Abstract
The fact that glioblastomas, which are one of the most devastating cancers, frequently express the Delta EGFR also called mutant variant III of EGFR (EGFRvIII) suggests that this cancer cell–specific receptor might serve as an ideal target for cancer therapy. To assess its potential as such a target, we constructed an oncolytic adenovirus with Retargeted Infectivity Via EGFR (Delta-24-RIVER) on the backbone of Delta-24. This new oncolytic adenovirus targets, as Delta-24 does, the disrupted Rb pathway in cancer cells; in addition, this adenovirus has also been retargeted through the abrogation of CAR binding (Y477A mutation in adenoviral fiber protein) and insertion of an EGFRvIII-specific binding peptide in the HI loop of the fiber protein. Compared to Delta-24, Delta-24-RIVER induced EGFRvIII-selective cytotoxicity in U-87 MG isogenic cell lines and in tetracycline-inducible EGFRVIII expressing U-251 MG cells. Accordingly, by tittering the viral progeny and examining fiber protein expression in the above cells, we showed that the replication of this new construct also correlated with EGFRvIII expression. Consistently, immunohistochemistry staining of the adenoviral capsid protein hexon in the virus-treated tumors revealed that the virus replicated more efficiently in EGFRvIII-expressing U-87 MG.ΔEGFR xenografts than in the tumors grown from U-87 MG cells. Importantly, treatment with Delta-24-RIVER prolonged the survival of animals with intracranial xenografts derived from U-87 MG.ΔEGFR cells. Therefore, our results constitute the first proof of the direct targeting of a cancer-specific receptor using an oncolytic adenovirus.
Keywords: oncolytic adenovirus, Delta-24, EGFR, glioma, therapy
Introduction
Glioblastoma multiforme (WHO grade IV), the most frequent histological type of primary brain tumor, accounts for 69% of all astrocytic and oligodendroglial tumors 1. Median survival is generally less than 1 year from the time of diagnosis, with a life expectancy of 2 years at the most in patients with the most favorable disease characteristics2. Recent advances in our understanding of brain tumor biology have suggested that treatment strategies that target molecular defects in the tumors will provide the most effective and selective therapies3,4. One of the most common molecular abnormalities in glioblastomas is amplification of the epithelial growth factor receptor (EGFR) gene, which exists in ~40% of primary glioblastomas 1. In addition, EGFR amplicons are often mutated, with the most frequent type (>50%) consisting of the variant Delta or III (EGFRvIII or Delta-EGFR) mutation, which comprises the deletion of exons 2 to 7 1,5. Importantly, this deletion generates a unique epitope in the extracellular component of the protein that is not present in normal cells and may be used as a cancer- specific target5.
Adenovirus-mediated oncolysis is a rapidly growing field that could lead to the development of novel and more effective anticancer therapies4,6,7. One problem that is hindering the development and application of this therapy is the varied tropism of the adenovirus resulting from the widespread distribution of its primary cellular receptor the coxsackie and adenovirus receptor (CAR). Of particular pertinence in gliomas is that most glioma cells are not optimum targets because they express low levels of CAR7. Moreover, normal tissues express high levels of CAR, with the result that the administration of oncolytic adenoviruses could result in potential uncontrolled infection and toxicity8. Therefore, strategies capable of CAR-independent infectivity and of retargeting the virus to cancer-specific receptors are required to improve the therapeutic index of oncolytic adenoviruses in gliomas. In one effort to overcome the problem, we have constructed a replication-competent adenovirus with Retargeted Infectivity Via EGFR (Delta-24-RIVER) and tested its infectivity, selectivity, and efficacy in gliomas cells in vitro and in vivo.
Methods
Cell lines and tissue culture
The human malignant glioma cell lines U-87 MG and U-251 MG and human lung carcinoma cell line A549 were obtained from the American Type Culture Collection (Manassas, VA). U-87.wtEGFR and U-87.ΔEGFR cell lines were kind gifts from Dr. WK Cavenee (Ludwig Institute for Cancer Research, University of California at San Diego, La Jolla, CA)9. The U-251.E18 cell line was a generous gift from Dr. AA Habib (The University of Texas Southwestern Medical Center, Dallas, TX)10. Cell lines were maintained in DMEM/F12 supplemented with 10% FBS, 100µg/ml penicillin, and 100µg/ml streptomycin in a humidified atmosphere containing 5% CO2 at 37°C. 293F28 and U-251.E18 cells were cultured in the same medium containing 100µg/ml Zeocin 10,11. In addition, U-251.E18 cells were maintained in the presence or absence of tetracycline (1µg/ml) to repress or activate, respectively, the expression of EGFRvIII10.
Peptide binding assay
The peptide (5-carboxyfluorescein)-HFLIIGFMRRALCGA was synthesized by SynPep (Dublin, CA). For peptide binding, cells were detached and dissociated using 0.05% EDTA in PBS by pipetting. After this, 106 cells were resuspended and blocked in 200µl of PBS containing 10% normal goat serum and 5% BSA for 20 min at 25°C with shaking at 100 rpm. Then, the cells were pelleted and resuspended in PBS containing 1% BSA and 1µM peptide and incubated at 25°C for 30 min with shaking at 100 rpm. Afterwards, the cells were washed once with 1% BSA in PBS and fixed with 4% paraformaldehyde in PBS for 10 min at 25°C. Finally, cells were washed twice with PBS and resuspended in 500µl of PBS. The binding of the peptide on the cell surface was analyzed with flow cytometry (FACSCalibur; Becton Dickinson, San Jose, CA).
Delta-24-RIVER construction and adenoviral infection
Delta-24-RIVER was generated by genetically modifying the adenovirus serotype 5, as depicted in Figure 1d. First, a duplex of oligonucleotides (5’-ACAACTGGATCTTCACACTTCCTGATCATTGGATTCATGCGTCGCGCACTTTG CGGTGCGGGATCATCTCC-3’ and 5’- GGAGATGATCCCGCACCGCAAAGTGCGCGACGCATGAATCCAATGATCAGG AAGTGTGAAGATCCAGTTGT-3’) encoding the previously described EGFRVIII-binding peptide, PEPCH112, was cloned within the HI loop of the knob domain, flanked by GSS flexible linkers. Specifically, the sequence was inserted into the EcoRV site of pXKΔHI, a shuttle vector designed to introduce the modifications in the HI loop of the fiber protein13. Second, a Y477A mutation was introduced using the QuikChange II XL site–directed mutagenesis kit (Stratagene, La Jolla, CA) to abrogate the binding of the viral fiber to the native receptor CAR, as previously described14, resulting in the generation of pXKΔHI.vIII. The Delta-24 deletion6, 7 was introduced into the adenoviral backbone through the homologous DNA recombination in E. coli BJ5183 (Stratagene) of the E1 fragment from pXC1-Delta-246 and the ClaI-linearized pVK500C15, which yielded the pVK.Delta-24 plasmid. The final adenoviral genome was generated by the homologous DNA recombination of pXKΔHI.vIII and SwaI-linearized pVK500C.Delta-24 in E. coli BJ5183. To rescue the Delta-24-RIVER virus, the resulting viral backbone vector was digested with PacI and then transfected into 293F28 cells, a cell line expressing high levels of wild-type viral fiber11, which helps in the first round of viral production. The virus was then propagated in A549 cells. The mutant-E1A region and the fiber modification were confirmed by PCR and restriction analysis, as previously described16, and sequencing.
