Abstract
Background
We investigated whether mitogen-activated protein kinases (MAPKs) are changed in the hearts of patients with diabetes after cardioplegia and cardiopulmonary bypass (CP/CPB) operations.
Methods
Biopsies from the right atrial appendage were harvested pre- and post-CP/CPB from nondiabetic (ND) patients (n = 8, hemoglobin A1c (HbA1c) = 5.4 ± 0.12); patients with controlled diabetes (CDM) (n = 8, HbA1c = 6.5 ± 0.15); and patients with uncontrolled diabetes (UDM) (n = 8, HbA1c = 9.6 ± 0.3) undergoing coronary artery bypass grafting. The expression and/or activation of the p38-MAPK, ERK1/2, JNK, and MKP-1 in the right-atrial tissues were analyzed by Western blotting. The vasomotor function of coronary arterioles was measured by videomicroscopy.
Results
The post-CP/CPB levels of total p38-MAPK were decreased in the 3 groups as compared with their pre-CP/CPB levels (P < .05). There were increases in phospho-p38-MAPK, phospho-ERK1/2, and MKP-1 in UDM patients as compared with ND and CDM patients at baseline (P < .05). Compared to pre-CP/CPB, the post-CP/CPB levels of phospho-p38-MAPK decreased in the UDM group but were unaltered in the ND and CDM groups; however, the post-CP/CPB levels of phospho-p38-MAPK still remained greater than the post-CP/CPB levels of the other 2 groups. Post-CP/CPB levels of phospho-ERK1/2 were increased in the ND and CDM groups but were decreased in the UDM group compared to their pre-CP/CPB levels, respectively (P < .05). There were no significant differences in phospho-JNK in 3 groups at baseline. Post-CP/CPB levels of phospho-JNK, however, were increased in the 3 groups and were more pronounced in the myocardia of the UDM group (P < .05). After CP/CPB, the protein levels of MKP-1 were unchanged in the 3 groups when compared with their pre-CP/CPB levels. Post-CP/CPB levels of MKP-1, however, remained greater in the UDM group than in the ND and CDM groups. The post-CP/CPB contractile responses to the thromboxane A2 analog U46619 were significantly impaired in all 3 groups compared with pre-CP/CPB contractile responses. These impairments were more pronounced in the UDM group.
Conclusion
Uncontrolled diabetes is associated with changes in expression of and activation of MAPKs and vasomotor dysfunction in the setting of CP/CPB.
Mitogen-activated protein kinases (MAPKs), the serine-threonine protein kinases, are expressed in multiple cell types, including cardiomyocytes, vascular smooth muscle cells, and endothelial cells.1,2 Extensive investigation has established roles for MAPKs, such as p38-MAPK, extracellular signal-regulated kinases (ERKs), and c-Jun NH2-terminal protein kinases (JNKs) in cardiovascular signal transduction pathways.1,2 These MAPKs are involved in several processes important in cardiac surgery, such as cytokine production, ischemia/ reperfusion, myocardial apoptosis, vascular permeability, vasomotor function, and injury.2–8 Preclinical and clinical studies from our and other laboratories have demonstrated previously that alterations in the activity of p38-MAPK, ERKs, and JNKs occur as a result of cardioplegia and cardiopulmonary bypass (CP/CPB).2–8
The expression and activation of MAPKs are also altered in the setting of hyperglycemia and diabetes in several animal models.9–14 Recent studies in our laboratory demonstrated that PKC-alpha and PKC-beta are upregulated and activated in the human diabetic (type 2) myocardium in the setting of CP/CPB.15–21 Whether diabetes, particularly if combining with CP/CPB and cardiac surgery, further affects MAPKs signaling in the human myocardium has not been investigated.
The goal of this study was to investigate the effects of diabetes and of diabetes combined with CP/CPB-related changes in myocardial MAPKs signaling. Specifically, this study was designed to test directly the effects of diabetes and CP/CPB on the expression/activation of MAPK protein in human atrial tissues harvested from patients undergoing coronary artery bypass graft (CABG) surgery.
METHODS
Human subjects and tissue harvesting
Samples of right atrial appendages were harvested from patients undergoing CABG surgery before and after exposure of the heart to blood cardioplegia (CP) and short-term reperfusion under conditions of CPB. Samples were handled in a nontraumatic fashion. Double 3–0 polypropylene purse-string sutures (Ethicon, Somerville, NJ) were placed in the atrial appendage. The first sample of atrial appendage was harvested pre-CP/CPB. During placement of the venous cannula, the superior suture was tightened to secure the venous cannula. The inferior suture remained loose to allow this portion of the atrium to be exposed to blood CP and CPB after removal of the aortic cross-clamp. An initial 600 to 800 mL of cold-blood (0°C to 4°C), hyperkalemic (15 mmol/L K+) cardioplegic solution was delivered antegrade into the aortic root. This method was followed at 8- to 20-min intervals with 200 to 300 mL of cold cardioplegic solution (15 mmol/L K+). The composition of cardioplegic solutions has been described in detail previously.20
The second sample of atrial tissue (post-CP/ CPB) was harvested between the purse-strings during removal of the venous cannula. Sections of atrial samples were put immediately into cold Krebs buffer (for an in vitro microvascular study), frozen in liquid nitrogen (immunoblotting), or fixed in 10% formalin for 24 h followed by paraffinization and sectioning into 5-µm slices (immunoflourescent staining).
