobrane® a promu l’adérence de la greffe à la plaie, a empêché de cisaillement, et a permis le drainage du fluide. Dans le même temps, Biobrane® a également facilité la guérison des sites donneurs adjacentes ou les brûlures d’épaisseur partielle. Mots-clés: Biobrane®, sites donneurs de greffe de peau, brûlures pédiatriques the flexibility of the Biobrane® which can then produce gentle pressure over the grafted areas. We noticed that Biobrane ® provided further stabilisation of SSG over recipient areas by its adherence to the wound bed through the slits in the mesh SSG or fenestrated sheet SSG. It was observed that Biobrane® peeled off sheet graft earlier than meshed grafts as healing occurred at a faster rate.
The transparency of the Biobrane® allowed regular checking of the state of SSG without disrupting the graft. Biobrane® also has the advantage of pores, which allows exudates to pass through to the outer dressing, whilst acting as a barrier against infection. Biobrane® is already used regularly to dress superficial partial thickness burns and SSG donor sites in our regional burns service, which means that this dressing is already familiar to the burn surgeons.
Biobrane® is an expensive dressing and it is not usually used to dress SSG alone in our regional burn centre. In this case series, there were relatively small grafted areas adjacent to the SSG donor sites or superficial partial thickness burns. There was no need for additional Biobrane ® dressings and there was less wastage of Biobrane® sheets, and thus there were reduced costs. By using one dressing (Biobrane®) for both grafted areas and donor sites or partial thickness burns, we negated the extra time needed when using different dressings, and thereby reduced both the risk of prolonged anaesthesia time and the associated financial implications. If we did the opposite of this by taking the usual dressings for grafts and stretching them over the surrounding areas, the proven benefit of Biobrane® would be lost on these areas.
These cases highlight anecdotally the advantages of using Biobrane® to cover both SSG and adjacent donor sites, or SSG and surrounding partial thickness burns. This series consists of seven cases with good results and further studies are required.
Conclusion
The use of Biobrane® to dress SSG adjacent to skin graft donor sites or partial thickness burns was found to be a safe, simple and efficient technique.
Acknowledgments
Conflict of interest. The authors of this paper hereby declare that they have no conflict of interest and that no funding has been involved.
References
- 1.Solanki NS, Nowak KM, Mackie IP, Greenwood JE. Using Biobrane®: Techniques to make life easier. Eplasty. 2010;10:70. [PMC free article] [PubMed] [Google Scholar]
- 2.Lesher AP, Curry RH, Evans J, Smith VA, Fitzgerald MT, Cina RA, Streck CJ, Hebra AV. Effectiveness of Biobrane® for treatment of partial-thickness burns in children. J Pediatr Surg. 2011;46:1759–63. doi: 10.1016/j.jpedsurg.2011.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahmanian-Schwarz A, Beiderwieden A, Willkomm LM, Amr A, Schaller HE, Lotter O. A clinical evaluation of Biobrane® and Suprathel® in acute burns and reconstructive surgery. Burns. 2011;37:1343–8. doi: 10.1016/j.burns.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Gerding RL, Emerman CL, Effron D, Lukens T, Imbembo AL, Fratianne RB. Outpatient management of partial-thickness burns: Biobrane® versus 1% silver sulfadiazine. Ann Emerg Med. 1990;19:121–4. doi: 10.1016/s0196-0644(05)81793-7. [DOI] [PubMed] [Google Scholar]
- 5.Housinger TA, Wondrely L, Warden GD. The use of Biobrane® for coverage of the pediatric donor site. J Burn Care Rehabil. 1993;14:26–8. doi: 10.1097/00004630-199301000-00007. [DOI] [PubMed] [Google Scholar]
- 6.El-Khatib HA, Hammouda A, Al-Ghol A, Habib B, Al-Basti Aldehyde-treated porcine skin versus Biobrane® as biosynthetic skin substitutes for excised burn wounds: Case series and review of the literature. Ann Burns Fire Disasters. 2007;20:78–82. [PMC free article] [PubMed] [Google Scholar]
- 7.Maguire S, Moynihan S, Mann M, Potokar T, Kemp AM. A systematic review of the features that indicate intentional scalds in children. Burns. 2008:1072–81. doi: 10.1016/j.burns.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Farina JA, Freitas FA, Ungarelli LF, Rodrigues JM, Rossi LA. Absence of pathological scarring in the donor site of the scalp in burns: An analysis of 295 cases. Burns. 2010;36:883–90. doi: 10.1016/j.burns.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Silverman HJ, Zuker RM, Morris S. Scalp as a donor site for grafts to facial and neck burns in children. Can J Surg. 1992;35:312–5. [PubMed] [Google Scholar]
- 10. Weinzweig, J , editor. Plastic Surgery Secrets Plus. Mosby Elsvier; 2010. Principle of skin grafts; p. 678. [Google Scholar]