Figure 1.
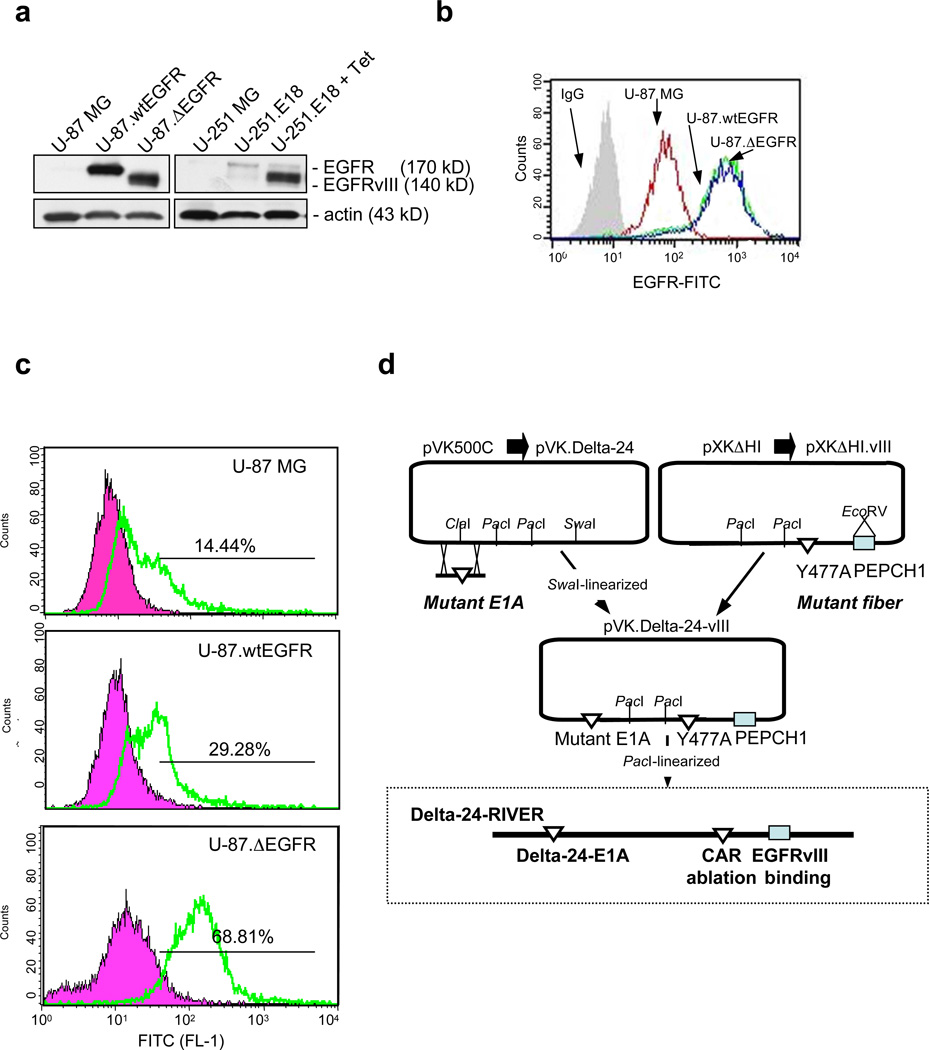
Generation of Delta-24-RIVER. (a) Expression of EGFR or EGFRvIII in U-87 MG and U-251 MG isogenic cell system, as assessed by Western blotting. Levels of wild-type EGFR in parental cells (U-87 MG and U-251 MG) and U251.E18 cells were almost undetectable. High levels of wild-type EGFR were detected in U-87.wtEGFR EGFR cells, and high levels of mutant EGFR were observed in U-87.ΔEGFR and U251.E18 cells cultured in the presence of tetracycline (Tet, 1µg/ml) for 24 hr. Actin was used as the loading control. (b) EGFR expression in the U-87 MG isogenic cell system. Levels of expression were analyzed by flow cytometry after exposure of the cultures to an antibody that recognizes both the wild-type and variant mutant form of EGFR. Note that U-87.EGFR and U-87.ΔEGFR cells expressed similar levels of EGFR or EGFRvII, respectively, on their surface. (c) Testing of the EGFRvIII-binding peptide to the isogenic U-87 MG system. Shown are representative findings from the peptide-binding assay performed in the three U-87 MG cell lines differing in their EGFR/EGFRvIII expression. For this assay, PECPH1 peptide linked to carboxyfluorescein was synthesized. Cultures were exposed to 1µM of the peptide, as explained in the Methods section, and analyzed for fluorescence levels using a flow cytometer. Note that PCEPH1 preferentially bound to EGFRvIII-overexpressing U-87 MG cells. (d) Schematic representation of the genetic modifications introduced into the backbone of adenovirus serotype 5. The Delta-24-RIVER genome contains three modifications: a mutant-E1A adenoviral region, consisting of 24-bp deletions in the Rb-binding region 6, 7; a Y477A mutation in the loop of the fiber, which is critical for binding to CAR14; and the coding sequence for the EGFRvIII-binding PEPCH1 peptide 12.
Flow cytometry
Suspensions of U-87 MG isogenic cells (2–5×105 cells) were first treated with FcR blocking reagent (Miltenyi Biotec, Auburn, CA) to block the unwanted binding of antibodies to the cells and then incubated with FITC-conjugated anti-human EGFR antibody (1:100; Calbiochem, La Jolla, CA) for 30 min at 4°C. The expression levels of CAR and integrin αvβ5 were determined as previously described17. We used conjugated IgG isotypes as negative controls. Samples were analyzed using FACSCalibur.
