Summary
Collagen based dressings for acute burn wound management have been extensively used in India, particularly in the city of Chennai. Due to the high levels of humidity in our city, closed dressings become infected and treatment with topical antimicrobials, like Silver Sulfadiazine cream, quickly become desiccated. Collagen membrane dressings were manufactured by the biomaterial laboratory of the Central Leather Research Institute (CLRI), Government of India in Chennai, and then the process was patented. Collagen was extracted from bovine skin and Achilles tendons, and then reconstituted. This was used on burn wounds as dressings after clearance from the Institutional Review Board and Ethics Committees of the Hospital and CLRI. Continued research in this field to enable resulted in the design of silver sulphadiazine loaded alginate microspheres which were embedded in the reconstituted collagen. Controlled delivery of silver sulphadiazine. This collagen membrane was used in chronic infected burns. Low molecular weight heparin was given subcutaneously to improve wound healing in burn injuries and collagen membrane dressings were also applied. After several trials the process technology was patented. The advantages and disadvantages of the collagen membrane cover is elaborated in a group of 487 pediatric burn patients. The trial was conducted at the burn unit of Kanchi Kamakoti Childs Trust Hospital (KKCTH) in Chennai, India.
Keywords: biological dressings, burn wounds, collagen based dressings, burn wound healing
Abstract
Pansements à base de collagène pour la gestion des blessures par brûlure ont été largement utilisés en Inde, en particulier dans la ville de Chennai. En raison des niveaux élevés d’humidité dans notre ville, les pansements fermées deviennent infectés et les traitements par les antimicrobiens topiques, comme la crème de sulfadiazine d’argent, deviennent vite desséchées. Le laboratoire de biomatériaux de l’Institut central de recherche sur le cuir (ICRC), gouvernement de l’Inde à Chennai, a fabriqué les pansements de membrane de collagène et le processus de fabrication a été breveté. Le collagène a été extrait de peau de bovin et de tendons d’Achille. Suite à l’autorisation de la commission de révision institutionnelle et les comités d’éthique de l’hôpital et l’ICRC, le collagène a été reconstitué et a été utilisé sur les brûlures comme pansements. Poursuite des recherches dans ce domaine a abouti à la conception des microsphères d’alginate chargées d’argent sulphadiazine qui ont été intégrées dans le collagène reconstitué. Cela a permis la libération contrôlée de sulfadiazine d’argent. Cette membrane de collagène a été utilisée dans les brûlures chroniques infectées. Les héparines de bas poids moléculaire ont été mises par injection sous-cutanée pour améliorer la cicatrisation des blessures par brûlure, et des pansements à membrane de collagène ont également été appliquées. Après plusieurs essais, la technologie de procédé a été breveté. Les avantages et les inconvénients de la couverture de la membrane de collagène est élaboré dans un groupe de 487 patients pédiatriques brûlés. Le procès s’est déroulé à l’unité des grands brûlés de l’hôpital Kanchi Kamakoti Childs Trust à Chennai, en Inde.
Introduction
The ultimate goal in the management of burn wounds is to obtain physiological closure in the shortest period of time. Traditional burn wound management involves cleansing, debridement and provision of a moist environment to encourage the process of natural healing. Hence, an ideal dressing material has to maintain a moist environment, act as a bacterial barrier, and as a medium for free exchange of gases, while providing a barrier against toxic contaminants. Research and development in the field of wound dressing has resulted in the fabrication and production of a wide variety of synthetic dressings.1,2 The various limitations of synthetic dressings lead researchers to seek dressings with superior qualities and functions, giving rise to interest in the development of biological dressings.3-7 Until 1990, Human Amnion was extensively used as a temporary biological cover for superficial partial thickness and deep partial thickness burns, providing good healing and epithelialization.8-1 Continued research for good functional biological dressings resulted in the evolution of collagen based dressings for burn wounds which have proven to be superior and more advantageous. 19-24
The development of collagen dressings is only logical given its unique structural and functional characteristics. Collagens are the most abundant and ubiquitous proteins in vertebrates. In addition to providing mechanical support to the connective tissue, the collagens form an essential substrate for cellular adhesion and migration.20,21 Therefore, collagen is considered to be an important factor in the regenerative process.25,26 Collagen is hemostatic, has low antigenicity, and supports cellular growth. The importance of collagen in burn wound healing has been appreciated for a long time. Wound healing and regeneration involves cell proliferation, cell migration, cell differentiation and interaction between the different components in which collagen may affect healing not only at the final stage but also in the very early stage of healing.25,27-30 Clinical trials were performed with the fabricated collagen membranes on burn wounds and the results show the advantages of the collagen membranes in burn wound healing.
