Abstract
Background
Several observational studies have investigated the association between -607 C/A polymorphism of IL-18 gene and cancer risk; however, the results were inconsistent. Therefore, we performed a meta-analysis to derive a more precise estimation of the association to help us better understand the relationship between -607 C/A polymorphism of IL-18 gene promoter and risk of cancer.
Methods
A literature search was carried out using PubMed, EMBASE, and China National Knowledge Infrastructure (CNKI) database between January 1966 and February 2013. Fixed-effect and random-effect models were used to estimate the pooled odds ratio (OR) and the corresponding 95% confidence intervals (CIs).
Results
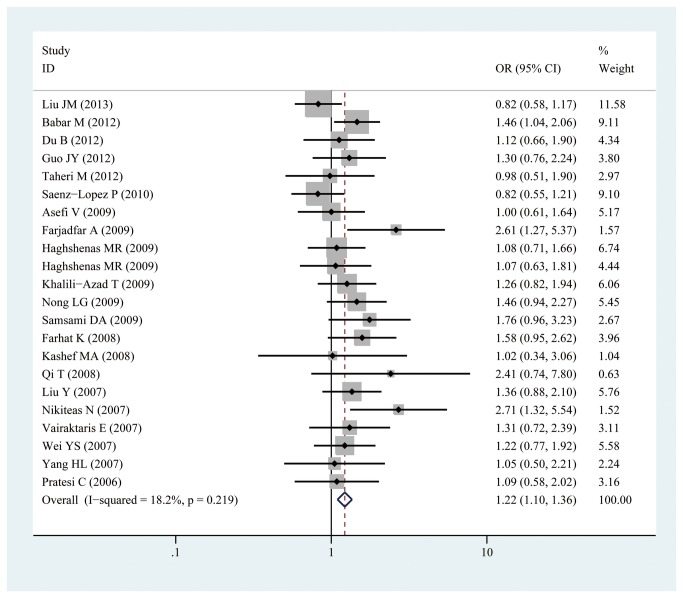
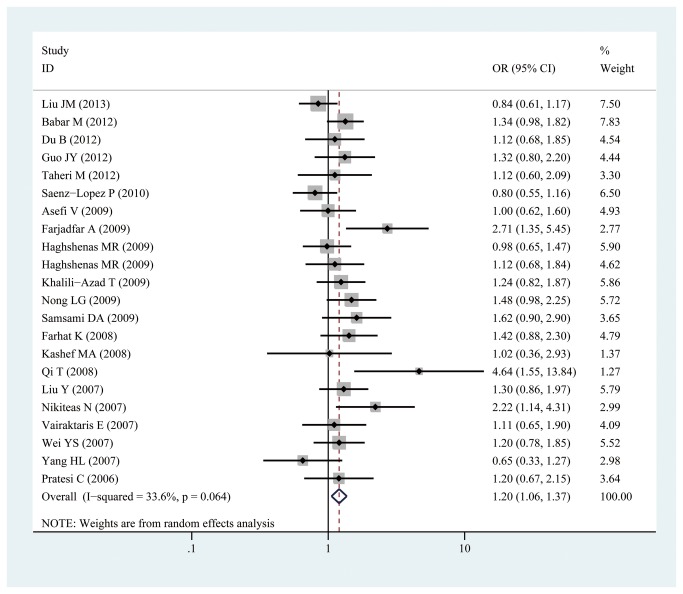
A total of 22 case-control studies including 4100 cancer cases and 4327 controls contributed to the analysis. Significant association between -607C/A polymorphism in IL-18 gene promoter and cancer risk was observed (CA vs CC:OR =1.221, 95% CI: 1.096, 1.360; Pheterogeneity=0.219; AA/CA vs. CC:OR =1.203, 95% CI: 1.057, 1.369; Pheterogeneity=0.064). In the subgroup analysis by ethnicity, -607C/A polymorphism significantly increased risk of cancer among Asian population (AA/CA vs. CC:OR =1.197, 95% CI: 1.023,1.401; Pheterogeneity=0.088); however, no significant association was found in Caucasian or African population. The -607C/A polymorphism was associated with a significantly increased risk of nasopharyngeal carcinoma (CA vs CC:OR =1.330, 95% CI: 1.029,1.719; Pheterogeneity=0.704; AA/CA vs. CC:OR =1.323, 95% CI: 1.037,1.687; Pheterogeneity=0.823) and esophageal cancer (AA/CA vs. CC:OR =1.289, 95% CI: 1.002,1.658; Pheterogeneity=0.700).
Conclusions
The present meta-analysis suggests that the -607C/A polymorphisms in IL-18 gene promoter is associated with a significantly increased risk of cancer, especially for nasopharyngeal carcinoma and esophageal cancer and in Asian population. More studies with larger sample size, well controlled confounding factors are warranted to validate this association.
Introduction
IL-1 family includes ten known members, all of which are characterized by gene structure, predicted three-dimensional fold, processing, receptor, signal transduction pathway and pro-inflammatory properties [1]. IL-18, also known as interferon-gamma inducing factor (IGIF), is a member of the IL-1 super-family [2]. IL-18 is secreted by a wide range of cells, including T and B lymphocytes, and antigen-presenting cells (APCs), including activated monocytes, macrophages, Kupffer cells, Langerhans cells, and NK cells [3-5]. IL-1 beta converting enzyme can convert IL-18 to a mature biologically active 18.3-kDa form through cleavage of the propeptide. IL-18 binds to the cell through a specific receptor, IL-18R, belonging to the toll-like receptor family [6]. IL-18 plays a central role in inflammation and immune response, and is generally acknowledged as a key defense cytokine against infectious agents. Because immune stimulating effects of IL-18 have also antineoplastic properties, it was tempting to propose IL-18 as a novel adjuvant therapy against cancer [7]. A number of single nucleotide polymorphisms (SNPs) of IL-18 gene have been identified and investigated [8]. There are three SNPs in the promoter region of IL-18 gene: -137, -607 and -656, relative to the transcriptional start site, which may alter the expression of IL-18 [9]. The C to A substitution at position −607 disrupts a consensus cAMP-responsive element protein-binding site, causing altered transcription factor binding and gene expression [9]. Several observational studies have investigated the association between -607 C/A polymorphism of IL-18 gene promoter and cancer risk; however, the results were inconsistent. For example, some studies found that -607 C/A polymorphism of IL-18 gene promoter was associated with increased risk of nasopharyngeal carcinoma [10] and lung cancer [11]. However, other studies found there was no association between -607 C/A polymorphism of IL-18 gene and risk of breast cancer [12] or head and neck squamous cell carcinoma [13]. Therefore, we performed a meta-analysis to derive a more precise estimation of the association to help us better understand the relationship between -607 C/A polymorphism of IL-18 gene and risk of cancer.