Cell viability assay
Cell viability was analyzed by crystal violet staining16. In this experiment, cells (U-87 MG and derived cells, 2×104 cells/well; U-251.E18, 5×104 cells/well) were seeded and 20 hr later infected with Delta-24-RIVER or Delta-24 at a multiplicity of infection (MOI) of 0 to 10. Experiments were concluded when the highest infection dose used produced a cytopathic effect of more than 80%. The infected cell monolayers were washed twice with PBS and fixed and stained with 0.1% crystal violet in 20% ethanol. Excess dye was removed by rinsing with water.
Viral replication assay
A549 cells were seeded at a density of 5× 105 cells/well in 6-well plates and infected with Delta-24-RIVER at 10 p.f.u. (plaque-forming units)/cell. Forty-eight hours after infection, the infectious viral progeny were titered using the Adeno-X-Rapid Titer assay kit (BD Biosciences, Palo Alto, CA) according to the manufacturer’s instructions. Final viral titers were determined as pfu/ml.
Real-Time PCR
Total RNA was isolated and reverse-transcribed using reverse transcriptase and quantitative-PCR analysis was performed on a chromo 4 sequence-detection system (Bio-Rad, Hercules, CA) using a SYBR-green PCR master mix (Applied Biosystems, Foster City, CA), and the following specific primers for fiber (forward: 5'-CGGCCTCCGAACGGTACT-3'; reverse: 5'-TCTTGCGCGCTTCATCTTG-3') and for the house-keeping gene β-actin (forward: 5'-GCTCTTTTCCAGCCTTCCTT-3'; reverse: 5'-GCCTCGGCTTGTCACATTTT-3'). The cycling conditions for PCR were as follows: 10 min at 95°C for 1 cycle; 15 s at 95°C, and 1 min at 60°C for 40 cycles. To determine relative gene expression, the comparative threshold cycle (CT) method was used18.
Immunoblotting
Equal amounts of proteins from the cell lysates were separated by SDS-PAGE and probed with antibodies against the following proteins: viral fiber protein (4D2; NeoMarkers, Inc., Fremont, CA), EGFR, and actin (Santa Cruz Biotechnology, Santa Cruz, CA). Protein bands were visualized using an ECL Western Blotting Detection System (GE Healthcare, Amersham Bioscience, Pittsburgh, PA).
Animal studies
To assess in vivo tropism, human glioma cells, either U-87 MG (5× 105) or U-87.ΔEGFR (2.5× 105) cells, were engrafted in the caudate nucleus of athymic mice, as previously described7, and on days 3, 5, and 7 after cell implantation, animals were locally treated (5.5× 107 p.f.u./dose) with intratumoral injections of Delta-24-RIVER. Animals were sacrificed 18–20 days after cell implantation and their brains studied for viral protein expression.
To assess the in vivo anticancer effects of Delta-24-RIVER, human glioma cells (105 cells) were intracranially implanted in athymic mice, and Delta-24-RIVER or UV-inactivated adenovirus was administered locally (5.5 ×107 p.f.u./dose, 5 doses) starting 3 days later. Any animals showing generalized or localized symptoms of toxicity were sacrificed. Surviving animals were sacrificed 61 days after cell implantation. The brains of the mice were removed, fixed in 4% formaldehyde for 24 hr at room temperature, and embedded in paraffin. Hematoxylin-eosin–stained sections were evaluated for evidence of tumor, necrosis, and viral nuclear inclusions. Animal studies were performed in veterinary facilities at The University of Texas M.D. Anderson Cancer Center, Houston, TX, in accordance with institutional guidelines.
Immunohistochemistry
Paraffin-embedded sections of mouse brain tumor were analyzed for expression of the adenoviral protein hexon by immunohistochemistry, as previously described7. Briefly, after antigen retrieval, sections were probed with rabbit polyclonal anti-hexon (1:150 dilution; Chemicon, Temecula, CA), counterstained with hematoxylin (Sigma, St. Louis, MO), and mounted on Immu-mount (Thermo Shandon Inc., Pittsburgh, PA) after washing.
Statistical Analysis
The in vivo anticancer effect of different treatments was assessed by plotting survival curves according to the Kaplan-Meier method, and groups were compared using the log-rank test.
Results
Construction of EGFRvIII-retargeted Delta-24
To test the possibility of constructing an EGFRvIII-retargeted adenovirus, we first tested the specificity of a small peptide PECPHC1 that was previously shown to bind preferentially to EGFRvIII in pull-down assays or to bind to immobilized recombinant proteins12. To this end, we selected two glioma isogenic cell systems that differed in their EGFR/EGFRvIII expression. The first isogenic system was a U-87 MG system consisting of parental (low EGFR expression), U-87.wtEGFR (high EGFR expression), and U-87.ΔEGFR (high EGFRvIII expression) cells, as shown by western blotting and FACS analysis (Fig 1a,b). Of interest, the overexpression of EGFR and EGFRvIII in the cellular membrane was of similar intensity in this system, as shown by FACS analysis (Fig 1b). In the second isogenic cell system, a tetracycline-inducible system was used to induce EGFRvIII expression in U-251 MG cells (ref #10 and Fig 1a).
The ability of the 5-FAM-PECPHC1 peptide to bind to EGFRvIII in vivo was analyzed in the U-87 MG isogenic system by exposing cultures to the peptide and analyzing the fluorescence levels by FACS analysis. The binding to EGFRvIII was around 5- and 2.5fold higher than the binding to EGFR in parental or EGFR-overexpressing U-87 MG cells, respectively (Fig 1c). We concluded from these results that, as previously noted in in vitro experiments12, the peptide preferentially binds in a living cell to the mutant variant of EGFR.
Delta-24-RIVER was constructed as described in Methods and depicted in Figure 1d. The virus encompasses three genetic modifications on the backbone of adenovirus serotype 5 to allow for selective infectivity and replication. The selective replicative phenotype is based in the generation of a 24-base pair deletion in the E1A gene, similar to the mutation in Delta-24 described elsewhere6. This mutation disables the binding of E1A to the cellular Rb protein, thereby halting viral replication in quiescent Rb-competent cells. The selective infectivity is achieved by two genetic modifications: the insertion of the coding sequence for the PEPHC1 peptide in the HI loop of the adenoviral fiber protein and the previously described Y477A mutation in the knob of the fiber protein, which abrogates the binding to CAR14 (Figure 1d). Thus, this new oncolytic adenovirus is designed to preferentially infect cells expressing EGFRvIII and to selectively replicate in cancer cells with a disrupted Rb pathway.
