Abstract
Background
Alpha-methylacyl-CoA racemase (AMACR) is a mitochondrial and peroxisomal enzyme that is overexpressed in prostate cancer. The aim of this study was to confirm and expand the findings that the PCa risk increased in men associated with AMACR expression across various geographic regions.
Methods
A systematic search of databases was carried out and other relevant articles were also identified. Then the meta-analyses were conducted according to the standard guidelines.
Results
A total of 22 studies with 4,385 participants were included on the basis of inclusion criteria. AMACR by IHC was significantly associated with increased diagnosis of PCa (OR = 76.08; 95% CI, 25.53–226.68; P<0.00001). Subgroup-analysis showed that findings didn’t substantially change when only Caucasians or Asians (OR = 51.23; 95% CI, 19.41–135.24; P<0.00001) were considered. Expression of AMACR by PCR in relation to PCa risk suggested that AMACR was associated with PCa (OR = 33.60; 95% CI, 4.67–241.77; P<0.00001). There was also no significant publication bias observed.
Conclusions
Our findings provide further evidences that the expression of AMACR contribute to PCa risk. AMACR protein overexpression was found in prostate cancers, low expression in any of the normal tissues or in benign prostatic tissue. AMACR is potentially an important prostate tumor marker.
Introduction
Prostate cancer (PCa) is the most frequently diagnosed non-cutaneous malignancy in men, and the second leading cause of male cancer-related mortality in the United States [1]. The incidence of prostate cancer in Asia, including in China and Japan, has been increasing, although it is lower than that in the Western world [2]. Diagnosis of prostate cancer glands can sometimes present a diagnostic challenge for pathologists, since prostate carcinoma can mimic benign prostate glands [3]. and the architectural or cytologic clues for the diagnosis of carcinoma may not always be seen in small foci of suspicious glands. Also, Tissue diagnosis of prostate cancer can be difficult in needle biopsies or in a small focus of cancer of radical prostatectomies, presenting one of the major challenges in surgical pathology. underdiagnosis of a small focus of prostatic adenocarcinoma might delay early treatment and cause severe adverse consequences for patients. Therefore, a PCa specific marker could be be of great importance and usefulness to adjunct to facilitate critical diagnostic decisions with high sensitivity and specificity [4].
Although prostate-specific antigen (PSA) is the main criteria for PCa diagnosis, it has poor specificity to cancer, highly expressed in noncancerous prostatic tissues as well as in cancerous tissues and often lead to over diagnosis and overtreatment. Consequently a new scenario is needed to identify potentially aggressive or lethal PCa to better support clinical decisions [5].
AMACR (alpha-methylacyl-CoAracemase), an enzyme currently used in prostate cancer diagnosis, which is a peroxisomal and mitochon drial enzyme that was preferentially overexpressed to approximately 80% of prostate cancer detected in prostate biopsies [6]–[7]. However, AMACR is not 100% sensitive, and its expression is not limited to prostatic adenocarcinoma but may also be seen in several of its histologic mimics [8], resulting in many potential caveats in its use [9]. Accordingly, evaluation of AMACR as new markers of prostatic adenocarcinoma is needed.
In an attempt to confirm the potential role of AMACR expression as a prognostic biomarker, we completed a meta-analysis of AMACR expression in men of Asia and European lineage across different geographic regions with PCa.
Evidence Acquisition
Search Strategy and Selection Criteria
We undertook a comprehensive literature review with search terms (Table 1) without language restriction. We restricted the search to Medline, Web of Science and the Cochrane Library. The last quest was updated on March 13, 2013. Bibliographies of relevant retrieved studies and recent reviews were also scanned for additional publications. When more than one studies with the same population were identified, only the most recent or complete one was included in this meta-analysis.