Methods
Identification of studies
Comprehensive searches were carried out using PubMed, EMBASE, and China National Knowledge Infrastructure (CNKI) databases between January 1966 and February 2013. There were no restriction of origin and languages. Search terms included: "Interleukin-18" or "IL-18" or "rs1946518" in combination with “polymorphism” or “variant” and ‘‘cancer’’ or ‘‘neoplasm’’ or ‘‘malignancy’’. The reference list of each comparative study and previous reviews were manually examined to find additional relevant studies.
Inclusion and exclusion criteria
Studies were selected according to the following inclusion criteria: (i) case-control studies; (ii) investigating the association between IL-18 rs1946518 (C>A) SNP and cancer risk; (iii)cancers diagnosed by histopathology; (iiii) providing detail genotype frequencies. Studies without detail genotype frequencies were excluded. Titles and abstracts of searching results were screened and full text papers were further evaluated to confirm eligibility. Two reviewers (WM and ZXY) independently selected eligible trials. Disagreement between the two reviewers was settled by discussing with the third reviewer(WL).
Data extraction
In the present study, the following characteristics were collected by two reviewers (WM and LY) independently using a purpose-designed form: name of first author, publishing time, country where the study was conducted, ethnicity, cancer types, source of control, number of cases and controls, genotype frequency in cases and controls. Different ethnicity descents were categorized as Asian, Caucasian, and African. Cancer types were classified as prostate cancer, esophageal cancer, nasopharyngeal carcinoma, colorectal cancer, breast cancer, cervical cancer, and other cancers (bladder cancer, renal cell carcinoma, head and neck squamous cell carcinoma, lung cancer, stomach cancer, ovarian cancer, choriocarcinoma, and oral cancer). Eligible studies were defined as hospital-based (HB) and population-based (PB) according to the control source.
Statistical analysis
Chi-square based Q test was used to check the statistical heterogeneity between studies, and the heterogeneity was considered significant when p<0.10 [14]. The fixed-effects model (based on Mantel-Haenszel method) and random-effects model (based on DerSimonian-Laird method) were used to pool the data from different studies. The fixed-effects model was used when there was no significant heterogeneity; otherwise, the random-effects model was applied [15]. The association strength between -607 C/A (rs1946518) polymorphism and cancer risk was measured by odds ratio (OR) with 95% confidence intervals (95% CI). The estimates of pooled ORs were achieved by calculating a weighted average of OR from each study. A 95% CI was used for statistical significance test and a 95% CI without 1 for OR indicating a significant increased or reduced cancer risk. The pooled ORs were calculated for homozygote comparison (AA versus CC), heterozygote comparison (CA versus CC), dominant (CA/AA versus CC) and recessive (AA versus CC/CA) modes, assuming dominant and recessive effects of the variant A allele, respectively. Subgroup analyses were performed according to (i) cancer types, (ii) ethnicities, (iii) source of control, and (iiii) sample size, to examine the impact of these factors on the association. To test the robustness of association, sensitivity analysis were carried out by excluding studies one-by-one and analyzing the effect size for all of rest studies. Cumulative meta-analysis was also performed to identify the change in trend of reporting risk over time. In cumulative meta-analysis, studies were chronologically ordered by publication year, then the pooled RRs were obtained at the end of each year. To better investigate the possible sources of between-study heterogeneity, a meta-regression analysis was performed [16]. Publication bias was assessed using Begg and Mazumdar adjusted rank correlation test and the Egger regression asymmetry test [17,18]. HWE(Hardy-Weinberg equilibrium) was tested by Pearson’s X2 test (P<0.05 means deviated from HWE). All analyses were performed using Stata version 11.0 (StataCorp, College Station, TX).
Results
Search results and characteristics of studies included in the meta-analysis
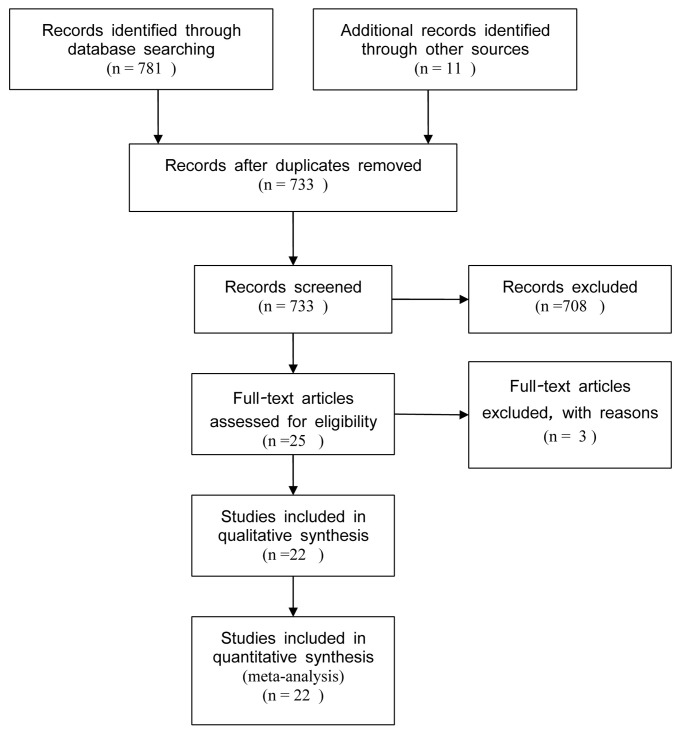
A total of 792 citations were identified during the initial search (shown in Figure 1). On the basis of the title and abstract, we identified 24 papers. After detailed evaluation, one study was excluded for incorrect data, and two studies were excluded for having not presented -607 C/A polymorphisms. In the study reported by Haghshenas MR and colleagues [19], they investigated rs1946518 polymorphisms and colorectal cancer, as well as stomach cancer, and the data was presented separately, thus both of them were considered as a separate study in this meta-analysis. At last, 22 case-control studies [10-13,19-35], including 4100 cancer cases and 4327 controls, were included in the meta-analysis(Baseline data and other details are shown in Table 1). 16 eligible studies were conducted in Asia [11-13,19,21,23-27,29,30,32,34,35], five in Europe [10,20,28,31,33], and the remaining one in Africa [22]. There were five studies including more than 500 participants and the others had a sample size less than 500 participants. Genotype distribution of controls in all studies was consistent with HWE.