Delta-24-RIVER–induced selective cytotoxicity of EGFRvIII-expressing glioma cells
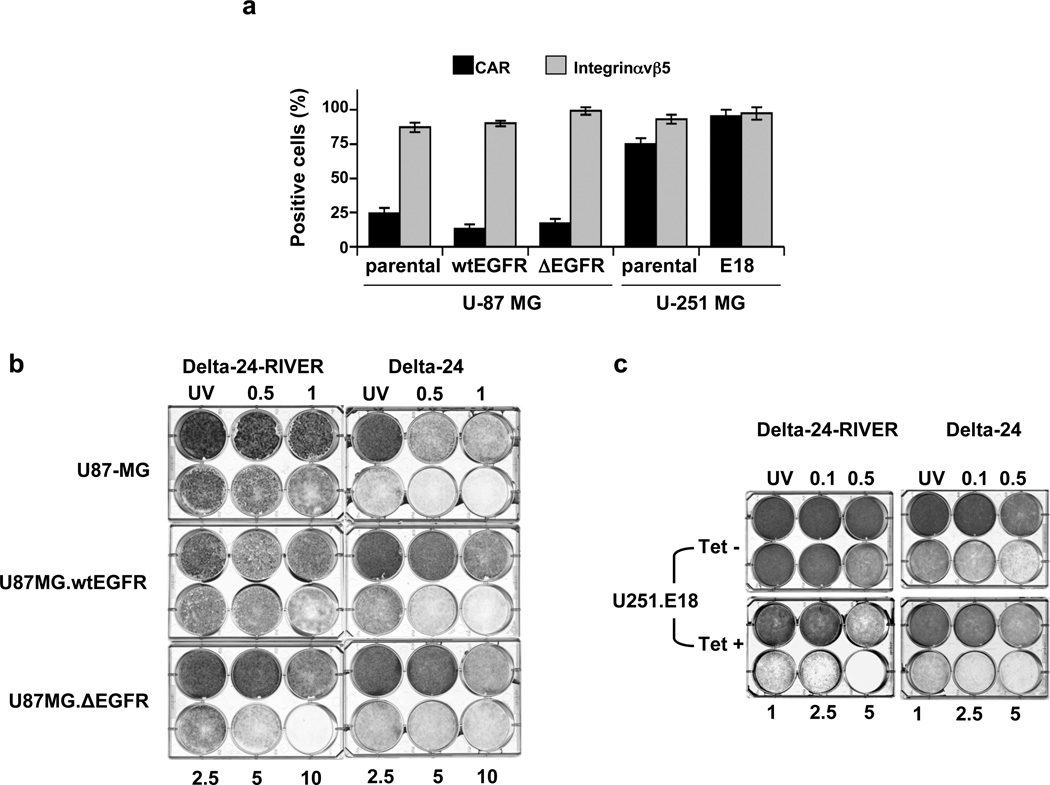
To determine whether Delta-24-RIVER–induced cytotoxicity is related to EGFRvIII expression rather than the expression of native adenoviral receptors, such as CAR and integrins19, we first examined the expression of these proteins on the cell surface using flow cytometry. The isogenic cells derived from U-87 MG cells expressed low levels of CAR (<30% of the examined cells), as did the parental cell line, whereas the U-251 MG– derived cells (U-251.E18) expressed high levels of the native receptor (>75%), similar to the levels of expression observed in the parental cells. As expected, all cell lines expressed high levels of integrin αvβ5 (>80%) on their surface (Fig 2a) 7.
Figure 2.
Delta-24-RIVER induces a preferential cytopathic effect in EGFRvIII-expressing human glioma cells. (a) Expression of adenoviral receptors. Flow cytometric analysis of CAR and integrin αvβ5 expression in the isogenic cell systems. Data are shown as the mean ± SD of data from three independent experiments. (b, c) Cell viability analysis of Delta-24-RIVER– and Delta-24–infected human glioma cell cultures. Low CAR–expressing U-87 MG– and U-87–derived cultures are represented in (b) and high CAR–expressing cell lines (U-251.E18 in the presence or absence of tetracycline, Tet, 1µg/ml) are represented in (c). Cultures were infected at the indicated doses. Experiments were concluded when one of the viruses induced cell death in more than 80% of cells at the highest dose. UV: UV-inactivated viruses at the highest dose used for infection.
To determine the oncolytic power of Delta-24-RIVER, we performed dose-dependence assays in which both isogenic systems (i.e., high-CAR– and low-CAR–expressing cell lines) were infected with Delta-24 or Delta-24-RIVER. Delta-24-RIVER displayed a specific oncolytic effect in EGFRvIII-expressing cancer cells independent of the levels of CAR expression. That is, Delta-24-RIVER had a complete cytopathic effect (CPE), at 10 p.f.u./cell, on the U-87.ΔEGFR monolayer, but less than a 30% CPE on U-87.wtEGFR and U-87 MG cultures (Fig 2b). Consistently, Delta-24-RIVER induced a complete CPE at a dose of 5 p.f.u./cell in U-251.E18 cells cultured in the presence of tetracycline but had almost no effect on U-251.E18 cells cultured in the absence of tetracycline (Fig 2c). Of importance, in cells expressing low levels of CAR (i.e., U-87 MG cells), Delta-24-RIVER was more efficient in causing CPE in EGFRvIII-expressing cells than was Delta-24.
Taken together, our data demonstrate that Delta-24-RIVER–mediated oncolysis was selective for EGFRvIII expression and independent of CAR expression, and that the retargeted oncolytic adenovirus was more potent than untargeted Delta-24 adenovirus in EGFRvIIII- and low-CAR–expressing cells.