Table 1. Characteristics of trials included in meta-analyses.
| Study | Year | methods | Ethnicity | Cases | Controls | Study design | Control source | ||
| Positive Total | Positive Total | ||||||||
| Rogers [14] | 2004 | IHC | Caucasian | 12 | 17 | 0 | 7 | cohort | biopsy negative |
| Shah [15] | 2013 | IHC | Caucasian | 48 | 51 | 2.5 | 3 | cohort | benign control |
| Trpkov [16] | 2009 | IHC | Caucasian | 120 | 124 | 16 | 20 | cohort | biopsy negative |
| Zhou [17] | 2004 | IHC | Caucasian | 176 | 215 | 4 | 11 | case series | benign control |
| Kaic [18] | 2009 | IHC | Caucasian | 9 | 16 | 0 | 4 | case series | benign control |
| Farinola [19] | 2004 | IHC | Caucasian | 16 | 23 | 2 | 16 | cohort | benign control |
| Puebla-Mora[20] | 2006 | IHC | Caucasian | 37 | 41 | 6 | 22 | cohort | benign control |
| Pertega-Gomes [21] | 2013 | IHC | Caucasian | 270 | 349 | 12 | 203 | cohort | benign control |
| Browne [22] | 2004 | IHC | Caucasian | 40 | 44 | 2 | 33 | cohort | benign control |
| Nassar [23] | 2005 | IHC | Caucasian | 34 | 38 | 0 | 15 | case series | benign control |
| Jiang [24] | 2005 | IHC | Caucasian | 78 | 82 | 0 | 56 | case series | benign control |
| Stewart [25] | 2007 | IHC | Caucasian | 272 | 320 | 0 | 292 | case series | benign control |
| Yamada [26] | 2013 | IHC | Asia | 42 | 60 | 9 | 19 | cohort | biopsy negative |
| Chen G [27] | 2004 | IHC | Asia | 71 | 78 | 3 | 68 | case series | benign control |
| Xiao [28] | 2004 | IHC | Asia | 103 | 105 | 19 | 135 | case series | benign control |
| Ng [29] | 2007 | IHC | Asia | 111 | 113 | 4 | 134 | case series | benign control |
| Yu [30] | 2007 | IHC | Asia | 42 | 42 | 0 | 30 | case series | benign control |
| Zielie [31] | 2004 | RT-PCR | Caucasian | 7 | 10 | 9 | 9 | case series | benign control |
| Jiang Z [32] | 2004 | RT-PCR | Caucasian | 441 | 454 | 254 | 277 | case series | benign control |
| Kristiansen [33] | 2008 | RT-PCR | Caucasian | 583 | 614 | 0 | 31 | case series | benign control |
| Schostak [34] | 2006 | RT-PCR | Caucasian | 37 | 57 | 8 | 55 | case series | benign control |
| Ouyang [35] | 2008 | RT-PCR | Caucasian | 30 | 43 | 14 | 49 | case series | benign control |
IHC = Immunohistochemistry; RT-PCR = Reverse Transcription-Polymerase Chain Reaction.
Studies were included if they fulfilled the following criteria: 1) cases were pathologically verified to have adenocarcinoma of the prostate (International Classification of Diseases-10: C61), 2) the control group consisted of subjects who were men and free of PCa, 3) studies investigating the association of AMACR with PCa risk as the main outcome.
Data Extraction and Quality Assessment
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [10] and Meta-analysis of Observational Studies in Epidemiology (MOOSE) [11] guidelines.
Study characteristics, ethnicity of included subjects, numbers of cases and control subjects, and positive staining were extracted for factors of interest. The authors of published studies were also contacted for requesting necessary data that were not provided. Quality assessment was undertaken independently by at least two authors (Ning Jiang, Shimiao Zhu, Jing Chen). Two authors (Liqun Zhou, Yuanjie Niu) independently did the literature search and extracted data. Any disagreements were resolved through discussion with authors (Niu and Zhou).
Data Analysis and Presentation
The effect estimates of choice were odds radio (OR) for dichotomous variables and the corresponding 95% confidence intervals (CI). The random effects model of DerSimonian and Laird was prespecified for use in all estimates because of the suspected a priori that studies were conducted by various authors with different populations and had different designs (eg, case-control and case series studies). Heterogeneity was evaluated using the Q test [12]. We also calculated the quantity I 2 statistic that represented the percentage of total variation across studies. As a guide, I 2 values of 25%, 50%, and 75% correspond to low, medium, and high levels of heterogeneity [13]. The funnel plot was addressed to reveal the potential publication bias. All analyses were conducted using Review Manage, version 5.2 (The Cochrane Collaboration, Oxford, U.K.).