Hemoglobin A1c was measured in all patients
The patients were then divided into the following 3 groups: (1) patients with normal HbA1c and no history of or treatment for diabetes were considered nondiabetics (ND); (2) patients with histories of diabetes with HbA1c >5.5 and <7 were considered controlled diabetics (CDM); and (3) patients with HbA1c ≥8.5 were considered uncontrolled diabetics (UDM). Of the 117 patients enrolled, 8 randomly chosen patients from each group were included for analysis in this study. Patients who also had valve surgery, cross-clamp time >160 min, or a CPB time >180 min were excluded from the study. All procedures were approved by the Institutional Review Board of Rhode Island Hospital, Alpert Medical School of Brown University, and informed consent was obtained from all enrolled patients, as required by the Institutional Review Board.
Immunoblot
Atrial tissue from 8 patients per group was dissected and cleaned of fat and connective tissues, then solubilized in SDS-PAGE buffer. Total protein (40 µg) was fractionated on an 8% to 16% SDS-PAGE gel and then transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA). Membranes were incubated for 1 h at room temperature with 1:1,000 dilutions of primary antibodies to total and/or modified p38 MAPK, ERK1/2, JNK, JNK-1 (Cell Signaling Technology, Danvers, MA), and MKP-1 (Santa Cruz Biotechnology, Santa Cruz, CA). The detailed methods have been described previously.15–20
Microvessel reactivity
Microvessel studies were performed by in vitro organ bath videomicroscopy, as described previously.15–20 Coronary microvessels (90 to 170 µm internal diameter) were dissected from harvested human atrial tissue by the use of a dissecting microscope (Olympus Optical, Tokyo, Japan). The microvessels were placed in an organ bath (University of Iowa Medical Instrumentation, Iowa City, IA) and cannulated with 10–0 nylon monofilament suture (Ethicon). Oxygenated (95%O2/5%CO2) KHB solution warmed to 37°C was circulated continuously through the organ bath. The microvessels were pressurized to 40 mm Hg in a nonflow state filled with KHB solution. Using an inverted microscope (Olympus Optical) connected to a video camera, the vessel image was projected onto a television monitor. An electronic dimension analyzer (Living System Instrumentation, Burlington, VT) was used to measure and record the internal diameter. After 60 min of equilibration, pre- and post-CP/CPB microvessels were exposed to the TXA-2 analog U-46619 (10−9 to 10−4 mol/L). The baseline diameter was defined as the diameter measured after cannulation and equilibration in the aerated KHB solution. Internal diameter was measured after treatment with U-46619 and normalized to the baseline diameter (% contraction). The microvessels were washed with the KHB solution and allowed to equilibrate 15 to 30 min between pharmacologic interventions.
Data analysis
Data are presented as the mean and standard error of the mean (SEM). Paired Student t tests were used to analyze Western blot data between pre- and post-CP/CPB within each group. Kruskal-Wallis tests followed by Dunn multiple comparison tests were employed to analyze clinical and Western blot data among the 3 groups (GraphPad Software, San Diego, CA). The Fisher exact test was used to analyze categorical data of patient characteristics. Microvascular responses are expressed as percent relaxation of the preconstricted diameter. Microvascular reactivity was analyzed using 2-way repeated-measures ANOVA with a post hoc Bonferroni test. P values < .05 were considered significant.
RESULTS
Patient characteristics
The patient characteristics are listed in the Table I. All patients with preoperative hypertension were on antihypertensive medication (β-blocker, aspirin, calcium channel blocker, or angiotensin-converting enzyme inhibitor). The preoperative HbA1c levels were 9.6 ± 0.3 in the UDM patients, 6.5 ± 0.2 in the CDM patients, and 5.4 ± 0.1 in the ND patients. There were no significant differences in perioperative norepinephrine administration in the 3 groups (Table I).
Table I.