Replication of Delta-24-RIVER in EGFRvIII-expressing glioma cells
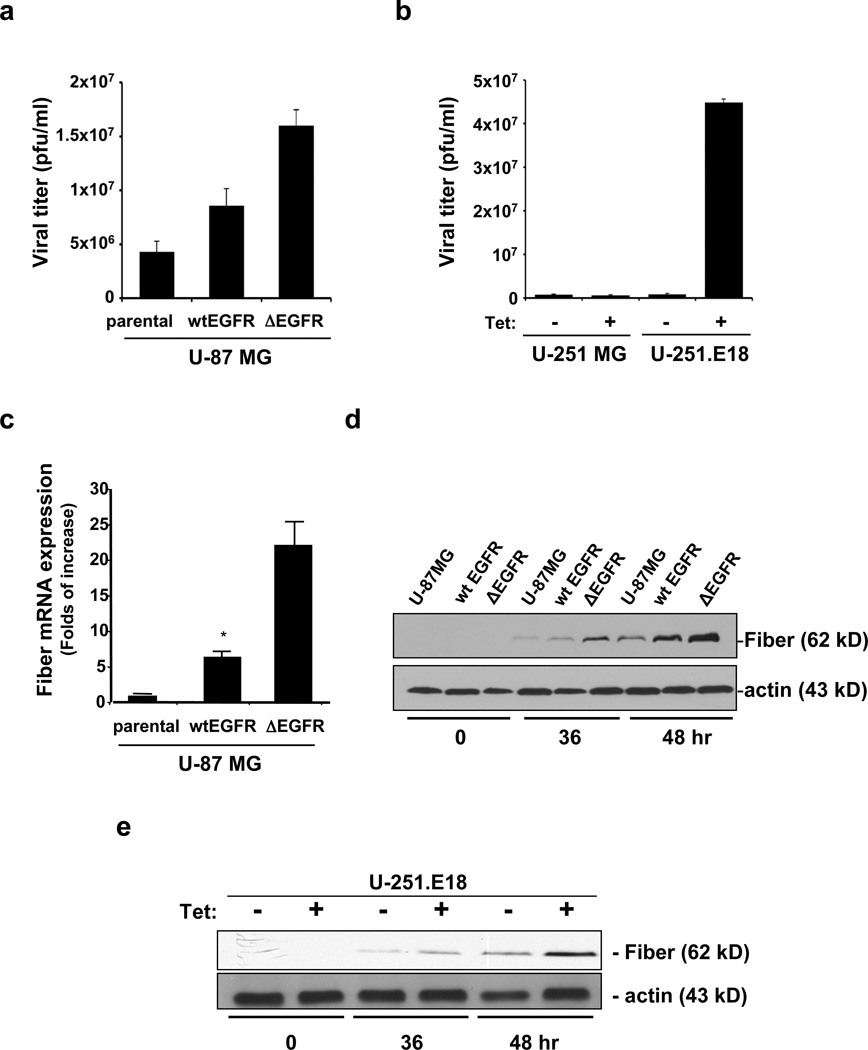
Since oncolytic adenoviruses induce cell death through viral replication and eventually lysis of the host cell6, we next determined whether the EGFRvIII-specific cytotoxicity induced by Delta-24-RIVER was related to increased adenoviral replication by performing a titration assay of the viral progeny. In both isogenic systems, we observed that Delta-24-RIVER replication was related to EGFRvIII expression. That is, the replication efficiency of Delta-24-RIVER in U-87.ΔEGFR cells was 2- and 4-fold more potent than that in U-87.wtEGFR and U-87 MG cells, respectively (Fig 3a). A similar positive relationship between selective CPE and adenoviral replication was observed in the U-251 MG derived cells. U-251 MG cells in which EGFRvIII expression was induced by the tetracycline-inducible system. Specifically, the replication efficiency of Delta-24-RIVER was 50- and 70-fold higher in the U-251.E18 cells expressing EGFRvIII (i.e., cultured in the presence of tetracycline) than in the EGFRvIII-negative U-251.E18 cells (i.e., cultured in the absence of tetracycline) and parental U-251 MG cells, respectively (Fig 3b).
Figure 3.
Delta-24-RIVER effectively replicates in EGFRvIII-expressing cells. (a, b) Cells were infected with Delta24-RIVER adenovirus at an MOI of 10, and analyses were performed 48 hr after infection. In the U-251 MG-derived system, cells were cultured in the presence or absence of tetracycline (Tet, 1µg/ml). Quantification of viral progeny. Viral titers were determined by the tissue culture infection dose50 (TICD50) method and expressed as p.f.u./ml. Data are represented as mean ± SD of three independent experiments. (c) Real-time PCR to analyze fiber mRNA levels in U-87 MG, parental and isogenic clones, 72 hours after Delta-24-RIVER infection. Data is represented as folds of increase of fiber mRNA expression relative to levels present in Delta-24-RIVER-infected parental cultures (mean ± SD). (d, e) Immunoblotting analysis of fiber protein expression during Delta-24-RIVER infection. Cell lysates were collected at the indicated time points. Actin was used as a loading control.
Using fiber protein expression as an indicator of adenoviral replication, we also examined viral fiber levels. Thus, by performing real-time PCR, we observed that fiber mRNA levels were 22.2 ± 3.4 and 9.5 ± 1.3 higher in Delta-24-RIVER-infected U-87.ΔEGFR and U-87.wtEGFR cells, respectively, than in the parental culture (Fig 3c). These results were confirmed by western blotting studies. Specifically, at 36 and 48 hr after viral infection in both U-87 MG isogenic and tetracycline-inducible U-251 MG cells, the fiber protein levels correlated with adenovirus-mediated CPE and replication (Fig 3d,e). Collectively, these results showed that the replication efficiency of Delta-24-RIVER was related preferentially to EGFRvIII expression, although viral replication was observed in cells expressing EGFR at high levels.