Figure 1. Flow diagram of the study selection process.
Table 1. Characteristics of studies included in the meta-analysis.
| First Author | Year | Country | Ethnicity | Control | No. of Cases | No. of Controls | Cancer Type | Sample size | Cases | Controls | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | CA | CC | AA | CA | CC | |||||||||
| Liu JM | 2013 | China | Asian | Population Based | 375 | 400 | Prostate Cancer | Large | 103 | 172 | 100 | 110 | 196 | 94 |
| Babar M | 2012 | UK | Caucasian | Population Based | 1070 | 194 | Esophageal Cancer | Large | 178 | 508 | 384 | 36 | 75 | 83 |
| Du B | 2012 | China | Asian | Hospital Based | 150 | 180 | Nasopharyngeal Carcinoma | Small | 34 | 80 | 36 | 40 | 93 | 47 |
| Guo JY | 2012 | China | Asian | Hospital Based | 170 | 160 | Colorectal Cancer | Small | 49 | 85 | 36 | 42 | 76 | 42 |
| Taheri M | 2012 | Iran | Asian | Population Based | 72 | 93 | Breast Cancer | Small | 11 | 32 | 29 | 8 | 45 | 40 |
| Saenz-Lopez P | 2010 | Spain | Caucasian | Population Based | 154 | 500 | Other Types | Large | 19 | 76 | 59 | 73 | 261 | 166 |
| Asefi V | 2009 | Iran | Asian | Hospital Based | 111 | 212 | Other Types | Small | 15 | 53 | 43 | 29 | 101 | 82 |
| Farjadfar A | 2009 | Iran | Asian | Hospital Based | 73 | 97 | Other Types | Small | 13 | 45 | 15 | 11 | 46 | 40 |
| Haghshenas MR | 2009 | Iran | Asian | Population Based | 142 | 311 | Colorectal Cancer | Small | 15 | 72 | 55 | 48 | 144 | 119 |
| Haghshenas MR | 2009 | Iran | Asian | Population Based | 87 | 311 | Other Types | Small | 16 | 40 | 31 | 48 | 144 | 119 |
| Khalili-Azad T | 2009 | Iran | Asian | Population Based | 200 | 206 | Breast Cancer | Small | 33 | 103 | 64 | 33 | 97 | 76 |
| Nong LG | 2009 | China | Asian | Population Based | 250 | 270 | Nasopharyngeal Carcinoma | Large | 71 | 132 | 47 | 68 | 133 | 69 |
| Samsami DA | 2009 | Iran | Asian | Hospital Based | 85 | 158 | Other Types | Small | 12 | 51 | 22 | 26 | 75 | 57 |
| Farhat K | 2008 | Tunisia | African | Population Based | 163 | 164 | Nasopharyngeal Carcinoma | Small | 28 | 94 | 41 | 34 | 77 | 53 |
| Kashef MA | 2008 | Iran | Asian | Population Based | 19 | 103 | Other Types | Small | 3 | 10 | 6 | 16 | 54 | 33 |
| Qi T | 2008 | China | Asian | Hospital Based | 50 | 50 | Cervical Cancer | Small | 28 | 17 | 5 | 9 | 24 | 17 |
| Liu Y | 2007 | China | Asian | Hospital Based | 265 | 280 | Prostate Cancer | Large | 72 | 143 | 50 | 78 | 137 | 65 |
| Nikiteas N | 2007 | Greece | Caucasian | Population Based | 84 | 89 | Colorectal Cancer | Small | 18 | 47 | 19 | 22 | 32 | 35 |
| Vairaktaris E | 2007 | Germany | Caucasian | Population Based | 149 | 89 | Other Types | Small | 28 | 66 | 55 | 22 | 32 | 35 |
| Wei YS | 2007 | China | Asian | Hospital Based | 235 | 250 | Esophageal Cancer | Small | 64 | 123 | 48 | 67 | 124 | 59 |
| Yang HL | 2007 | China | Asian | Population Based | 107 | 80 | Cervical Cancer | Small | 24 | 50 | 33 | 36 | 26 | 18 |
| Pratesi C | 2006 | Italy | Caucasian | Population Based | 89 | 130 | Nasopharyngeal Carcinoma | Small | 21 | 42 | 26 | 23 | 64 | 43 |
Main results
Given that the P value of Q-tests was less than 0.10 under the allelic, homozygous, recessive, and dominant genetic models, the random-effects model was used. By contrast, the P value of Q-tests was more than 0.10 under the heterozygous genetic model (P for heterogeneity = 0.219); thus, the fixed-effects model was adopted. Significant associations between -607C/A polymorphisms in IL-18 gene promoter and cancer risk were observed in the heterozygous model (CA vs CC:OR =1.221, 95% CI: 1.096, 1.360; Pheterogeneity=0.219, Figure 2) and the dominant model (AA/CA vs. CC:OR =1.203, 95% CI: 1.057, 1.369; Pheterogeneity=0.064, Figure 3) in this meta-analysis. However, no significant association between -607C/A polymorphisms in IL-18 gene promoter and cancer risk was observed under the allelic model(A vs C:OR =1.088, 95% CI: 0.987,1.200; Pheterogeneity=0.003), homozygous model(AA vs CC:OR =1.139, 95% CI: 0.948, 1.369; Pheterogeneity=0.023), and recessive model (AA vs. CC/CA: OR =0.995, 95% CI: 0.851, 1.163; Pheterogeneity=0.025) (shown in Table 2).