Patient characteristics
| Patient characteristics | Nondiabetes | Controlled diabetes | Uncontrolled diabetes | P values |
|---|---|---|---|---|
| Age (y)* | 65 ± 5 | 69 ± 4.6 | 66 ± 4.0 | .8 |
| Male/female (n) | 5/3 | 6/2 | 5/3 | .97 |
| HbA1c (%)* | 5.4 ± 0.1 | 6.5 ± 0.2 | 9.4 ± 0.3† | .001 |
| Patient blood glucose (mg/dL, pre-CPB)* | 102 ± 10 | 138 ± 14 | 270 ± 15† | .0001 |
| Patient blood glucose (mg/dL, during-CPB)* | 135 ± 12 | 160 ± 16 | 196 ± 17† | .03 |
| Preoperative insulin (n) (µ/h*) | 0 (0) | 1 (2.0 ± 1) | 8 (10 ± 3)† | .004 |
| Intraoperative insulin (n) (µ/h*) | 0 (0) | 2 (10 ± 2) | 8 (15 ± 4)† | .001 |
| Obesity (BMI >30) | 3 | 3 | 3 | 1.00 |
| Atrial fibrillation (n) | 0 | 0 | 0 | 1.00 |
| Hypercholesterolemia (n) | 8 | 8 | 8 | 1.0 |
| Hypertension (n) | 8 | 8 | 8 | 1.0 |
| Duration of CPB (min)* | 120 ± 11 | 125 ± 12 | 133 ± 15 | .7 |
| Cross-clamp time (min)* | 103 ± 10 | 110 ± 12 | 116 ± 10 | .5 |
| CABG only (n) | 10 | 10 | 10 | 1.0 |
| Number of grafts | 2.9 ± 0.8 | 3.1 ± 0.8 | 3.3 ± 0.9 | .9 |
| Perioperative inotropic agent NE (n) | 7 (1.57 ± 0.47) | 7 (1.8 ± 0.48) | 7 (1.74 ± 0.51) | .9 |
Data expressed as mean ± standard error of mean.
Versus nondiabetics.
BMI, Body mass index; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; NE, norepinephrine; NS, no significance.
MAPKs protein expression
Total p38-MAPK and phospho-p38-MAPK protein expression
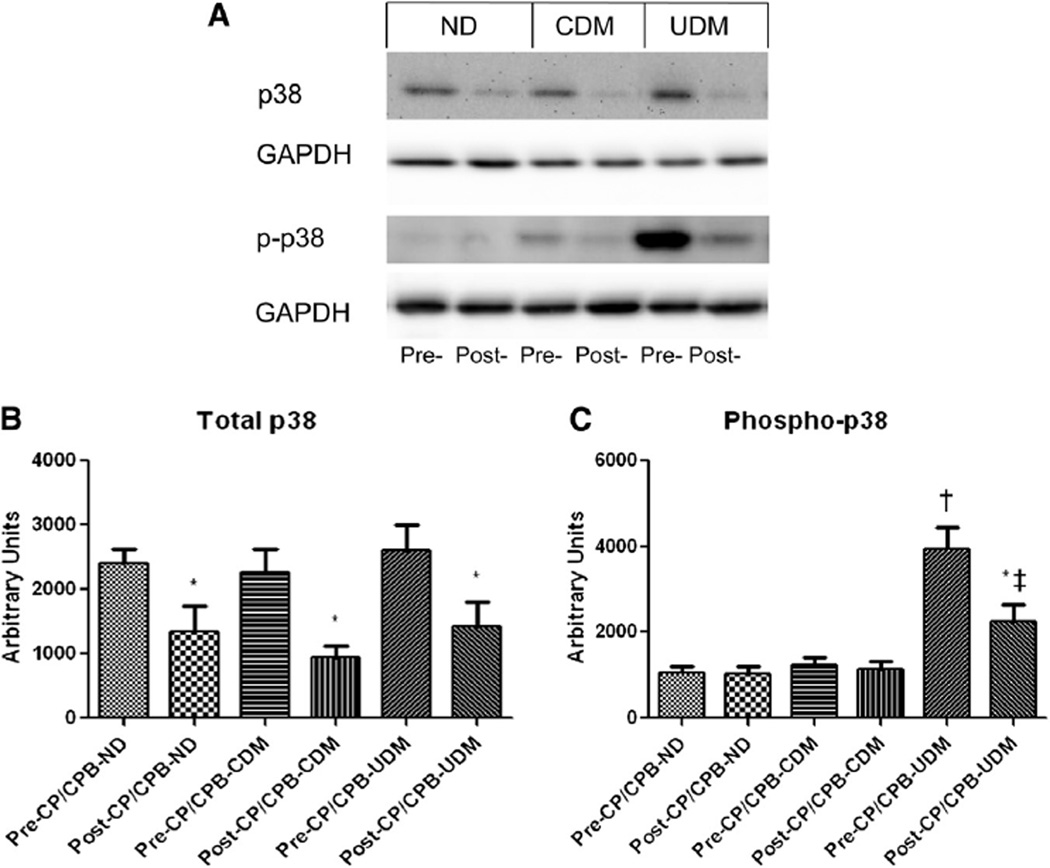
There were no significant differences in the total protein levels of the total p38-MAPK among 3 groups at baseline (Fig 1). The post-CP/CPB level of the total p38-MAPK was decreased (ND: P = .02, CDM: P = .01, UDM: P = .04, respectively), as compared with their pre-CP/CPB, but there were no significant differences in the post-CP/CPB levels of p38-MAPK among 3 groups. There were increases in phos-pho-p38-MAPK in UDM patients as compared with ND or CDM at baseline (versus ND: P = .01 or versus CDM: P = .04). Post-CP/CPB levels of phospho-p38-MAPK were unaltered in ND and CDM groups but decreased in the UDM group compared with pre-CP/CPB levels (P = .01). It is important to note that the post-CP/CPB levels of phospho-p38-MAPK remained greater in the UDM group than in the ND or CDM groups (versus ND: P = .02 and versus CDM: P = .03).