Selective in vivo anti-glioma effect of Delta-24-RIVER
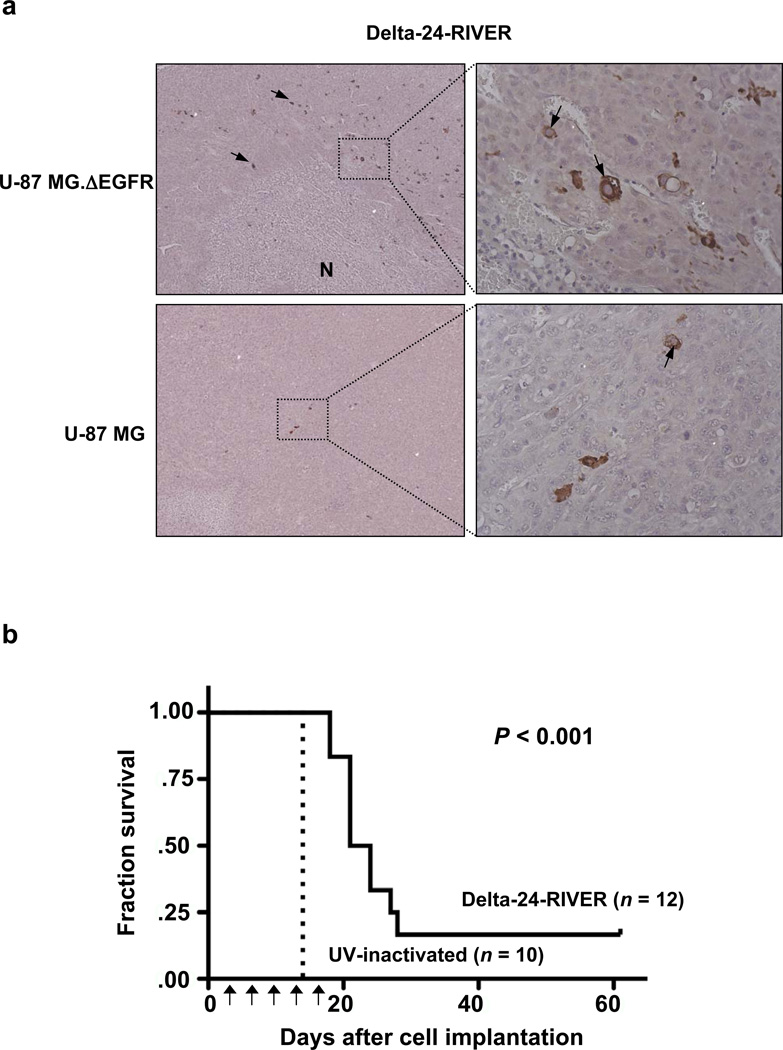
To examine the tropism of Delta-24-RIVER in vivo, we first performed a study consisting on the adenoviral local treatment of intracranial xenografts derived from U-87 MG or U- 87.ΔEGFR cells. Animals were sacrificed (18–20 days after cell implantation) and their brains were analyzed microscopically, which revealed the presence of hypercellular tumors. Further examination of these tumors revealed the presence of prominent viral inclusions in the U-87.ΔEGFR cell–derived xenografts, but such inclusions were very rare in U-87 MG cell–derived xenografts. This feature was consistent with the findings from the immunohistochemistry assay performed to assess the expression of the viral protein hexon. That is, while hexon was expressed in many cells around necrotic areas, only occasional glioma cells expressed the viral protein. The presence of virus around necrotic areas suggests, as we previously reported, the existence of a wave of oncolytic viral spread around and within the tumor. However, in the parental U-87 MG tumors, few cells were hexon positive (Fig 4a). These results indicate that Delta-24-RIVER replicates more efficiently in glioma cells expressing EGFRvIII, as we also observed in the in vitro experiments.
Figure 4.
Delta-24-RIVER displays an efficient in vivo antiglioma effect. (a) Retargeted tropism of Delta-24-RIVER. Histopathological appearance of Delta-24-RIVER–treated intracranial tumors derived from the implantation of U-87 MG (left) or U-87 MG.ΔEGFR (right) cells. The animals were sacrificed 18–20 days after implantation. The tissue sections were immunostained with anti-hexon antibody, and diaminobenzidine was used as the chromogen, followed by counterstaining with hematoxylin. Left, viral protein expression (see arrows) was mainly present in U-87.ΔEGFR–derived tumors and located near the necrotic area (N) (×200). Right, Close-ups of the boxed areas showing hexon staining in cytoplasmatic compartment of the glial cells (x400). (b) Kaplan–Meier curves for overall survival in Delta-24-RIVER-inactivated (n = 12) and UV-inactivated (UVi) Delta-24-RIVER–treated (n = 10) athymic mice bearing intracranial U-87.ΔEGFR xenografts. The intracranial implantation of glioma cells was followed by adenoviral intratumoral injection, as explained in the Methods section (arrows, treatment doses). The P value was determined by log-rank test and represents a comparison of overall survival in the Delta-24-RIVER-treated mice with that in UVi-inactivated Delta-24-RIVER-treated mice. Note that two Delta-24-RIVER–treated animals and none of the UVi-treated animals were censored at the end of the experiment.
Next, we examined the efficacy of Delta-24-RIVER in the treatment of EGFRvIII-expressing U-87 MG cells. In this study, EGFR-expressing xenografts were grown in the brains of athymic mice, and mice were then treated with Delta-24-RIVER (n = 12) or UV-inactivated Delta-24-RIVER (n = 10). Animals were sacrificed when they began to exhibit signs of generalized or localized disease. We observed that Delta-24-RIVER treatment resulted in the prolonged survival of glioma-bearing animals (mean survival time, 26.1 days) compared with animals treated with the control adenovirus (mean survival time, 14 days; log-rank test < 0.0001). Of significance, although all control animals died by day 14 of the experiment, two of the 12 (17%) Delta-24-RIVER–treated mice were still alive without noticeable neurologic deficits at the end of the study (day 61) (Fig 4b). Thus, we conclude that Delta-24-RIVER replicates preferentially in EGFRvIII-expressing glioma cells in vivo and induces a significant therapeutic effect in animals with intracranial tumors derived from these cells.
Discussion
This study is the first to show the anti-glioma effect of an oncolytic adenovirus, Delta-24-RIVER, targeting both the prototype tumor suppressor gene Rb and the most relevant oncogene EGFR and its mutant variant III in malignant brain tumors. To construct the virus, we used detargeting strategies to ablate CAR binding14 and modified the HI loop to incorporate the sequence of a peptide previously shown to interact with EGFRvIII12. We showed that Delta-24-RIVER efficiently infects low CAR–expressing/high EGFRvIII–expressing glioma cells in vitro and in vivo. Using several systems of isogenic glioma cell lines with different expressions of EGFR and EGFRvIII, we demonstrated that expression of the mutant cancer receptor was sufficient to sensitize glioma cells to adenoviral infection. Our results are significant for two reasons: first, because EGFR amplification is one of the hallmarks of the molecular signature of the most frequent primary malignant brain tumors, and second, because expression of the cancer-specific receptor EGFRvIII has not been documented in normal cells. Collectively, our data showed the resulting oncolytic adenovirus is capable of highly specific infectivity, without a lessening of the replication phenotype, and has a potent anti-glioma effect in vitro and in vivo.