Evidence Synthesis
Literature Search and Characteristics of Studies
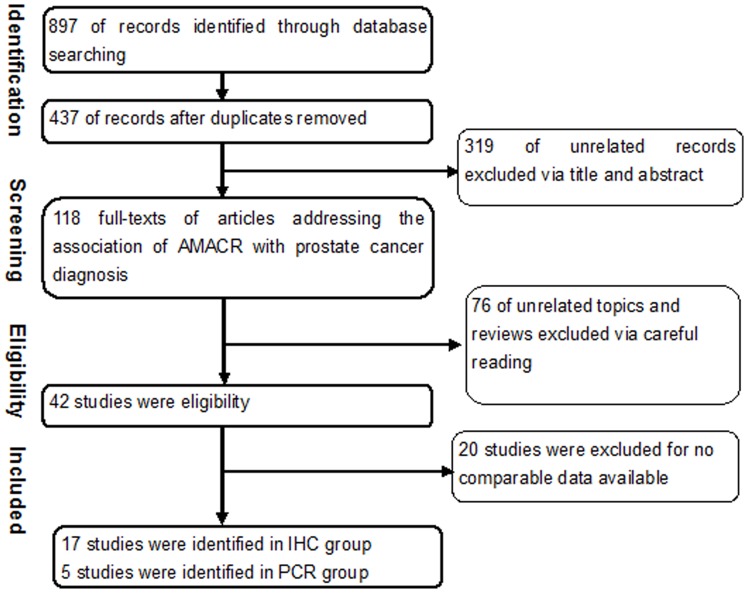
The literature searches yielded a total 897 studies. After review of the abstracts, 118 studies were identified as potentially eligible for inclusion. After full review, 17 studies [14]–[30]using immunohistochemical method (IHC) and 5 studies [31]–[35] using Polymerase Chain Reaction (PCR) were deemed eligible and were included in the study. The list of studies excluded and reasons for exclusion are shown in Figure 1.
Figure 1. Flowchart of selecting process for meta-analysis.
The included studies were published from 2004 to 2012. Five conducted in Asia, the others in western countries. Most of included studies chose benigh prostate hyperplasia. The details were listed in Table 1.
Meta-analysis Results
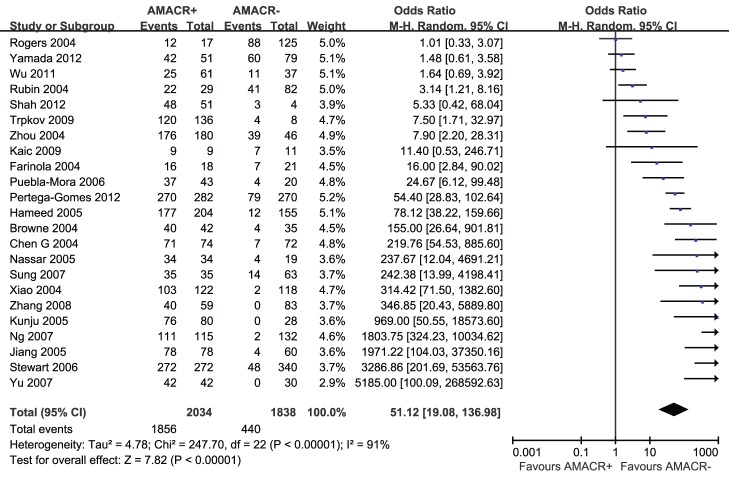
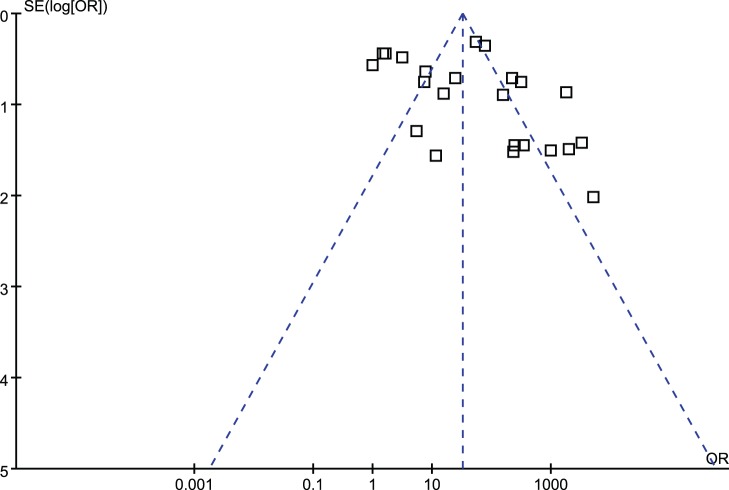
The pooled result revealed that positive AMACR by IHC was significantly associated with increased diagnosis of PCa (OR = 76.08; 95% CI, 25.53–226.68; P<0.00001) (Figure 2). Funnel plot asymmetry couldn’t be observed (Figure 3), which suggested no significant publication bias existing.