Materials and methods
A group of 853 pediatric burn patients were treated at the pediatric burn unit of KKCTH, Chennai, India from 1992–2011. 487 of these patients had various types of collagen dressings.
The use of amniotic membrane collagen membrane was terminated in 1997, due to the Government of India’s notification that blood connected products should not be used on humans. Since then, we have used bovine collagen membrane, obtained from animal skin and Achilles, and reconstituted in the biomaterial laboratory of the CLRI in Chennai, which is a National Research Institute of the Government of India. This product has also been patented.
When applied in one site or in multiple sites there was no significance. Healing was identified in terms of number of days for healing and scar quality.
We had 47 children below the age of 1 year, among which 18 cases were intentional burns.
In the age group of 2-10, we had 280 patients. These were school children, suffering mainly from scalds. In the adolescent group, collagen dressings were used for scald, flame and electric burns. Collagen dressings were used after primary excision of limited deep burns, and when there was paucity of skin graft. Collagen served the purpose of wound cover at every stage.
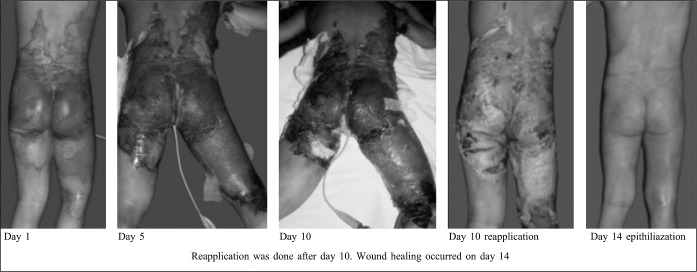
In some patients, the membrane had to be reapplied to complete wound healing due to collection of serosanguinous fluid under the membrane, or in cases of obvious wound infection. Secondary application is advantageous and, along with systemic antibiotic therapy, wound epithelialization occurs. Sometimes, this requires a few more days than normal healing, which takes 12- 15 days.
Table I. Type of biological dressings used in this study. Collagen based dressings were used in a total of 487 children.
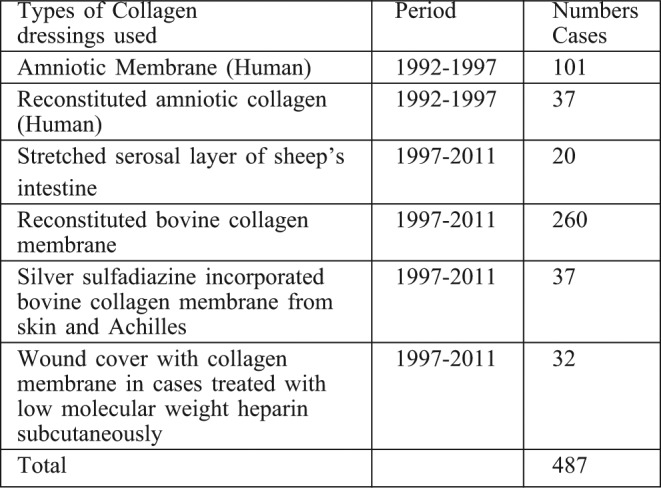
Table II. Number of times that the collagen was applied during 1992-2011.
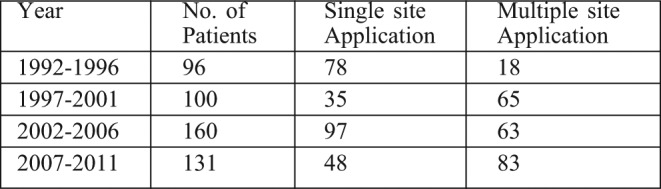
Table III. Age distribution of patients treated with collagen dressings.