Because of the paucity of CAR in glioma cells, oncolytic adenoviruses need to be retargeted to achieve a significant anticancer effect in vivo. However, this is not an easy task, as few modifications of the fiber might compromise both an efficient assembly and a potent viral replication4. As a result, there are few oncolytic adenoviruses that can be targeted to gliomas and fully develop a replication phenotype4, 7. Other previously reported oncolytic adenoviruses with the ability to infect CAR-negative tumor cells include those with a cyclic RGD peptide inserted into the fiber HI loop, lysine residues added to the carboxy-terminus of the fiber protein, and Ad5/Ad3 or Ad5/Ad35 fiber chimeras20–23. Perhaps, the best-characterized of these adenoviruses is Delta-24-RGD7, 23, which will soon be tested clinically. With regard to tropism modifications, Delta-24-RGD and Delta-24-RIVER are at opposite ends of the oncolytic adenovirus spectrum. Specifically, the RGD-4C–modification has been designed to induce binding and internalization via RGD-related integrins and thereby powerfully enhance the tropism with universal infectivity7, 23. On the other hand, Delta-24-RIVER has been designed to infect EGFRvIII-expressing cells with exquisite specificity, with only a minor and, indeed, desirable leakage into cells overexpressing EGFR. Thus, glioblastomas with amplified EGFR and encompassing EGFRvIII mutations should be optimum targets for Delta-24-RIVER treatment; this strategy is also preferable to Delta-24-RGD treatment in patients with these tumors because it can be administered at high doses with negligible toxicity.
Because EGFR overexpression has been reported for many types of humans cancers, including gliomas, efforts have been made to develop agents that can target this receptor; this has included a search for small-molecule inhibitors specific to EGFRvIII and the generation of EGFRvIII-specific antibody-based therapies5, 24, 25. The disadvantage of these two approaches, however, is that they target a specific mutant protein in a pathway that is normally overridden by the activation of pathways with overlapping functions3. The oncolytic effect in this context is more potent than the pharmacological one, because, once the cell is infected, the adenovirus is able to control the main regulators of the cell machinery and thus able to induce cell death independently of the genetic makeup of the host.
Although previous efforts have been made to target EGFR using viral vectors26, 27,28, 29, our group is the first to have generated a new oncolytic adenovirus, Delta-24-RIVER, with tropism redirected to EGFRvIII that is able to acquire a full replication phenotype in cells expressing EGFRvIII but only low levels of CAR. Using two isogenic cell line systems, we dissected the pathways responsible for the infectivity of the adenovirus to clearly show the need for EGFRvIII for optimum infectivity. In addition, we showed that cells expressing high levels of wild-type EGFR (mimicking the amplification and/or overexpression of EGFR observed in most malignant gliomas) are receptive to infection by Delta-24-RIVER, albeit with less efficiency than the ones expressing the mutant form, but enough to induce an anti-glioma effect. These observations have important implications for therapy because it is thought that cells expressing different forms of EGFR are part of the brain tumor cell population and that these cells are responsible for tumor recurrence after therapy and the ultimate death of the patient30.
In summary, Delta-24-RIVER has a selective and potent anti-glioma effect. Our ultimate goal is to translate our laboratory findings regarding oncolytic adenoviruses into therapies appropriate for clinical use. Delta-24-RIVER is well suited to this objective, and our findings are grounds for the initiation of a phase I clinical trial to test its toxicity and efficacy in patients with recurrent malignant gliomas. Because EGFRvIII has been shown to confer resistance to both radiotherapy and chemotherapy31, 32, the targeting of this molecule is of great clinical relevance 33. In fact, this agent could be used in resistant-tumors to anti-EGFR therapies, since a recent study of GBM patients found that EGFRvIII in combination with the loss of the PTEN tumor suppressor gene resulted in resistance to second generation cancer therapeutics targeting EGFR34. Another aspect to consider in the clinical setting is the role that will play the immunity on the efficacy of Delta-24-RIVER. In this regard, as is contemplated in other virotherapy strategies35, the combination of immunosuppressant together with oncolytic adenoviruses might be considered in the future.
Acknowledgments
We thank Betty Notzon (Department of Scientific Publications, The University of Texas M.D. Anderson Cancer Center) for editorial support and Verlene Henry and Jennifer Edge (Brain Tumor Center, The University of Texas M.D. Anderson Cancer Center) for technical assistance in the in vivo experiments.
These studies were supported by R01-CA090879 and R01 CA116621 from National Cancer Institute (to JF and VK, respectively), Marcus Foundation (to JF and CAC), Nick Eric Wichman Foundation grant (to JF and CGM), an Institutional Research Grant to M. D. Anderson (to HJ), and NCI CA-16672 grant (to M.D. Anderson Cancer Center: DNA Analysis, Research Animal Support, and Media Preparation Facilities).
References
- 1.Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170(5):1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 4.Jiang H, McCormick F, Lang FF, Gomez-Manzano C, Fueyo J. Oncolytic adenoviruses as antiglioma agents. Expert Rev Anticancer Ther. 2006;6(5):697–708. doi: 10.1586/14737140.6.5.697. [DOI] [PubMed] [Google Scholar]
- 5.Kuan CT, Wikstrand CJ, Bigner DD. EGFRvIII as a promising target for antibody-based brain tumor therapy. Brain Tumor Pathol. 2000;17(2):71–78. doi: 10.1007/BF02482738. [DOI] [PubMed] [Google Scholar]
- 6.Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19(1):2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 7.Fueyo J, Alemany R, Gomez-Manzano C, Fuller GN, Khan A, Conrad CA, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95(9):652–660. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- 8.Barnett BG, Crews CJ, Douglas JT. Targeted adenoviral vectors. Biochim Biophys Acta. 2002;1575(1–3):1–14. doi: 10.1016/s0167-4781(02)00249-x. [DOI] [PubMed] [Google Scholar]
- 9.Mishima K, Johns TG, Luwor RB, Scott AM, Stockert E, Jungbluth AA, et al. Growth suppression of intracranial xenografted glioblastomas overexpressing mutant epidermal growth factor receptors by systemic administration of monoclonal antibody (mAb) 806, a novel monoclonal antibody directed to the receptor. Cancer Res. 2001;61(14):5349–5354. [PubMed] [Google Scholar]