Figure 2. Forest plot of heterozygote comparison for overall comparison (CA vs. CC).
Figure 3. Forest plot of dominant model for overall comparison (CA/AA vs. CC).
Table 2. Stratified analyses of the -607C/A polymorphisms in IL-18 gene promoter with cancer risk.
| A vs. C |
AA vs. CC |
CA vs. CC |
AA vs. CC/CA
|
AA/CA vs. CC |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | OR(95% CI) | Phet | Study | OR(95% CI) | Phet | Study | OR(95% CI) | Phet | Study | OR(95% CI) | Phet | Study | OR(95% CI) | Phet | |
| Overall | 23 | 1.088 (0.987-1.200) | 0.003 | 23 | 1.139 (0.948-1.369) | 0.023 | 23 | 1.221 (1.096-1.360)* | 0.219 | 23 | 0.995 (0.851-1.163) | 0.025 | 23 | 1.203 (1.057-1.369)* | 0.064 |
| Ethnicity | |||||||||||||||
| Asian | 17 | 1.107 (0.972-1.260) | 0.001 | 17 | 1.196 (0.936-1.530) | 0.007 | 17 | 1.191 (1.047-1.356)* | 0.487 | 17 | 1.035 (0.854-1.255) | 0.011 | 17 | 1.197(1.023,1.401)* | 0.088 |
| Caucasian | 5 | 1.041 (0.909-1.193) | 0.336 | 5 | 1.023 (0.779-1.343) | 0.473 | 5 | 1.294 (0.906-1.848) | 0.041 | 5 | 0.890 (0.696-1.138) | 0.620 | 5 | 1.198 (0.888-1.618) | 0.083 |
| African | 1 | 1.076 (0.790-1.464) | NA | 1 | 1.065 (0.558-2.030) | NA | 1 | 1.578 (0.951-2.620) | NA | 1 | 0.793 (0.455-1.382) | NA | 1 | 1.421 (0.877-2.301) | NA |
| Source of controls | |||||||||||||||
| Hospital based | 9 | 1.247 (1.022-1.523)* | 0.005 | 9 | 1.329 (0.924-1.912) | 0.009 | 9 | 1.353 (1.115-1.642)* | 0.435 | 9 | 1.135 (0.871-1.479) | 0.044 | 9 | 1.362(1.134,1.635)* | 0.116 |
| Population based | 14 | 1.021 (0.941-1.107) | 0.124 | 14 | 1.012 (0.832-1.231) | 0.196 | 14 | 1.165 (1.024-1.327)* | 0.189 | 14 | 0.911 (0.764-1.087) | 0.154 | 14 | 1.114(0.986,1.258) | 0.190 |
| Sample size | |||||||||||||||
| Small | 18 | 1.092 (0.955-1.249) | <0.001 | 18 | 1.149 (0.896-1.472) | 0.008 | 18 | 1.223 (1.036-1.445)* | 0.085 | 18 | 1.006 (0.813-1.246) | 0.008 | 18 | 1.200 (1.006-1.430)* | 0.016 |
| Large | 5 | 1.032 (0.918-1.161) | 0.269 | 5 | 1.051 (0.836-1.320) | 0.313 | 5 | 1.134 (0.863-1.490) | 0.042 | 5 | 0.980 (0.824-1.164) | 0.810 | 5 | 1.107 (0.862-1.421) | 0.052 |
| Cancer types | |||||||||||||||
| Prostate cancer | 2 | 0.993 (0.852-1.157) | 0.385 | 2 | 0.993 (0.732-1.346) | 0.331 | 2 | 1.039 (0.639-1.690) | 0.081 | 2 | 0.985 (0.773-1.254) | 0.896 | 2 | 1.027 (0.675-1.565) | 0.109 |
| Esophageal cancer | 2 | 1.095 (0.926-1.293) | 0.852 | 2 | 1.111 (0.799-1.544) | 0.783 | 2 | 1.371 (1.045-1.800)* | 0.528 | 2 | 0.945 (0.713-1.253) | 0.591 | 2 | 1.289 (1.002-1.658)* | 0.700 |
| Nasopharyngeal carcinoma | 4 | 1.144 (0.985-1.328) | 0.845 | 4 | 1.305 (0.961-1.772) | 0.759 | 4 | 1.330 (1.029-1.719)* | 0.704 | 4 | 1.082 (0.842-1.391) | 0.547 | 4 | 1.323 (1.037-1.687)* | 0.823 |
| Colorectal cancer | 3 | 1.066 (0.883-1.286) | 0.262 | 3 | 1.092 (0.664-1.795) | 0.213 | 3 | 1.460 (0.898-2.371) | 0.097 | 3 | 0.896 (0.638-1.259) | 0.359 | 3 | 1.337 (0.865-2.068) | 0.118 |
| Breast cancer | 2 | 1.147 (0.904-1.456) | 0.726 | 2 | 1.332 (0.800-2.216) | 0.438 | 2 | 1.169 (0.814-1.678) | 0.532 | 2 | 1.225 (0.716-2.096) | 0.274 | 2 | 1.204 (0.854-1.696) | 0.784 |
| Cervical cancer | 2 | 1.396 (0.208-9.382) | <0.001 | 2 | 1.890 (0.069-51.901) | <0.001 | 2 | 1.397 (0.644-3.031) | 0.241 | 2 | 1.403 (0.090-21.882) | <0.001 | 2 | 1.653 (0.241-11.339) | 0.003 |
| Other cancers | 8 | 1.044 (0.910-1.196) | 0.200 | 8 | 0.978 (0.717-1.334) | 0.223 | 8 | 1.100 (0.803-1.507) | 0.014 | 8 | 0.935 (0.738-1.183) | 0.804 | 8 | 1.075 (0.795-1.454) | 0.012 |
OR: odds ratio; CI: confidence intervals; Phet: P value for heterogeneity; * OR with statistical significance
Subgroup analyses, sensitivity analysis and cumulative meta-analysis
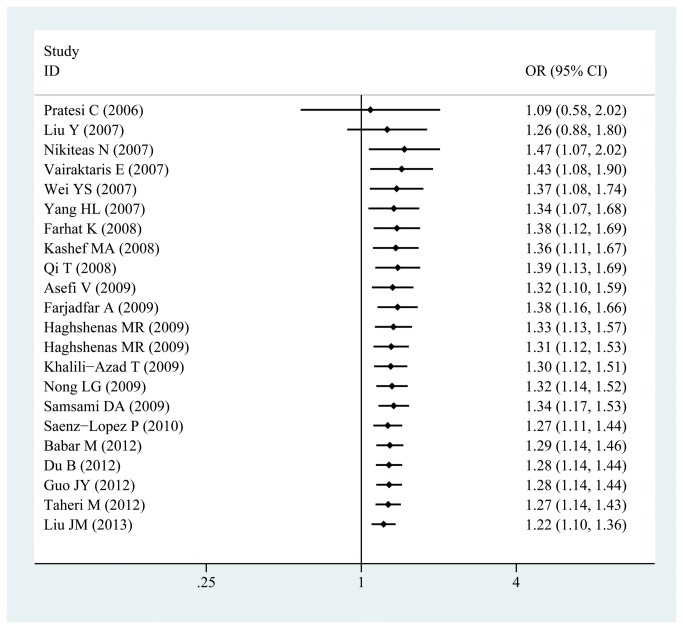