Fig 1.
(A) Representative immunoblots of human atrial tissues for total and phospo-p38 MAPK (B) Immunoblot quantification shows no changes in total p38 MAPK protein expression among 3 groups at baseline but decreased post-CP/ CPB in all groups. (C) Immunoblot quantification shows that pre- or post-CP/CPB phospho-p38-MAPK were increased in the UDM group, as compared with the ND and CDM groups; *P < .05 versus pre-CP/CPB; †P < .05 versus pre-CP/ CPB-ND or pre-CP/CPB-CDM; ‡P < .05 versus post-CP/CPB-ND or post-CP/CPB-CDM; n = 8/group.
Total ERK and phospho-ERK protein expression
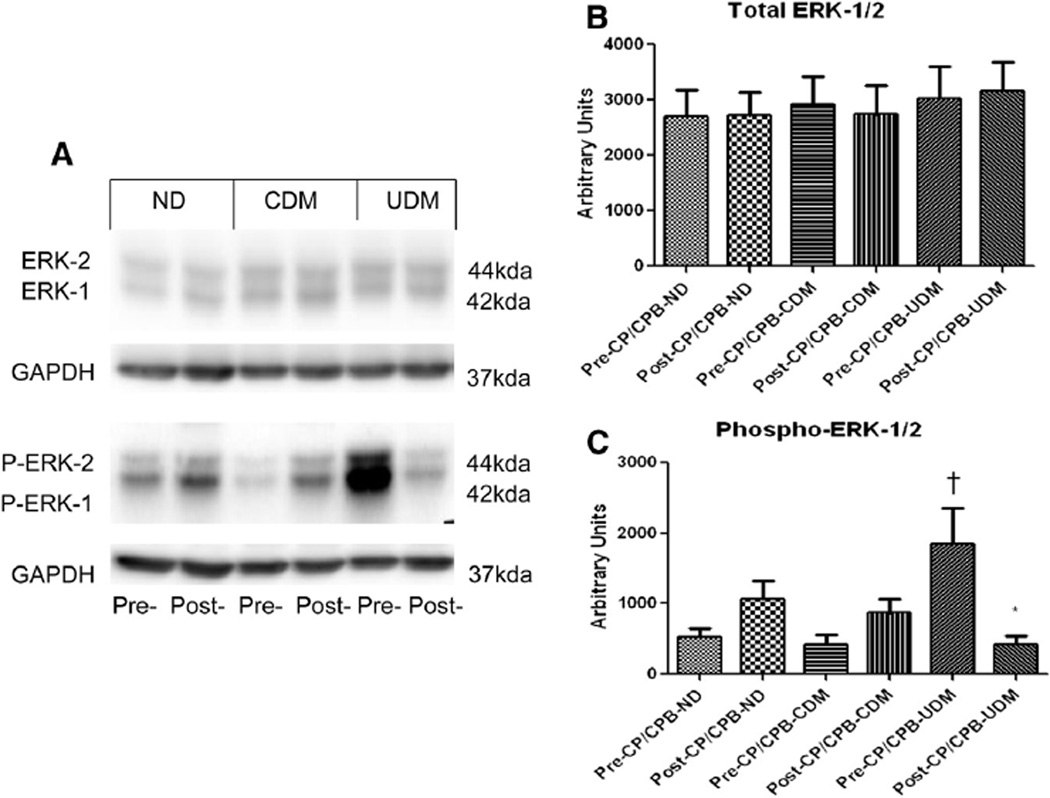
There were no significant differences in the total protein levels of ERK1/2 among the 3 groups at baseline (Fig 2, A and B). Post-CP/CPB levels of ERK1/2 were not altered in the 3 groups, as compared with their pre-CP/CPB levels (P ≥ .1 each) (Fig 2, B). There were significant increases in phospho-ERK1/2 in UDM patients compared with ND or CDM at baseline (versus ND: P = .01 and versus CDM: P = .04; Fig 2, A and C). Post-CP/CPB levels of phospho-ERK1/2 were significantly increased in the ND and CDM groups but decreased in the UDM group compared with their pre-CP/CPB levels (P < .05, Fig 2, A and C).
Fig 2.
(A) Representative immunoblots of human atrial tissues for total and phospo-ERK1/2. (B) Immunoblot quantification shows no changes of total ERK1/2 protein expression among 3 groups pre- or post-CPB in all groups. (C) Immunoblot quantification shows that pre-CP/CPB (baseline) levels of phospho-ERK1/2 were increased in the UDM group, as compared with the ND and CDM groups, but the post-CP/CPB (baseline) levels of phospho-ERK1/2 were decreased in the UDM group as compared with the ND and CDM groups; *P < .05 versus pre-CP/CPB; †P < .05 versus pre-CP/CPB-ND or pre-CP/CPB-CDM; n = 8/group.