Figure 2. Forest plots for overall analysis of association of positive AMACR by immunohistochemistry with prostate cancer risk and under random-effects model.
M-H = Mantel-Haenszel method; CI = confidence interval.
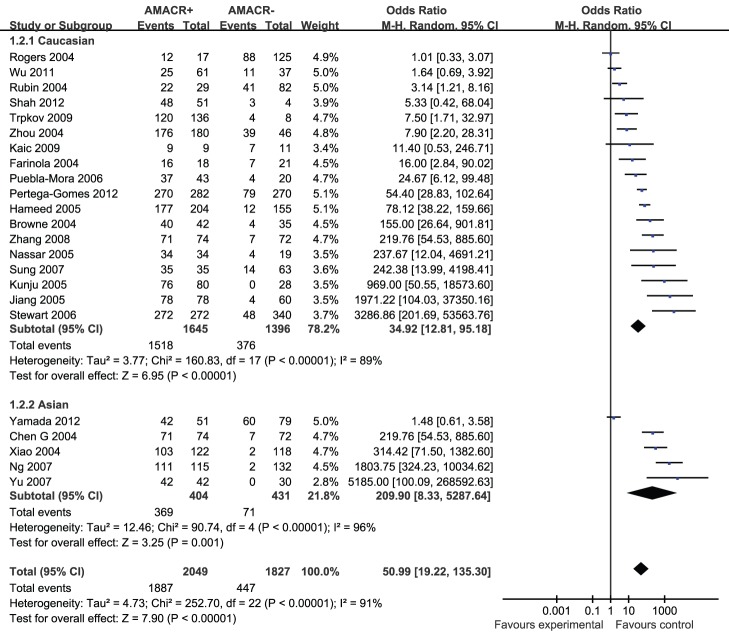
Figure 3. Forest plots for subgroup-analysis of association of positive AMACR by immunohistochemistry associated with prostate cancer risk in Caucasians and Asians.
M-H = Mantel-Haenszel method; CI = confidence interval.
In consideration of the potential different expression of AMACR in different races, we yielded enthnicity-based subgroup-analyses (Figure 4). Subgroup-analysis showed that findings didn’t substantially change when only Caucasians (OR = 51.23; 95% CI, 19.41–135.24; P<0.00001), or Asians were included (OR = 209.90; 95% CI, 8.33–5287.64; P<0.00001). Both the results of subgroup-analyses showed that heterogeneity was usually a variation affecting the degree of risk rather than direction of effect.
Figure 4. Forest plots for analysis of association of positive AMACR by RT-PCR with prostate cancer risk in; M-H = Mantel-Haenszel method; CI = confidence interval.
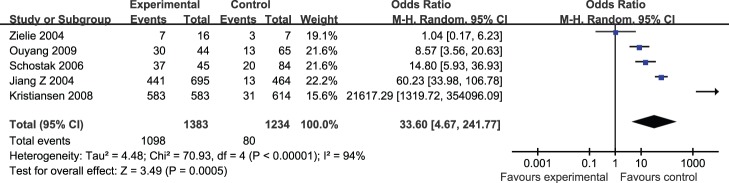
We next explored the positive AMACR by PCR in relation to PCa risk. Pooled results suggested that positive AMACR was associated with PCa (OR = 33.60; 95% CI, 4.67–241.77; P<0.00001) (Figure 5). There was also no significant publication bias observed.
Figure 5. Funnel plots illustrating meta-analysis of overall analysis.
SE = standard error; OR = odds ratio.