In a stratified analysis by specific cancer types, -607C/A polymorphisms in IL-18 gene promoter was significantly associated with an increased risk of nasopharyngeal carcinoma (CA vs CC:OR =1.330, 95% CI: 1.029,1.719; Pheterogeneity=0.704; AA/CA vs. CC:OR =1.323, 95% CI: 1.037,1.687; Pheterogeneity=0.823) and esophageal cancer(CA vs CC:OR =1.371, 95% CI: 1.045,1.800; Pheterogeneity=0.528; AA/CA vs. CC:OR =1.289, 95% CI: 1.002,1.658; Pheterogeneity=0.700) in the heterozygous model and dominant model. No evidence of association was found in any genetic model between-607C/A polymorphisms in IL-18 gene promoter and the risk of prostate cancer, colorectal cancer, breast cancer, cervical cancer, and other cancers(shown in Table 2). According to ethnicity, the polymorphism presented a significantly increased risk of cancer among Asian population in the heterozygous model and dominant model(CA vs CC:OR =1.191, 95% CI: 1.047,1.356; Pheterogeneity=0.487; AA/CA vs. CC:OR =1.197, 95% CI: 1.023,1.401; Pheterogeneity=0.088); however, no significant association was found in Caucasian and African population (shown in Table 2). In the stratified analysis by source of control groups, we found that the -607C/A polymorphisms in IL-18 gene promoter was associated with a significantly increased risk in hospital-based controls in the allelic model (A vs C:OR =1.247, 95% CI: 1.022, 1.523; Pheterogeneity=0.005), heterozygous model(A vs C:OR =1.353, 95% CI: 1.115, 1.642; Pheterogeneity=0.435), and dominant model(A vs C:OR =1.362, 95% CI: 1.134,1.635; Pheterogeneity=0.116). However, among studies with population-based controls, a significant association was only observed in heterozygous model(A vs C:OR =1.165, 95% CI: 1.024, 1.327; Pheterogeneity=0.189). When stratifying the sample size, a significant association was observed among studies with small sample size in the heterozygous model and dominant model (CA vs CC:OR =1.223, 95% CI: 1.036,1.445; Pheterogeneity=0.085; AA/CA vs. CC:OR =1.200, 95% CI: 1.006,1.430; Pheterogeneity=0.016), but not observed among studies with large sample size in any genetic models. To test the robustness of association, sensitivity analysis was carried out by excluding studies one-by-one and analyzing effect size for all of rest studies. Sensitivity analysis indicated that no significant variation in combined RR by excluding any of the study, confirming the stability of present results. Cumulative meta-analyses were carried out in the heterozygous and dominant genetic models. Between 2006 and 2013, with each accumulation of more studies, the 95% CIs for the pooled ORs became increasingly narrower, indicating that the precision of the estimation was progressively boosted by continually adding more samples(shown in Figure 4).
Figure 4. Cumulative meta-analysis of association between -607C/A polymorphisms in IL-18 gene promoter and cancer risk under the heterozygous model(CA vs. CC).
Meta-regression and Publication bias
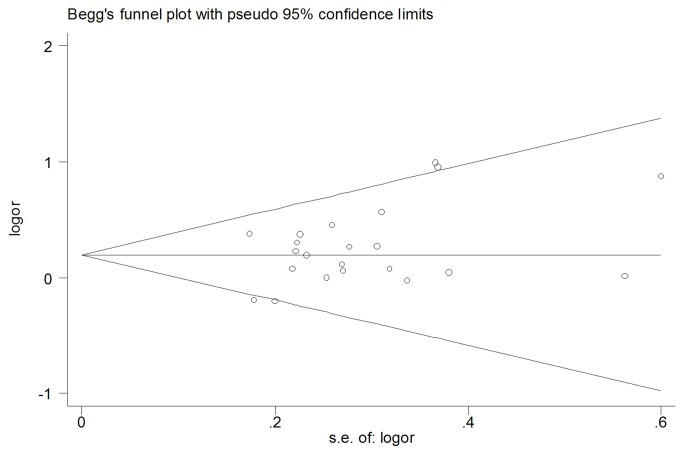
As shown in Table 2, significant heterogeneity was present in all models except for heterozygous model, hence, meta-regression was conducted to detect the source of heterogeneity. Ethnicity, source of controls, sample size and cancer type, which may be potential sources of heterogeneity, were tested by a meta-regression method. The results showed that, in the dominant model (AA/CA vs. CC) for instance, the heterogeneity could only be explained by cancer type(p=0.014), but not ethnicity, sample size, or the source of controls. The potential publication bias of the literatures was evaluated by funnel plot and Egger’s test. No visual publication bias was found in the funnel plot (Figure 5). And Egger’s test suggested that no publication bias was detected in all the comparison models (P >0.05)
Figure 5. Funnel plot for publication bias in the studies investigating the association between -607C/A polymorphisms in IL-18 gene promoter and risk of cancer(heterozygous genetic model: CA vs. CC).