MKP-1 and JNK protein expression
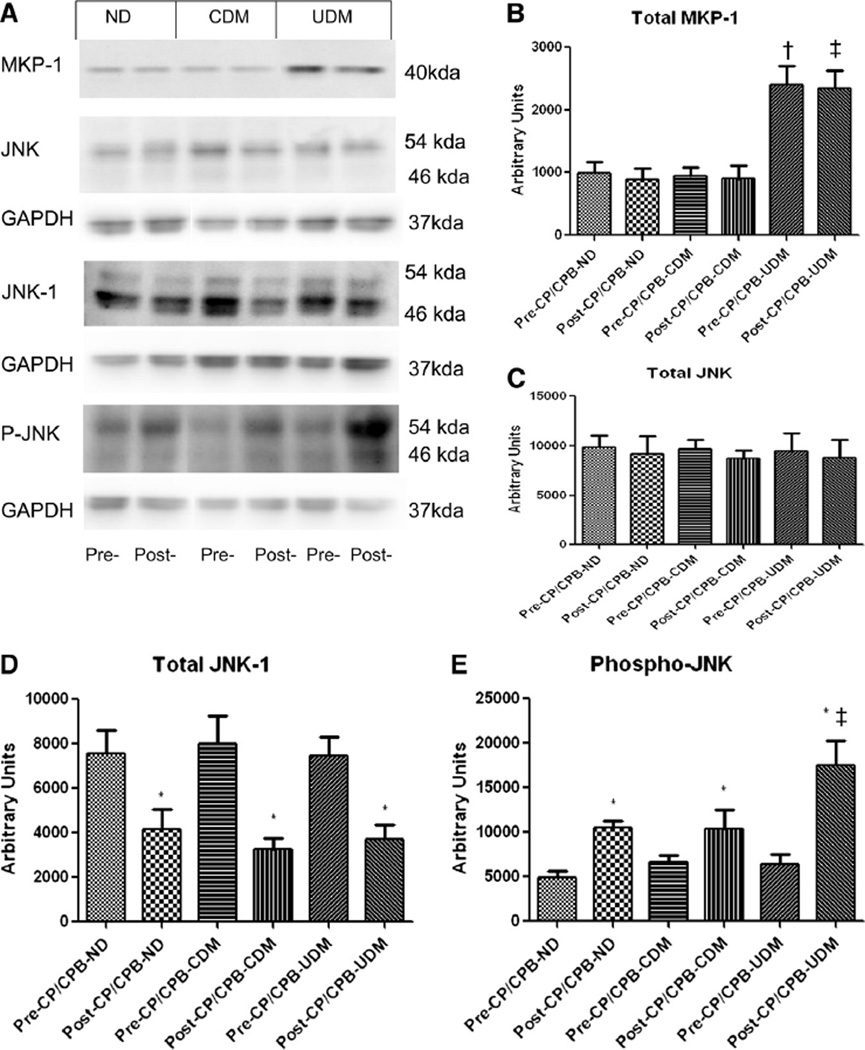
Total protein levels of MKP-1 were increased in the UDM heart compared to ND and CDM hearts at the baseline (Fig 3, B; P = .01 and P = .04). After CP/CPB, the protein levels of MKP-1 were unchanged in the 3 groups compared with pre-CP/CPB levels; however, in UDM hearts, the post-CP/CPB levels of MKP-1 were still greater than those of ND or CDM hearts (P = .01). There was no significant difference in total protein expression of JNK and JNK-1 expression among the 3 groups at baseline (P = .2). Total protein levels of JNK were unchanged post-CP/CPB in the 3 groups (P = .11). In contrast, total protein levels of JNK-1 were decreased post-CP/CPB in the 3 groups as compared with their pre-CP/CPB levels (P < .05, respectively).
Fig 3.
(A) Representative immunoblots of human atrial tissues for MKP-1, JNK, JNK1, and phospo-JNK. (B) Immunoblot quantification shows that pre- or post-CP/CPB MKP-1 was increased in the UDM group, as compared with the ND and CDM groups. (C) Immunoblot quantification shows no changes in total JNK protein expression among 3 groups at baseline and after CP/CPB. (D) Immunoblot quantification shows no changes in total JNK-1 protein expression among 3 groups at baseline but decreased post-CP/CPB in 3 groups as compared to their pre-CP/CPB, P < .05, respectively). (E) Immunoblot quantification shows increases in phospho-JNK expression post-CP/CPB in 3 groups; *P < .05 versus pre-CP/CPB; †P < .05 versus ND or CDM; n = 8/group.