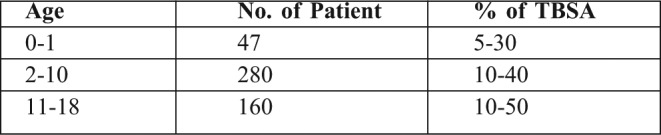
Preparation of collagen dressings
Preparation of collagen as a dressing can be divided into two major approaches. In the first approach, biological structures, such as the serosal layer of bovine small intestine and amnion, were treated with mild enzymes to remove non-collagenous materials and strengthen the remaining collagen without disturbing the existing structure and intermolecular cross linkages.20,21 In the second approach, the collagen was solubilized and purified, then reconstituted in dry form. The first approach has the advantage of exploiting normal biological and three dimensional structures in a biomaterial, but has the disadvantage of producing material which has relatively fixed and predetermined configurations. The second approach has wider potential because the desired size and porosity of the membrane can be designed. This form is preferred because of its ease of handling. Hence, we designed reconstituted collagen based dressings from human amnion, bovine skin and Achilles tendons. Recently mesh collagen membrane has also become available.
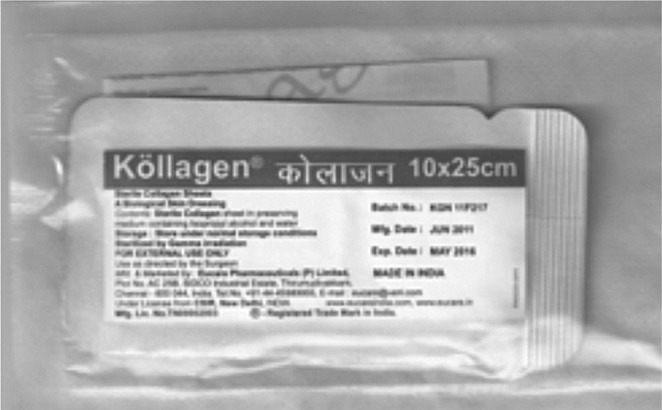
All the collagen membrane were sterilized with ethylene oxide treatment and packed.
Picture shows the sterilized and packed bovine collagen dressing.
Picture shows the sterilized and packed bovine collagen dressing.
Results
Comparative advantages of the types of Collagen Membrane used
The Amniotic Membrane and the reconstituted amniotic membranes were extensively used before 1997. Wound healing was excellent and in some cases scar outcome was also was satisfactory.
Commercial Kollagen (collagen of bovine origin) manufactured in the laboratory (CLRI in Chennai, which is a National Research Institute of the Government of India), is available and this is a patented product.
The collagen membrane proved satisfactory in the healing of superficial and superficial partial thickness burns.
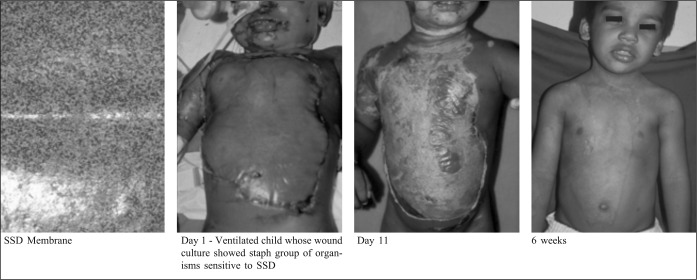
Silver sulphadiazine incorporated membrane was used in limited cases, where skin grafting was delayed due to wound infection from pseudomonas, acinetobacter, and the staph group of organisms. These were treated with the appropriate antibiotics and showed sensitivity to silver sulphadiazine plates
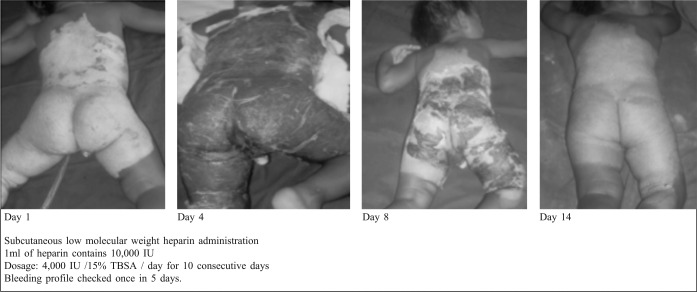
Low molecular weight heparin treatment reduced burn oedema and inflammatory cytokine activity.31 These burns were covered with collagen membrane to enhance wound healing. Scar outcome was very good.
Fig. 1. Superficial partial thickness burns - Amniotic membrane application.