No publication bias was observed among studies using Begg’s P value (P = 0.167) and Egger’s (P = 0.387) test, which suggested there was no evidence of publication bias.
Discussion
The present meta-analysis, which included 4100 cancer cases and 4327 controls from 21 publications with 22 case-control studies, explored the relationship between -607C/A polymorphisms in IL-18 gene promoter and cancer risk. For overall comparison of pooled ORs, significantly increased risk was observed in the heterozygous model(CA vs CC) and the dominant model(AA/CA vs. CC). Under the allelic, homozygous and recessive genetic models, there was no significant association between -607C/A polymorphisms in IL-18 gene promoter and cancer risk. Overall, a significant association exists between -607C/A polymorphisms in IL-18 gene promoter and cancer risk. This finding indicates that the genetic variant in IL-18 gene promoter region may crucially modify the susceptibility of cancers. The C to A substitution at position −607 disrupts a consensus cAMP-responsive element protein-binding site, causing altered transcription factor binding and gene expression [9]. IL-18 serum levels have been reported to be elevated in a variety of cancers compared with control group [19,36-39]. Hence, the -607C/A polymorphisms in IL-18 gene promoter may modify the susceptibility of cancers though changing the expression of IL-18 gene. The mechanism needs further investigation.
When identifying eligible studies by reading full text, the study conducted by Jaiswal PK and colleagues [40] was excluded for incorrect data. The OR and 95% CI under heterozygous genetic model (OR =0.59, 95% CI: 0.39, 0.92)we got based on the genotype frequency in cases and controls(CC: 81, CA: 89 in cases; CC: 61, CA: 113 in controls) were totally opposed to that they got(OR =1.59, 95% CI: 1.01-2.95). Hence, we excluded this study for its unbelievable result.
In the stratified analysis based on ethnicity, a significant increased risk of cancer was found in Asian population, but not in Caucasian or African population. One probable reason is that different environment they live in and different genetic backgrounds may account for these differences. As we know, different populations carry different genotype and/or allele frequencies of this locus polymorphism and may lead to various degrees of cancer susceptibility [41]. And different ethnic groups live with multiple life styles and environmental factors and thus yield diverse gene-environment interactions [42]. In addition, there are only one study and five studies investigating the association between -607C/A polymorphisms in IL-18 gene promoter and cancer risk among African and Caucasian population, respectively. Insufficient number of patients limited us to detect stable effects in these two populations. Additional studies are warranted to further validate ethnic difference in the effect of -607C/A polymorphisms in IL-18 gene promoter on cancer risk, especially in Africans. During sub-group analyses, we found that the source of controls also affected the association between -607C/A polymorphisms in IL-18 gene promoter and cancer risk. A significant association was observed in hospital-based controls under allelic and dominant genetic models, but not the population-based controls. The reason may be that the hospital-based studies have some inherent selection biases as such controls may just represent a sample of ill-defined reference population and may not be very representative of the study population or the general population. In stratified analysis by cancer site, we found that -607C/A polymorphisms in IL-18 gene promoter was statistically related with an increased risk of esophageal cancer and nasopharyngeal carcinoma. However, no evidence of association was found in any genetic model between-607C/A polymorphisms in IL-18 gene promoter and the risk of prostate cancer, colorectal cancer, breast cancer, cervical cancer, or other cancers. One possible reason is that carcinogenic mechanism underlying the etiology may differ by different tumor sites and that the -607C/A polymorphisms in IL-18 gene promoter may play a different role in different cancers. Futher, the number of studies which investigated the association between -607C/A polymorphisms in IL-18 gene promoter and risk of different types of cancer was too small(≤3), which limited us to detect stable effects on different cancer types. So, more studies focusing on different cancer types are need in the future.
The strength of the present analysis lies in inclusion of 22 studies, reporting data of 4100 cancer cases and 4327 controls. Publication bias, which, due to the tendency of not publishing small studies with null results, was not found in our meta-analysis. Furthermore, our findings were stable and robust in sensitivity analyses. Cumulative meta-analyses showed that, with each accumulation of more studies, the 95% CIs for the pooled ORs became increasingly narrower, indicating that the precision of the estimation was progressively boosted by continually adding more samples. Some limitations might be included in the meta-analysis. Firstly, we did not search for unpublished studies, so only published studies were included in our meta-analysis. Therefore, publication bias may have occurred although no publication bias was indicated from both visualization of the funnel plot and Egger’s test. Secondly, the results were based on unadjusted ORs, while a more precise estimation should take into account the effect of multiple confounders such as age, smoking status, drinking status and environmental factors on the association. Lack of information for data analysis may cause serious confounding bias. Thirdly, the small sample size is the major defect in this meta-analysis. In the stratified analysis by ethnicity and cancer type, the sample size of studies among Caucasians, Africans and among several cancer types is small, which limited us to detect stable effects in these populations and cancer types. Further studies are warranted to further evaluate the association in different ethnicities and cancer types in the future. Additionally, heterogeneity was significant in our meta-analysis, which may attenuate the strength of this study.
In conclusion, the present meta-analysis suggests that the -607C/A polymorphisms in IL-18 gene promoter is associated with a significantly increased risk of cancer, especially for nasopharyngeal carcinoma and esophageal cancer and in Asian population. More studies with larger sample size, well controlled confounding factors are warranted to further evaluate the association in different ethnicities and different cancer types in the future.
Supporting Information
PRISMA checklist.
(DOC)
Funding Statement
The authors have no support or funding to report.