Microvascular reactivity
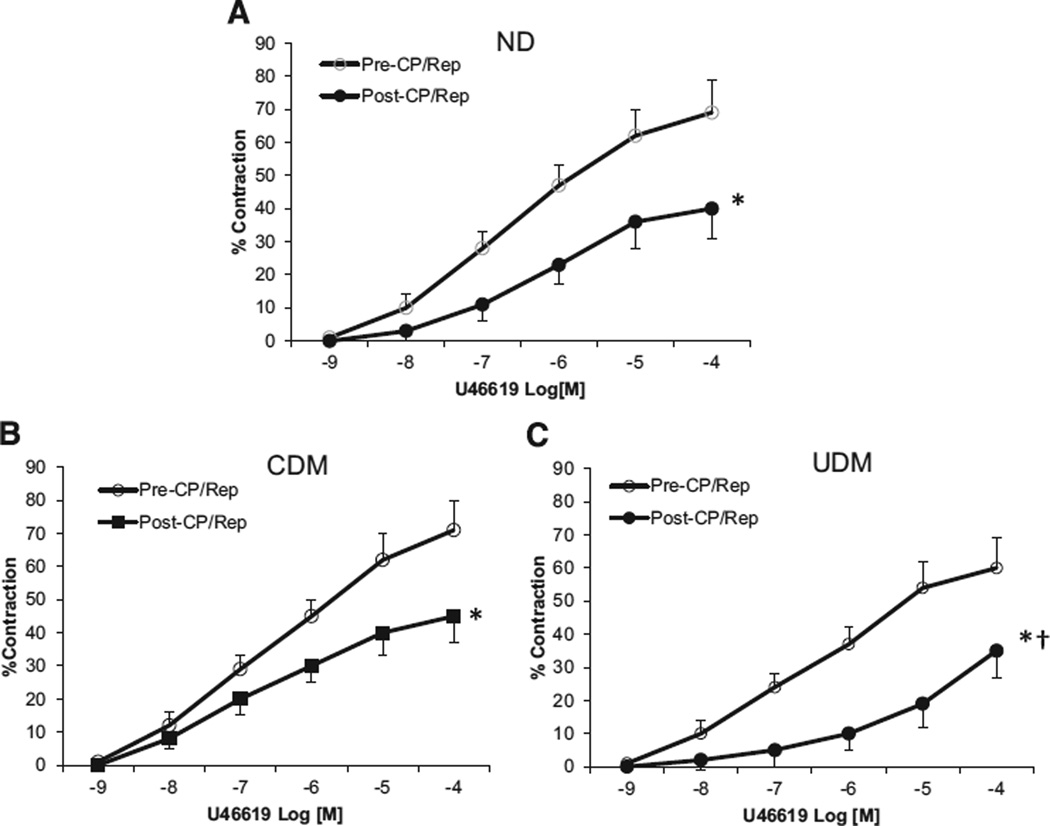
The data of TXA-2 analog U46619-induced dose-dependent contractile response of coronary arterioles are summarized in Fig 4. At baseline, no differences in response to U46619 were observed among the 3 groups. In contrast, the post-CP/CPB contractile response to U46619 was impaired in all 3 groups compared to those of pre-CP/CPB responses (P < .05 versus pre-CP/CPB, respectively). These impairments were even more pronounced in the group with uncontrolled diabetes (P < .05 versus ND or UDM, respectively; Fig 4).
Fig 4.
Coronary arteriolar vasoconstriction in response to TXA-2 analog U-46619 before and after CP/CPB. (A) ND, Nondiabetics; (B) CDM, Controlled diabetics; (C) UDM, Uncontrolled diabetics; *P < .05 versus pre-CP/CPB; †P < .05 versus ND or CDM; n = 8/group.
DISCUSSION
Previous experiments in our laboratory have shown that CPB is associated with oscillations or alterations in ERK1/2 activation in pig myocardium. 4–6 For instance, in a CPB model of pigs, there was an observed increase in phospho-ERK1/2 after 30 min of CPB followed by a decrease after 90 min of CPB in pig atrial myocardium. 4,5 Notably, the total protein levels of ERK1/ 2 in pig myocardium were unchanged after 90 min CPB. Further deactivation of ERK1/2 below its baseline level was observed during post-CPB reperfusion. Oscillations in ERK1/2 activity were also observed in pig skeletal muscle and mesenteric vessels.4 In contrast, the other study showed increased levels of activated ERK1/2 in atrial myocardial tissue from patients undergoing CABG surgery.3 This difference may represent a pattern of oscillations between activation and deactivation of ERK1/2 pathways during CP/CPB and reperfusion. It is important to note that it may also be due to the patients studied, who had differing comorbidities, such as hypertension, aging, hypercholesterolemia, and diabetes. Indeed, the present study demonstrates that activated-ERK1/2 of UDM patients was significantly increased compared to ND or CDM at baseline. Also important is that the post-CP/CPB level of activated ERK1/2 was significantly increased in the myocardium of nondiabetic and controlled-diabetic patients but was significantly decreased in the poorly controlled diabetics compared to their pre-CP/CPB levels. Consistent with our animal studies, the total protein levels of ERK1/2 in human atrial myocardium were unchanged before and after CP/CPB in the 3 groups.7,8 These results may suggest that diabetes, especially poorly controlled diabetes, is associated with modifications in ERK1/2 activation in the setting of CP/ CPB and cardiac surgery.