Discussion
In this study, we explored the association between the AMACR and PCa risk in 22 studies from various geographic regions including European and Asia. AMACR expression by IHC was significantly associated with increased diagnosis of PCa (OR = 76.08; 95% CI, 25.53–226.68; P<0.00001). The overall-analysis provided strong replication of the initial findings, confirming the AMACR for PCa.
AMACR is a well-characterized enzyme that plays a key role in peroxisomal b-oxidation of dietary branched fatty acids and C27-bile acid intermediates. It catalyzes the conversion of (R)-a-methyl-branched-chain fatty acyl-CoA esters to their (S)-stereoisomers. AMACR was identified as being overexpressed in prostate carcinoma cells when compared with benign or normal prostate epithelial cells [6]. The function of AMACR in prostate cancer has not been clarified yet. Several investigators have examined the mechanistic relationships between AMACR expression and hormone status. It has been reported that AMACR expression in hormone-sensitive cell lines and found its expression remained unchanged after exposure to antiandrogen drugs, suggesting that AMACR expression may not be directly regulated by the androgen pathway [36]. a-methylacyl-CoA racemase could not affect the stabilization of androgen receptor or modulate the expression of the androgen receptor–targeted gene, it indicating that the expression of AMACR is independent of androgen receptor–mediated signaling [37]. But Suzue et al [38] analyzed patients who had received hormonal therapy and found that those with localized prostate carcinoma had significantly diminished levels of AMACR expression. However, the exact mechanism by which hormonal therapy influences the expression level of AMACR remains elusive. Further studies are needed to further explore the mechanisms.
Strengths of this study include its large sample size. Because of this, the geographic regions were distinguished in subgroup-analyses. However, our results are based on unadjusted estimates, some un-provided parameters known to be associated with prostate carcinogenesis, such as inherent nature, might substantially confound the presented results.
Conclusion
Meta-analysis of the comprehensive literature revealed that the AMACR expression was strongly associated with PCa risk in man from various regions. There was no varying between Caucasian and Asian man.
Supporting Information
PRISMA checklist.
(DOC)
Funding Statement
This work was supported by the Science Foundation of Tianjin (No. 11JCZDJC19700)2010KZ95 and grant number 09ZCZDSF04300; and by the National Natural Science Foundation of China, grant numbers: 2012CB518304,2012DFG32220. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jemal A, Siegel R, Xu J, Ward E (2010) Cancer Statistics, 2010. CA Cancer J Clin 60: 277–300. [DOI] [PubMed] [Google Scholar]
- 2. Shin HR, Carlos MC, Varghese C (2012) Cancer control in the Asia Pacific region: current status and concerns. Jpn J Clin Oncol 42(10): 867–81. [DOI] [PubMed] [Google Scholar]
- 3. Donovan MJ, Cordon-Cardo C (2013) Predicting high-risk disease using tissue biomarkers. Curr Opin Urol 23(3): 245–5. [DOI] [PubMed] [Google Scholar]
- 4. Humphrey PA (2007) Diagnosis of adenocarcinoma in prostate needle biopsy tissue. J Clin Pathol 60(1): 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thomson AH, Kulkarni S, Bahl A (2008) Primary cryotherapy with salvage external beam radiotherapy for locally recurrent prostate cancer. Clin Oncol (R Coll Radiol) 20(5): 385. [DOI] [PubMed] [Google Scholar]
- 6. Kunju LP, Chinnaiyan AM, Shah RB (2005) Comparison of monoclonal antibody (P504S) and polyclonal antibody to alpha methylacyl-CoA racemase (AMACR) in the work-up of prostate cancer. Histopathology 47(6): 587–96. [DOI] [PubMed] [Google Scholar]