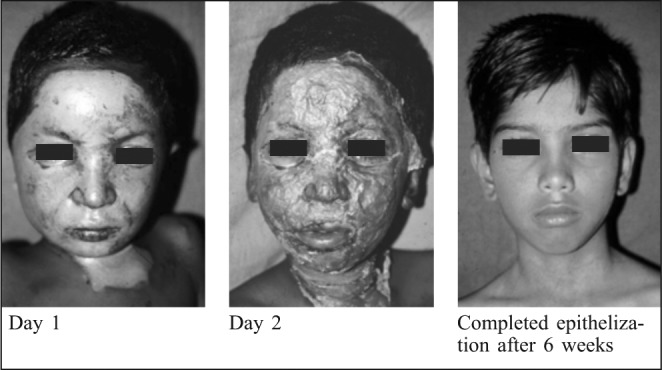
Fig. 2. Superficial partial thickness burns: Reconstituted amniotic collagen membrane.
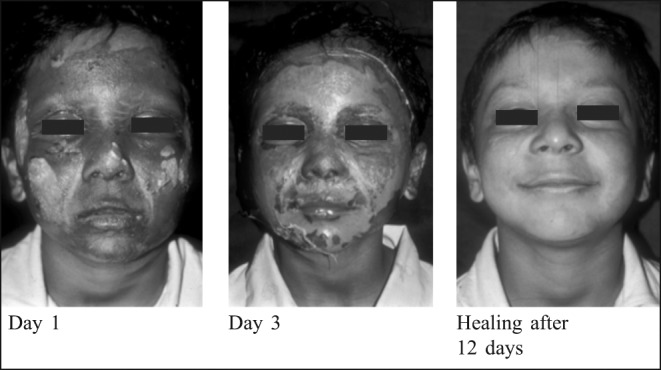
Fig. 3. Reconstituted bovine collagen membrane (Kollagen): Deep partial thickness burns.
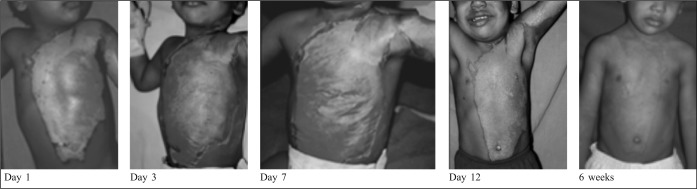
Fig. 4. Deep partial thickness burns: Repeated application of bovine reconstituted collagen membrane.
Fig. 5. Deep partial thickness burns: Silver sulphadizne (SSD) incorporated reconstituted bovine collagen membrane.
Table V. Scar outcome.
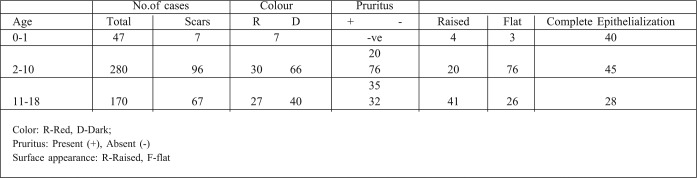
Fig. 6. Reconstituted bovine collagen membrane on superficial partial thickness burns treated subcutaneously with low molecular weight heparin.
Scar outcome after collagen membrane treatment
The scar outcome can be assessed only after two to three months. When complete epithelialization and pigmentation occurs, there is usually no scar within 3 months. After 3 months, however, change of color, symptoms like pruritus, pain and thickness will begin. These symptoms may stop within a year, or some areas, or all the areas, might become hypertrophic or keloidal. Unfortunately, the type of dressing alone cannot dictate scar outcome.
In the younger age group 0-1, healing is very good. Out of 47 cases, only seven had post burn scar. They were reddish in color and 4 of them had raised surfaces, almost becoming hypertrophic. The other 40 cases healed with good epithelialization and, even if they were symptomatic after 1 year, there were no scars.
In the 2-10 year age group, which included pubertal children, scars developed in 96 cases. They were symptomatic with pruritus. The older children of this group were capable of expressing this stage.
In the 11-18 year age group, there were 67 scars among 170 patients, with most injuries resulting from electric and chemical burns. Only 28 cases completely epithelialized, and these were mainly scald burns. The scar outcome is related to the age group: hypertrophic scars and keloids were noted mainly in the adolescent age group, while flame, electrical and chemical burns caused the most scarring.
Discussion
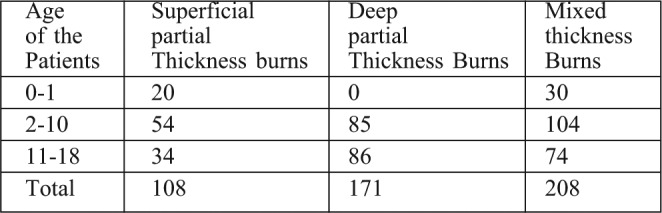
Before 1996, we had only human amniotic membrane as biological dressings. This membrane was efficient in wound healing but had the disadvantage of being a blood product, which could transmit HIV and hepatitis infections. Research and development into biological dressings led to the fabrication of collagen based dressings for burn wound cover. These were manufactured from bovine sources, mainly from animal skin and Achilles. Initially, these research products had to go through limited clinical trials. Table IV delineates the types of collagen dressings used.