Talmer and colleagues reported that activated p38 MAPK was significantly increased in atrial myocardial tissue following CP/CPB in patients undergoing CABG surgery.3 In our present study, however, post-CP/CPB–activated-p38-MAPK was unaltered in nondiabetic and controlled-diabetic myocardium but was significantly decreased in the poorly controlled diabetic myocardium as compared with the pre-CP/CPB. Interestingly, post-CP/CPB levels of phospho-p38-MAPK were still greater in poorly controlled diabetics compared with nondiabetics and controlled diabetics. Additionally, the baseline level of phospho-p38-MAPK was increased in the uncontrolled-diabetic myocardium. The discrepancy between the findings of Talmer et al3 and the present study also represent a pattern of oscillation between activation and deactivation of p38-MAPK pathways during CPB and reperfusion. Our results may suggest that diabetes, especially uncontrolled diabetes, plays an important role in the regulation of myocardial p38-MAPK expression and activation in the setting of CP/CPB.
MKP-1, which may be activated by ischemia and reperfusion, potentially dephosphorylates and deactivates ERK1/2 post-CPB.1,2,4,5 In the present study, we found that basal MKP-1 levels were increased in the myocardia of patients with uncontrolled diabetes. In addition, the post-CP/CPB levels of MKP-1 in the uncontrolled diabetic myocardium were still greater than those of nondiabetics. Consistent with the previous study of Talmer et al,3 our results also indicate that JNKs were significantly activated after CP/CPB in the 3 groups. Importantly, the activated JNKs were more pronounced in the uncontrolled diabetic myocardium after CP/CPB. Further, JNK-1 protein expression was downregulated after CP/CPB in nondiabetic and diabetic myocardium.
Previous studies have shown that peripheral microvessel reactivity and coronary myogenic tone were significantly reduced after treatment with the MAPKs inhibitors PD98059 and SB203580. 6–8 These findings suggest that MAPKs play an important role in the regulation of microvascular reactivity and myogenic tone. Recently, we have found that diabetes decreased the contractile response of human arterioles to endothelin-1.19 In this study, the basal contractile response of coronary arterioles to TXA-2 analog U46619 was significantly decreased post-CPB. The impairment was more pronounced in the group with uncontrolled diabetes. Thus, the altered MAPK activation after CP/CPB may, in part, mediate coronary microvascular dysfunction in diabetic patients. Dysfunction of the coronary microcirculation due to diabetes and CP/CPB may result in detrimental effects in myocardial perfusion and function.2,18,20,21
In conclusion, CP/CPB was associated with alterations in myocardial expression and activation of MAPKs as well as vasomotor dysfunction of coronary arterioles in patients with CABG. Uncontrolled diabetes or diabetes combined with CP/ CPB further altered expression and activation of MAPKs, such as p38-MAPK, ERK1/2, JNK, and MKP-1. Poorly controlled diabetes worsens the recovery of vasomotor function of coronary arterioles in patients undergoing CABG surgery.
Acknowledgments
We thank all nurses, physician assistants, and perfusionists at the Lifespan Hospitals for collecting tissue samples and patient data.
Supported in part by National Institutes of Health R01 grants-HL-46716 and HL-69024 (FWS) and NIH training grant 5T32-HL-094300-03 (AAS).
Footnotes
Presented at 8th Annual Academic Surgical Congress, February 5–7, 2013, New Orleans, LA.