- 7. Zhou M, Aydin H, Kanane H, Epstein JI (2004) How often does alpha-methylacyl-CoA-racemase contribute to resolving an atypical diagnosis on prostate needle biopsy beyond that provided by basal cell markers? Am J Surg Pathol 28(2): 239–43. [DOI] [PubMed] [Google Scholar]
- 8. Gupta A, Wang HL, Policarpio-Nicolas ML, Tretiakova MS, Papavero V, et al. (2004) Expression of alpha-methylacyl-coenzyme A racemase in nephrogenic adenoma. Am J Surg Pathol 28(9): 1224–9. [DOI] [PubMed] [Google Scholar]
- 9. Osunkoya AO, Hansel DE, Sun X, Netto GJ, Epstein JI (2008) Aberrant diffuse expression of p63 in adenocarcinoma of the prostate on needle biopsy and radical prostatectomy: report of 21 cases. Am J Surg Pathol 32(3): 461–7. [DOI] [PubMed] [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283: 2008–12. [DOI] [PubMed] [Google Scholar]
- 12. Handoll HH (2006) Systematic reviews on rehabilitation interventions. Arch Phys Med Rehabil 87: 875. [DOI] [PubMed] [Google Scholar]
- 13. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rogers CG, Yan G, Zha S, Gonzalgo ML, Isaacs WB, et al. (2004) Prostate cancer detection on urinalysis for alpha methylacyl coenzyme a racemase protein. J Urol 172(4 Pt 1): 1501–3. [DOI] [PubMed] [Google Scholar]
- 15. Shah RB, Tadros Y, Brummell B, Zhou M (2013) The diagnostic use of ERG in resolving an “atypical glands suspicious for cancer” diagnosis in prostate biopsies beyond that provided by basal cell and α-methylacyl-CoA-racemase markers. Hum Pathol M44(5): 786–94. [DOI] [PubMed] [Google Scholar]
- 16. Trpkov K, Bartczak-McKay J, Yilmaz A (2009) Usefulness of cytokeratin 5/6 and AMACR applied as double sequential immunostains for diagnostic assessment of problematic prostate specimens. Am J Clin Pathol 132(2): 211–20. [DOI] [PubMed] [Google Scholar]
- 17. Zhou M, Aydin H, Kanane H, Epstein JI (2004) How often does alpha-methylacyl-CoA-racemase contribute to resolving an atypical diagnosis on prostate needle biopsy beyond that provided by basal cell markers? Am J Surg Pathol 28(2): 239–43. [DOI] [PubMed] [Google Scholar]
- 18. Kaić G, Tomasović-Loncarić C (2009) Alpha-methylacyl-CoA racemase (AMACR) in fine-needle aspiration specimens of prostate lesions. Diagn Cytopathol 37(11): 803–8. [DOI] [PubMed] [Google Scholar]
- 19. Farinola MA, Epstein JI (2004) Utility of immunohistochemistry for alpha-methylacyl-CoA racemase in distinguishing atrophic prostate cancer from benign atrophy. Hum Pathol 35(10): 1272–8. [DOI] [PubMed] [Google Scholar]
- 20. Puebla-Mora AG, Heras A, Cano-Valdez AM, Domínguez-Malagón H (2006) Human telomerase and a-methylacyl-coenzyme A racemase in prostatic carcinoma. A comparative immunohistochemical study. Ann Diagn Pathol 10(4): 205–8. [DOI] [PubMed] [Google Scholar]
- 21. Pértega-Gomes N, Vizcaíno JR, Gouveia C, Jerónimo C, Henrique RM, et al. (2013) Monocarboxylate transporter 2 (MCT2) as putative biomarker in prostate cancer. Prostate 73(7): 763–9. [DOI] [PubMed] [Google Scholar]
- 22. Browne TJ, Hirsch MS, Brodsky G, Welch WR, Loda MF, et al. (2004) Prospective evaluation of AMACR (P504S) and basal cell markers in the assessment of routine prostate needle biopsy specimens. Hum Pathol 35(12): 1462–8. [DOI] [PubMed] [Google Scholar]
- 23. Nassar A, Amin MB, Sexton DG, Cohen C (2005) Utility of alpha-methylacyl coenzyme A racemase (p504s antibody) as a diagnostic immunohistochemical marker for cancer. Appl Immunohistochem Mol Morphol 13(3): 252–5. [DOI] [PubMed] [Google Scholar]