References
- 1. Bazan JF, Timans JC, Kastelein RA (1996) A newly defined interleukin-1? Nature 379: 591. doi: 10.1038/379591a0. PubMed: 8628393. [DOI] [PubMed] [Google Scholar]
- 2. Dinarello CA (1999) Interleukin-18. Methods 19: 121-132. doi: 10.1006/meth.1999.0837. PubMed: 10525448. [DOI] [PubMed] [Google Scholar]
- 3. Baxevanis CN, Gritzapis AD, Papamichail M (2003) In vivo antitumor activity of NKT cells activated by the combination of IL-12 and IL-18. J Immunol 171: 2953-2959. PubMed: 12960319. [DOI] [PubMed] [Google Scholar]
- 4. Lebel-Binay S, Berger A, Zinzindohoué F, Cugnenc P, Thiounn N et al. (2000) Interleukin-18: biological properties and clinical implications. Eur Cytokine Netw 11: 15-26. PubMed: 10705295. [PubMed] [Google Scholar]
- 5. Tschoeke SK, Oberholzer A, Moldawer LL (2006) Interleukin-18: a novel prognostic cytokine in bacteria-induced sepsis. Crit Care Med 34: 1225-1233. doi: 10.1097/01.CCM.0000208356.05575.16. PubMed: 16540967. [DOI] [PubMed] [Google Scholar]
- 6. Sims JE (2002) IL-1 and IL-18 receptors, and their extended family. Curr Opin Immunol 14: 117-122. doi: 10.1016/S0952-7915(01)00306-5. PubMed: 11790541. [DOI] [PubMed] [Google Scholar]
- 7. Vidal-Vanaclocha F, Mendoza L, Telleria N, Salado C, Valcárcel M et al. (2006) Clinical and experimental approaches to the pathophysiology of interleukin-18 in cancer progression. Cancer Metastasis Rev 25: 417-434. doi: 10.1007/s10555-006-9013-3. PubMed: 17001512. [DOI] [PubMed] [Google Scholar]
- 8. Tsuboi K, Miyazaki T, Nakajima M, Fukai Y, Masuda N et al. (2004) Serum interleukin-12 and interleukin-18 levels as a tumor marker in patients with esophageal carcinoma. Cancer Lett 205: 207-214. doi: 10.1016/j.canlet.2003.10.010. PubMed: 15036653. [DOI] [PubMed] [Google Scholar]
- 9. Giedraitis V, He B, Huang WX, Hillert J (2001) Cloning and mutation analysis of the human IL-18 promoter: a possible role of polymorphisms in expression regulation. J Neuroimmunol 112: 146-152. doi: 10.1016/S0165-5728(00)00407-0. PubMed: 11108943. [DOI] [PubMed] [Google Scholar]
- 10. Pratesi C, Bortolin MT, Bidoli E, Tedeschi R, Vaccher E et al. (2006) Interleukin-10 and interleukin-18 promoter polymorphisms in an Italian cohort of patients with undifferentiated carcinoma of nasopharyngeal type. Cancer Immunol Immunother 55: 23-30. doi: 10.1007/s00262-005-0688-z. PubMed: 16059673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farjadfar A, Mojtahedi Z, Ghayumi MA, Erfani N, Haghshenas MR et al. (2009) Interleukin-18 promoter polymorphism is associated with lung cancer: a case-control study. Acta Oncol 48: 971-976. doi: 10.1080/02841860902878145. PubMed: 19642044. [DOI] [PubMed] [Google Scholar]
- 12. Taheri M, Hashemi M, Eskandari-Nasab E, Fazaeli A, Arababi F et al. (2012) Association of -607 C/A polymorphism of IL-18 gene (rs1946518) with breast cancer risk in Zahedan, Southeast Iran. Prague Med Rep 113: 217-222. PubMed: 22980562. [DOI] [PubMed] [Google Scholar]
- 13. Asefi V, Mojtahedi Z, Khademi B, Naeimi S, Ghaderi A (2009) Head and neck squamous cell carcinoma is not associated with interleukin-18 promoter gene polymorphisms: a case-control study. J Laryngol Otol 123: 444-448. doi: 10.1017/S0022215108003733. PubMed: 18940019. [DOI] [PubMed] [Google Scholar]
- 14. Lau J, Ioannidis JP, Schmid CH (1997) Quantitative synthesis in systematic reviews. Ann Intern Med 127: 820-826. doi: 10.7326/0003-4819-127-9-199711010-00008. PubMed: 9382404. [DOI] [PubMed] [Google Scholar]
- 15. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177-188. doi: 10.1016/0197-2456(86)90046-2. PubMed: 3802833. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JP, Thompson SG (2004) Controlling the risk of spurious findings from meta-regression. Stat Med 23: 1663-1682. doi: 10.1002/sim.1752. PubMed: 15160401. [DOI] [PubMed] [Google Scholar]
- 17. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088-1101. doi: 10.2307/2533446. PubMed: 7786990. [DOI] [PubMed] [Google Scholar]
- 18. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629-634. doi: 10.1136/bmj.315.7109.629. PubMed: 9310563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haghshenas MR, Hosseini SV, Mahmoudi M, Saberi-Firozi M, Farjadian S et al. (2009) IL-18 serum level and IL-18 promoter gene polymorphism in Iranian patients with gastrointestinal cancers. J Gastroenterol Hepatol 24: 1119-1122. doi: 10.1111/j.1440-1746.2009.05791.x. PubMed: 19638090. [DOI] [PubMed] [Google Scholar]
- 20. Babar M, Ryan AW, Anderson LA, Segurado R, Turner G et al. (2012) Genes of the interleukin-18 pathway are associated with susceptibility to Barrett’s esophagus and esophageal adenocarcinoma. Am J Gastroenterol 107: 1331-1341. doi: 10.1038/ajg.2012.134. PubMed: 22664470. [DOI] [PubMed] [Google Scholar]
- 21. Du B, Zhao J, Wei Y (2012) Interleukin-18 genegenetic polymorphisms and risk of nasopharyngeaI carcinomain in Han population from Sichuan China. Med. J West China 24: 1683-1685. [Google Scholar]
- 22. Farhat K, Hassen E, Bouzgarrou N, Gabbouj S, Bouaouina N et al. (2008) Functional IL-18 promoter gene polymorphisms in Tunisian nasopharyngeal carcinoma patients. Cytokine 43: 132-137. doi: 10.1016/j.cyto.2008.05.004. PubMed: 18555694. [DOI] [PubMed] [Google Scholar]