Table IV. Depth of burns selected for the study in different age groups.
This qualitative study on the advantages of collagen membrane on pediatric burn wounds targeted the time taken for complete epithelialization. This study looked at whether the dressings were removed by the physician or came off by themselves, as well as scar quality. Ideal selection of burn wounds in terms of depth (super and deep partial thickness burns, without-infection) has been the single determinant factor in successful healing. The ease of application and removal of the membrane, painless dressing, and cost effectiveness compared to Integra®, Biobrane ® and Acticoat® - which are the other bio-membranes available - are the real advantages in a developing country.
Single application was done in 78% of the total number of cases, and reapplication was carried out in 22% of the patients. Infection was observed as redness on the wound and purulent discharge under the adherent membrane in 17% of cases, who arrived at our center after 72 hours. 73% of all cases healed without any infection. In 15 days, the wounds healed well and, within 6 weeks, epithelialization with pigmentation was complete.
The advantages of reconstituted collagen membrane over the amniotic or serosal membranes are: ease of availability in various sizes, ease of removal, ability to remain stable at room temperature for 3 years, and, in due course, the ability to incorporate drugs and growth factors that can be delivered in a controlled manner.
There are also some disadvantages of reconstituted collagen membrane: a single stretch of membrane is not advisable to be placed on flexor surfaces as it cracks when it becomes dry; and wounds visible through the membrane have given rise to apprehension amongst care givers.
Conclusion
Reconstituted collagen membrane has proven to be highly advantageous for burn patients in developing countries. It is being used extensively in India thanks to its superior ability to adapt to hot and humid environments, as well as the fact that it is also cost effective.
References
- 1.Park GB. Burn wound coverings: A review. Biomater Med Devices Artif Organs. 1978;6:1–35. doi: 10.3109/10731197809118690. [DOI] [PubMed] [Google Scholar]
- 2. Thomas, S , editor. Wound Management and Dressings. The Pharmaceutical Press; London: 1990. Semipermeable film dressing. pp. 25–34. [Google Scholar]
- 3.Lineen E, Namias N. Biologic dressing in burns. J Craniofac Surg. 2008;19:923–5. doi: 10.1097/SCS.0b013e318175b5ab. [DOI] [PubMed] [Google Scholar]
- 4.Peters WJ. Biological dressings in burns: A review. Ann Plast Surg. 1980;4:133–7. doi: 10.1097/00000637-198002000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Wolf DL, Capozzi A, Pennisi VR. Evaluation of biological dressings. Ann Plast Surg. 1980;5:186–90. doi: 10.1097/00000637-198009000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Norton L, Chvapil M. Comparison of newer synthetic and biological wound dressing. J Trauma. 1981;21:463–8. [PubMed] [Google Scholar]
- 7.Burget A, Nathan P, Holder IA, Macmillan BG. The effect of a collagen dressing on contaminated surgical wounds in rats. Langenbecks Archiv für Chirugie. 1976;343:69–73. doi: 10.1007/BF01261571. [DOI] [PubMed] [Google Scholar]
- 8.Ramakrishnan KM, Jayaraman V. Management of partial-thickness burn wounds by amniotic membrane: A cost effective treatment in developing countries. Burns. 1997;23:33–6. doi: 10.1016/s0305-4179(97)90099-1. [DOI] [PubMed] [Google Scholar]
- 9.Hadjiiski O, Anatassov N. Amniotic membranes for temporary burn coverage. Ann Burns Fire Disasters. 1996;9:88–92. [Google Scholar]
- 10.Gruss JS, Jirsch DW. Human amniotic membrane: A versatile wound dressing. Can Med Assoc J. 1978;118:1237–46. [PMC free article] [PubMed] [Google Scholar]
- 11.Colocho G, Graham WP, Greene AE, Matheson DW, Lynch D. Human amniotic membrane as a physiologic wound dressing. Arch Surg. 1974;109:370–3. doi: 10.1001/archsurg.1974.01360030022006. [DOI] [PubMed] [Google Scholar]