- 24. Jiang Z, Li C, Fischer A, Dresser K, Woda BA (2005) Using an AMACR (P504S)/34betaE12/p63 cocktail for the detection of small focal prostate carcinoma in needle biopsy specimens. Am J Clin Pathol 123(2): 231–6. [DOI] [PubMed] [Google Scholar]
- 25. Stewart J, Fleshner N, Cole H, Sweet J (2007) Comparison of annexin II, p63 and alpha-methylacyl-CoA racemase immunoreactivity in prostatic tissue: a tissue microarray study. J Clin Pathol 60(7): 773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamada H, Tsuzuki T, Maeda N, Yamauchi Y, Yoshida S, et al. (2013) Alpha methylacyl-CoA racemase (AMACR) in prostate adenocarcinomas from Japanese patients: is AMACR a “race”-dependent marker? Prostate 73(1): 54–9. [DOI] [PubMed] [Google Scholar]
- 27. Chen GY, Liu L, Zhou XG, Zhang CH, Huang SF (2004) Assessment of P504S immunohistochemistry in diagnosis and differential diagnosis of prostatic adenocarcinoma. Zhonghua Bing Li Xue Za Zhi 33(5): 419–23. [PubMed] [Google Scholar]
- 28. Xiao Y, Chen J, Luo YF, Cao JL, Ling Q, et al. (2004) Detection of P504S (alpha-methylacyl-CoA racemase) in prostatic adenocarcinomas. Zhonghua Yi Xue Za Zhi 84(16): 1362–6. [PubMed] [Google Scholar]
- 29. Ng VW, Koh M, Tan SY, Tan PH (2007) Is triple immunostaining with 34betaE12, p63, and racemase in prostate cancer advantageous? A tissue microarray study. Am J Clin Pathol 127(2): 248–53. [DOI] [PubMed] [Google Scholar]
- 30. Yu T, Zhu SX, Zheng S, Chen SP (2007) Detection of AMACR (P504S), P63 and 34betaE12 cocktail in the early diagnosis of prostate cancer. Zhonghua Nan Ke Xue 13(3): 222–5. [PubMed] [Google Scholar]
- 31. Zielie PJ, Mobley JA, Ebb RG, Jiang Z, Blute RD, et al. (2004) A novel diagnostic test for prostate cancer emerges from the determination of alpha-methylacyl-coenzyme a racemase in prostatic secretions. J Urol 172(3): 1130–3. [DOI] [PubMed] [Google Scholar]
- 32. Jiang Z, Wu CL, Woda BA, Iczkowski KA, Chu PG, et al. (2004) Alpha-methylacyl-CoA racemase: a multi-institutional study of a new prostate cancer marker. Histopathology 45(3): 218–25. [DOI] [PubMed] [Google Scholar]
- 33. Kristiansen G, Fritzsche FR, Wassermann K, Jäger C, Tölls A, et al. (2008) GOLPH2 protein expression as a novel tissue biomarker for prostate cancer: implications for tissue-based diagnostics. Br J Cancer 99(6): 939–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schostak M, Miller K, Krause H, Schrader M, Kempkensteffen C, et al. (2006) Kinetic fluorescence reverse transcriptase-polymerase chain reaction for alpha-methylacyl CoA racemase distinguishes prostate cancer from benign lesions. Cancer Detect Prev 30(5): 449–54. [DOI] [PubMed] [Google Scholar]
- 35. Ouyang B, Bracken B, Burke B, Chung E, Liang J, et al. (2009) A duplex quantitative polymerase chain reaction assay based on quantification of alpha-methylacyl-CoA racemase transcripts and prostate cancer antigen 3 in urine sediments improved diagnostic accuracy for prostate cancer. J Urol 181(6): 2508–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuefer R, Varambally S, Zhou M, Lucas PC, Loeffler M, et al. (2002) alpha-Methylacyl-CoA racemase: expression levels of this novel cancer biomarker depend on tumor differentiation. Am J Pathol 161(3): 841–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zha S, Ferdinandusse S, Denis S, Wanders RJ, Ewing CM, et al. (2003) Alpha-methylacyl-CoA racemase as an androgen-independent growth modifier in prostate cancer. Cancer Res 63(21): 7365–76. [PubMed] [Google Scholar]
- 38. Suzue K, Montag AG, Tretiakova M, Yang XJ, Sahoo S (2005) Altered expression of alpha-methylacyl-coenzyme A racemase in prostatic adenocarcinoma following hormone therapy. Am J Clin Pathol 123(4): 553–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist.
(DOC)