REFERENCES
- 1.Rose BA, Force T, Wang YB. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90:1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan TA, Bianchi C, Ruel M, Voisine P, Sellke FW. Mitogen-activated protein kinase pathways and cardiac surgery. J Thorac Cardiovasc Surg. 2004;127:806–811. doi: 10.1016/j.jtcvs.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Talmor D, Applebaum A, Rudich A, Shapira Y, Tirosh A. Activation of mitogen-activated protein kinases in human heart during cardiopulmonary bypass. Circ Res. 2000;12:1004–1007. doi: 10.1161/01.res.86.9.1004. [DOI] [PubMed] [Google Scholar]
- 4.Araujo EG, Bianchi C, Faro R, Sellke FW. Oscillation in the activities of MEK/ERK1/2 during cardiopulmonary bypass in pigs. Surgery. 2001;130:182–191. doi: 10.1067/msy.2001.115826. [DOI] [PubMed] [Google Scholar]
- 5.Araujo EG, Bianchi C, Sato K, Faro R, Li XA, Sellke FW. Inactivation of the MEK/ERK pathway in the myocardium during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2001;121:773–781. doi: 10.1067/mtc.2001.112933. [DOI] [PubMed] [Google Scholar]
- 6.Khan TA, Bianchi C, Araujo EG, Ruel M, Voisine P, Sellke FW. Activation of pulmonary mitogen-activated protein kinases during cardiopulmonary bypass. J Surg Res. 2003;115:56–62. doi: 10.1016/s0022-4804(03)00236-1. [DOI] [PubMed] [Google Scholar]
- 7.Khan TA, Bianchi C, Ruel M, Voisine P, Li J, Liddicoat JR, et al. Mitogen-activated protein kinase inhibition and cardioplegia-cardiopulmonary bypass reduce coronary myogenic tone. Circulation. 2003;108:II348–II353. doi: 10.1161/01.cir.0000087652.93751.0e. [DOI] [PubMed] [Google Scholar]
- 8.Khan TA, Bianchi C, Araujo EG, Ruel M, Voisine P, Li J, et al. Cardiopulmonary bypass reduces peripheral microvascular contractile function by inhibition of mitogen-activated protein kinase activity. Surgery. 2003;134:247–254. doi: 10.1067/msy.2003.229. [DOI] [PubMed] [Google Scholar]
- 9.Ebel D, Toma O, Appler S, Baumann K, Fräissdorf J, Preckel B, et al. Ischemic preconditioning phosphorylates mitogen-activated kinases and heat shock protein 27 in the diabetic rat heart. Horm Metab Res. 2009;41:10–15. doi: 10.1055/s-0028-1087171. [DOI] [PubMed] [Google Scholar]
- 10.Riad A, Unger D, Du J, Westermann D, Mohr Z, Sobirey M, et al. Chronic inhibition of p38MAPK improves cardiac and endothelial function in experimental diabetes mellitus. Eur J Pharmacol. 2007;554:40–45. doi: 10.1016/j.ejphar.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 11.Wenzel S, Soltanpour G, Schlüter KD. No correlation between the p38 MAPK pathway and the contractile dysfunction in diabetic cardiomyocytes: hyperglycaemia-induced signaling and contractile function. Pflugers Arch. 2005;451:328–337. doi: 10.1007/s00424-005-1476-5. [DOI] [PubMed] [Google Scholar]
- 12.Adhikary L, Chow F, Nikolic-Paterson DJ, et al. Abnormal p38 mitogen-activated protein kinase signalling in human and experimental diabetic nephropathy. Diabetologia. 2004;47:1210–1222. doi: 10.1007/s00125-004-1437-0. [DOI] [PubMed] [Google Scholar]
- 13.Westermann D, Rutschow S, Van Linthout S, et al. Inhibition of p38 mitogen-activated protein kinase attenuates left ventricular dysfunction by mediating pro-inflammatory cardiac cytokine levels in a mouse model of diabetes mellitus. Diabetes. 2007;6:641–646. doi: 10.1007/s00125-006-0385-2. [DOI] [PubMed] [Google Scholar]
- 14.Strniskova M, Barancik M, Neckar J, Ravingerova T. Mitogen-activated protein kinases in the acute diabetic myocardium. Mol Cell Biochem. 2003;249:59–65. [PubMed] [Google Scholar]
- 15.Sodha NR, Feng J, Clements RT, Bianchi C, Boodhwani M, Ramlawi R, et al. Protein kinase C-α modulates microvascular reactivity in the human coronary and skeletal microcirculation. Surgery. 2007;142:243–252. doi: 10.1016/j.surg.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Feng J, Liu YH, Khabbaz KR, Hagberg R, Osipov RM, Sodha NR, et al. Endothelin-1 induced contractile responses of human coronary arterioles via endothelin-A receptors and PKC-α signaling pathways. Surgery. 2010;147:798–804. doi: 10.1016/j.surg.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng J, Chu LM, Robich M, Clements RT, Khabbaz K, Hagberg R, et al. Endothelin-1 induced contraction and signaling in the human skeletal muscle microcirculation. Circulation. 2010;122:S150–S155. doi: 10.1161/CIRCULATIONAHA.109.928226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng J, Liu YH, Chu LM, Clements RT, Khabbaz KR, Robich MP, et al. Thromboxane-induced contractile response of human coronary arterioles is diminished post-cardioplegic arrest. Ann Thorac Surg. 2011;92:829–836. doi: 10.1016/j.athoracsur.2011.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng J, Liu YH, Khabbaz K, Hagberg R, Robich MP, Clements RT, et al. Decreased contractile response to endothelin-1 of peripheral microvasculature from diabetic patients. Surgery. 2011;149:247–252. doi: 10.1016/j.surg.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng J, Liu YH, Chu LM, Singh AK, Dobrilovic N, Fingleton JG, et al. Changes in microvascular reactivity after cardiopulmonary bypass in patients with poorly controlled versus controlled diabetes. Circulation. 2012;126:S73–S80. doi: 10.1161/CIRCULATIONAHA.111.084590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruel M, Khan TA, Voisine P, Bianchi C, Sellke FW. Vasomotor dysfunction after cardiac surgery. Eur J Cardiothorac Surg. 2004;26:1002–1014. doi: 10.1016/j.ejcts.2004.07.040. [DOI] [PubMed] [Google Scholar]