- 12.Quinby WC Jr, Hoover HC, Scheflan M, et al. Clinical trials of amniotic membranes in burn wound care. Plast Reconstr Surg. 1982;70:711–7. doi: 10.1097/00006534-198212000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Ramakrishnan KM, Doss CR, Rao DK, et al. Human amniotic membrane as a temporary biological dressing in complicated burns a developing country. J Burn care Rehabit. 1983:202–204. [Google Scholar]
- 14.Atanassov , Mazgalova J, Todorov R, Stereva K, Trencheva W. Use of amniotic membranes as biological dressings in contemporary treatment of burns. Ann Medit Burns Club. 1994 Dec;7 [Google Scholar]
- 15.Sawhney CP. Amniotic membrane as a biological dressings in the management of burns. Burns. 1989;15:339–42. doi: 10.1016/0305-4179(89)90015-6. [DOI] [PubMed] [Google Scholar]
- 16.Bujang–Safawi E, Halim AS, Khoo TL, Dorai AA. Dried irradiated human amniotic membrane as a biological dressing for facial burns: A 7 year case series. Burns. 2010;36:876–82. doi: 10.1016/j.burns.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Singh R, Chackarkar MP. Dried gamma-irradiated amniotic membrane as dressing in burn wound care. J Tissue Visibility. 2011;20:49–54. doi: 10.1016/j.jtv.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Sharma SC, Bagree MM, Bhat AL, Banga BB, Singh MP. Amniotic membrane is an effective burn dressing material. Jon J Surg. 1985;15:140–3. doi: 10.1007/BF02469744. [DOI] [PubMed] [Google Scholar]
- 19.Bose B. Burn wound dressing with human amniotic membrane. Ann R Coll Surg. 1979;61:444–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Purna Sai K, Babu M, et al. Collagen based dressings: A review. Burns. 2000;26:54–62. doi: 10.1016/s0305-4179(99)00103-5. [DOI] [PubMed] [Google Scholar]
- 21.Balasubramani M, Kumar TR, Babu M. Skin substitutes: A review. Burns. 2001;27:534–44. doi: 10.1016/s0305-4179(01)00018-3. [DOI] [PubMed] [Google Scholar]
- 22.Singh O, Gupta SS, Soni M, Moses S, Shukla S, Mathur RK. Collagen dressing versus conventional dressings in burn and chronic wounds: A retrospective study. J Cutan Aesthet Surg. 2011;4:12–16. doi: 10.4103/0974-2077.79180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazovic G, Colic M, Grubor M, Jovanovic M. The Application of collagen sheet in open wound healing. Ann Burns Fire Disasters. 2005;18:151–156. [PMC free article] [PubMed] [Google Scholar]
- 24.Brett D. A review of collagen and collagen-based wound dressings. Wounds. 2008;20:12. [PubMed] [Google Scholar]
- 25.Bhattacharya S, Tripathi HN, Gupta V, Nigam B, Khanna A. Collagen sheet dressings for cutaneous lesions of toxic epidermal necrolysis. Indian J Plast Surg. 2011;44:474–7. doi: 10.4103/0970-0358.90826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada KM. Cell surface interaction with extracellular materials. Ann Rev Biochem. 1983:509–48. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]
- 27.Shoshan S, Gross J. Biosynthesis and metabolism of collagen and its role in tissue repair processes. Israel J Med Sci. 1974;10:537–61. [PubMed] [Google Scholar]
- 28.Pruitt BA, Levine NS Jr. Characteristics and uses of biologic dressings and skin substitutes. Arch Surg. 1984;119:312–22. doi: 10.1001/archsurg.1984.01390150050013. [DOI] [PubMed] [Google Scholar]
- 29.Chem PL, Baum CL, Arpey CJ, et al. Biologic dressings: Current applications and limitations in dermatologic surgery. Dermatol Surg. 2009;35:891–906. doi: 10.1111/j.1524-4725.2009.01153.x. [DOI] [PubMed] [Google Scholar]
- 30.Bao L, Yang W, Mao X, Mou S, Tang S. Agar/collagen membrane as skin dressing for wounds. Biomed Mater. 2008;3:4. doi: 10.1088/1748-6041/3/4/044108. [DOI] [PubMed] [Google Scholar]
- 31.Ravikumar T, Shanmugasundaram N, Mathangi Ramakrishnan K, Babu M. Low molecular weight heparin-induced pharmacological modulation of burn wound healing. Ann Burns and Fire Disasters. 2006;19:123–9. [PMC free article] [PubMed] [Google Scholar]