- 10.Ramnarain DB, Park S, Lee DY, Hatanpaa KJ, Scoggin SO, Otu H, et al. Differential gene expression analysis reveals generation of an autocrine loop by a mutant epidermal growth factor receptor in glioma cells. Cancer Res. 2006;66(2):867–874. doi: 10.1158/0008-5472.CAN-05-2753. [DOI] [PubMed] [Google Scholar]
- 11.Belousova N, Korokhov N, Krendelshchikova V, Simonenko V, Mikheeva G, Triozzi PL, et al. Genetically targeted adenovirus vector directed to CD40-expressing cells. J Virol. 2003;77(21):11367–11377. doi: 10.1128/JVI.77.21.11367-11377.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campa MJ, Kuan CT, O'Connor-McCourt MD, Bigner DD, Patz EF., Jr Design of a novel small peptide targeted against a tumor-specific receptor. Biochem Biophys Res Commun. 2000;275(2):631–636. doi: 10.1006/bbrc.2000.3347. [DOI] [PubMed] [Google Scholar]
- 13.Belousova N, Krendelchtchikova V, Curiel DT, Krasnykh V. Modulation of adenovirus vector tropism via incorporation of polypeptide ligands into the fiber protein. J Virol. 2002;76(17):8621–8631. doi: 10.1128/JVI.76.17.8621-8631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alemany R, Curiel DT. CAR-binding ablation does not change biodistribution and toxicity of adenoviral vectors. Gene Ther. 2001;8(17):1347–1353. doi: 10.1038/sj.gt.3301515. [DOI] [PubMed] [Google Scholar]
- 15.Mahasreshti PJ, Kataram M, Wu H, Yalavarthy LP, Carey D, Fisher PB, et al. Ovarian cancer targeted adenoviral-mediated mda-7/IL-24 gene therapy. Gynecol Oncol. 2006;100(3):521–532. doi: 10.1016/j.ygyno.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 16.Jiang H, Alemany R, Gomez-Manzano C, Medrano DR, Lemoine MG, Olson MV, et al. Downmodulation of E1A protein expression as a novel strategy to design cancer-selective adenoviruses. Neoplasia. 2005;7(8):723–729. doi: 10.1593/neo.04793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang H, Gomez-Manzano C, Aoki H, Alonso MM, Kondo S, McCormick F, et al. Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: role of autophagic cell death. J Natl Cancer Inst. 2007;99(18):1410–1414. doi: 10.1093/jnci/djm102. [DOI] [PubMed] [Google Scholar]
- 18.Ariani F, Mari F, Pescucci C, Longo I, Bruttini M, Meloni I, et al. Real-time quantitative PCR as a routine method for screening large rearrangements in Rett syndrome: Report of one case of MECP2 deletion and one case of MECP2 duplication. Hum Mutat. 2004;24(2):172–177. doi: 10.1002/humu.20065. [DOI] [PubMed] [Google Scholar]
- 19.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275(5304):1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 20.Bernt KM, Ni S, Gaggar A, Li ZY, Shayakhmetov DM, Lieber A. The effect of sequestration by nontarget tissues on anti-tumor efficacy of systemically applied, conditionally replicating adenovirus vectors. Mol Ther. 2003;8(5):746–755. doi: 10.1016/j.ymthe.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Rivera AA, Davydova J, Schierer S, Wang M, Krasnykh V, Yamamoto M, et al. Combining high selectivity of replication with fiber chimerism for effective adenoviral oncolysis of CAR-negative melanoma cells. Gene Ther. 2004;11(23):1694–1702. doi: 10.1038/sj.gt.3302346. [DOI] [PubMed] [Google Scholar]
- 22.Shinoura N, Yoshida Y, Tsunoda R, Ohashi M, Zhang W, Asai A, et al. Highly augmented cytopathic effect of a fiber-mutant E1B-defective adenovirus for gene therapy of gliomas. Cancer Res. 1999;59(14):3411–3416. [PubMed] [Google Scholar]
- 23.Suzuki K, Fueyo J, Krasnykh V, Reynolds PN, Curiel DT, Alemany R. A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin Cancer Res. 2001;7(1):120–126. [PubMed] [Google Scholar]
- 24.Trembath DG, Lal A, Kroll DJ, Oberlies NH, Riggins GJ. A novel small molecule that selectively inhibits glioblastoma cells expressing EGFRvIII. Mol Cancer. 2007;6:30. doi: 10.1186/1476-4598-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorimer IA. Mutant epidermal growth factor receptors as targets for cancer therapy. Curr Cancer Drug Targets. 2002;2(2):91–102. doi: 10.2174/1568009023333926. [DOI] [PubMed] [Google Scholar]
- 26.Lorimer IA, Lavictoire SJ. Targeting retrovirus to cancer cells expressing a mutant EGF receptor by insertion of a single chain antibody variable domain in the envelope glycoprotein receptor binding lobe. J Immunol Methods. 2000;237(1–2):147–157. doi: 10.1016/s0022-1759(99)00219-7. [DOI] [PubMed] [Google Scholar]
- 27.Snitkovsky S, Niederman TM, Carter BS, Mulligan RC, Young JA. A TVA-single-chain antibody fusion protein mediates specific targeting of a subgroup A avian leukosis virus vector to cells expressing a tumor-specific form of epidermal growth factor receptor. J Virol. 2000;74(20):9540–9545. doi: 10.1128/jvi.74.20.9540-9545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen C, Vongpunsawad S, Nakamura T, James CD, Schroeder M, Cattaneo R, et al. Retargeted oncolytic measles strains entering via the EGFRvIII receptor maintain significant antitumor activity against gliomas with increased tumor specificity. Cancer Res. 2006;66(24):11840–11850. doi: 10.1158/0008-5472.CAN-06-1200. [DOI] [PubMed] [Google Scholar]
- 29.Grill J, Van Beusechem VW, Van Der Valk P, Dirven CM, Leonhart A, Pherai DS, et al. Combined targeting of adenoviruses to integrins and epidermal growth factor receptors increases gene transfer into primary glioma cells and spheroids. Clin Cancer Res. 2001;7(3):641–650. [PubMed] [Google Scholar]
- 30.Ayuso-Sacido A, Graham C, Greenfield JP, Boockvar JA. The duality of epidermal growth factor receptor (EGFR) signaling and neural stem cell phenotype: cell enhancer or cell transformer? Curr Stem Cell Res Ther. 2006;1(3):387–394. doi: 10.2174/157488806778226849. [DOI] [PubMed] [Google Scholar]
- 31.Lammering G, Valerie K, Lin PS, Hewit TH, Schmidt-Ullrich RK. Radiation-induced activation of a common variant of EGFR confers enhanced radioresistance. Radiother Oncol. 2004;72(3):267–273. doi: 10.1016/j.radonc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Nagane M, Levitzki A, Gazit A, Cavenee WK, Huang HJ. Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proc Natl Acad Sci U S A. 1998;95(10):5724–5729. doi: 10.1073/pnas.95.10.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang PH, Cavenee WK, Furnari FB, White FM. Uncovering therapeutic targets for glioblastoma: a systems biology approach. Cell Cycle. 2007;6(22):2750–2754. doi: 10.4161/cc.6.22.4922. [DOI] [PubMed] [Google Scholar]
- 34.Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353(19):2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 35.Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci U S A. 2006;103(34):12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]