- 23. Guo JY, Qin AQ, Li RK, Yang CM, Huang FD et al. (2012) [Association of the IL-18 gene polymorphism with susceptibility to colorectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi 15: 400-403. [PubMed] [Google Scholar]
- 24. Kashef MA, Dehaghani AS, Naeimi S, Fattahi MJ, Ghaderi A (2008) Interleukin-18 gene promoter polymorphisms in women with gestational trophoblastic diseases. J Reprod Med 53: 853-859. PubMed: 19097518. [PubMed] [Google Scholar]
- 25. Khalili-Azad T, Razmkhah M, Ghiam AF, Doroudchi M, Talei AR et al. (2009) Association of interleukin-18 gene promoter polymorphisms with breast cancer. Neoplasma 56: 22-25. doi: 10.4149/neo_2009_01_22. PubMed: 19152241. [DOI] [PubMed] [Google Scholar]
- 26. Liu JM, Liu JN, Wei MT, He YZ, Zhou Y et al. (2013) Effect of IL-18 gene promoter polymorphisms on prostate cancer occurrence and prognosis in Han Chinese population. Genet Mol Res 12: 820-829. doi: 10.4238/2013.March.15.2. PubMed: 23546966. [DOI] [PubMed] [Google Scholar]
- 27. Liu Y, Lin N, Huang L, Xu Q, Pang G (2007) Genetic polymorphisms of the interleukin-18 gene and risk of prostate cancer. DNA Cell Biol 26: 613-618. doi: 10.1089/dna.2007.0600. PubMed: 17688413. [DOI] [PubMed] [Google Scholar]
- 28. Nikiteas N, Yannopoulos A, Chatzitheofylaktou A, Tsigris C (2007) Heterozygosity for interleukin-18 -607 A/C polymorphism is associated with risk for colorectal cancer. Anticancer Res 27: 3849-3853. PubMed: 18225542. [PubMed] [Google Scholar]
- 29. Nong LG, Luo B, Zhang L, Nong HB (2009) Interleukin-18 gene promoter polymorphism and the risk of nasopharyngeal carcinoma in a Chinese population. DNA Cell Biol 28: 507-513. doi: 10.1089/dna.2009.0912. PubMed: 19622039. [DOI] [PubMed] [Google Scholar]
- 30. Qi T, Wang Q, Zheng L, Yang HL, Bao J (2008) [Correlation of serum IL-18 level and IL-18 gene promoter polymorphisms to the risk of cervical cancer]. NAN Fang Yi Ke Xue Xue Bao 28: 754-757. [PubMed] [Google Scholar]
- 31. Sáenz-López P, Carretero R, Vazquez F, Martin J, Sánchez E et al. (2010) Impact of interleukin-18 polymorphisms-607 and -137 on clinical characteristics of renal cell carcinoma patients. Hum Immunol 71: 309-313. doi: 10.1016/j.humimm.2009.11.010. PubMed: 19961892. [DOI] [PubMed] [Google Scholar]
- 32. Samsami Dehaghani A, Shahriary K, Kashef MA, Naeimi S, Fattahi MJ et al. (2009) Interleukin-18 gene promoter and serum level in women with ovarian cancer. Mol Biol Rep 36: 2393-2397. doi: 10.1007/s11033-009-9469-7. PubMed: 19263242. [DOI] [PubMed] [Google Scholar]
- 33. Vairaktaris E, Serefoglou ZC, Yapijakis C, Agapi C, Vassiliou S et al. (2007) The interleukin-18 -607A/C polymorphism is not associated with risk for oral cancer. Anticancer Res 27: 4011-4014. PubMed: 18225563. [PubMed] [Google Scholar]
- 34. Wei YS, Lan Y, Liu YG, Tang H, Tang RG et al. (2007) Interleukin-18 gene promoter polymorphisms and the risk of esophageal squamous cell carcinoma. Acta Oncol 46: 1090-1096. doi: 10.1080/02841860701373595. PubMed: 17851835. [DOI] [PubMed] [Google Scholar]
- 35. Yang HL, Pin BH, Wang Q, Zheng L, Tang W et al. (2007) [Association of interleukin-18 gene rs1946519 and rs360718 single nucleotide polymorphism with cervical cancer]. Nan Fang Yi Ke Da Xue Xue Bao 27: 1006-1008. [PubMed] [Google Scholar]
- 36. Günel N, Coşkun U, Sancak B, Günel U, Hasdemir O et al. (2002) Clinical importance of serum interleukin-18 and nitric oxide activities in breast carcinoma patients. Cancer 95: 663-667. doi: 10.1002/cncr.10705. PubMed: 12209760. [DOI] [PubMed] [Google Scholar]
- 37. Kawabata T, Ichikura T, Majima T, Seki S, Chochi K et al. (2001) Preoperative serum interleukin-18 level as a postoperative prognostic marker in patients with gastric carcinoma. Cancer 92: 2050-2055. doi: 10.1002/1097-0142(20011015)92:8. PubMed: 11596019. [DOI] [PubMed] [Google Scholar]
- 38. Thong-Ngam D, Tangkijvanich P, Lerknimitr R, Mahachai V, Theamboonlers A et al. (2006) Diagnostic role of serum interleukin-18 in gastric cancer patients. World J Gastroenterol 12: 4473-4477. PubMed: 16874857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bushley AW, Ferrell R, McDuffie K, Terada KY, Carney ME et al. (2004) Polymorphisms of interleukin (IL)-1alpha, IL-1beta, IL-6, IL-10, and IL-18 and the risk of ovarian cancer. Gynecol Oncol 95: 672-679. doi: 10.1016/j.ygyno.2004.08.024. PubMed: 15581980. [DOI] [PubMed] [Google Scholar]
- 40. Jaiswal PK, Singh V, Srivastava P, Mittal RD (2013) Association of IL-12, IL-18 variants and serum IL-18 with bladder cancer susceptibility in North Indian population. Gene 519: 128-134. doi: 10.1016/j.gene.2013.01.025. PubMed: 23403235. [DOI] [PubMed] [Google Scholar]
- 41. Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K (2002) A comprehensive review of genetic association studies. Genet Med 4: 45-61. doi: 10.1097/00125817-200203000-00002. PubMed: 11882781. [DOI] [PubMed] [Google Scholar]
- 42. Dick DM (2011) Gene-environment interaction in psychological traits and disorders. Annu Rev Clin Psychol 7: 383-409. doi: 10.1146/annurev-clinpsy-032210-104518. PubMed: 21219196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist.
(DOC)