Abstract
Brain irradiation has several well-known long-term side effects, including radiation-induced neoplasms and vasculopathy. In this case report, we describe an extremely rare case of meningioma and 15 cavernomas developing in a 29-year-old man, 19 years after cranial irradiation for posterior cranial fossa medulloblastoma. To our knowledge, this is the first case of a radiation-induced meningioma accompanied by this many radiation-induced cavernous angiomas.
Background
Prophylactic or therapeutic cranial irradiation is part of the treatment protocol for certain neoplasmatic conditions. Brain irradiation can lead to several well-known complications, including neoplasm formation and vasculopathy. As long-term cure rates increase, such side effects also begin to appear more frequently.
We describe a case of a radiation-induced meningioma (RIM) accompanied by 15 radiation-induced cavernomas (RICs). Synchronous development of tumours and vascular lesions is rare. Concomitant development of meningioma and cavernoma(s) has been reported as a long-term side effect of the brain irradiation1–3 and, to our knowledge, this is the first case report of RIM accompanied by such a large number of RICs.
Case presentation
A 29-year-old man was admitted to our hospital for epileptic seizures. Nineteen years earlier he had undergone a gross total resection of medulloblastoma of the posterior cranial fossa and had received prophylactic irradiation to the neuraxis. Follow-up MRIs over the next 5 years showed no signs of relapse or pathological findings other than the gliotic/porencephalic operated region.
The present MR scan showed a markedly enhancing extra-axial tumour compatible with meningioma in the right frontal lobe, and 15 hypointense lesions on T2* gradient echo images compatible with cavernous angiomas. Among the 15 cavernous angiomas, the largest two displayed high signal intensity on T1-weighted images (T1W), compatible with a subacute haemorrhage (figures 1 and 2).
Figure 1.
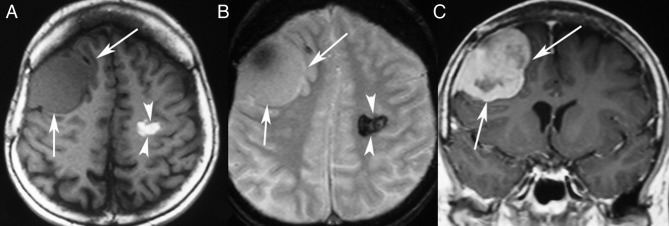
MR scan 19 years after the whole brain irradiation for posterior fossa medulloblastoma. Axial T1-weighted (T1W) (A) and T2*W (B) images showing an extra-axial mass in the right frontal lobe isoattenuating with the brain parenchyma. An intraparenchymal lesion is also shown in the left centrum semiovale with haemorrhagic features, displaying high signal intensity in T1W images and very low signal intensity in T2*W images. Contrast-enhanced coronal T1W image (C) showing heterogeneous marked enhancement of the extra-axial lesion, which proved to be a radiation-induced meningioma.
Figure 2.
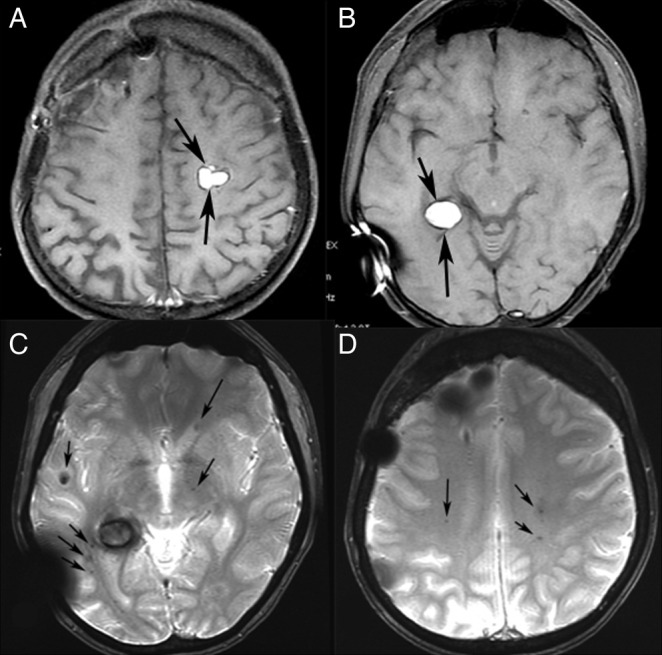
MR scan after complete removal of meningioma. T1-weighted axial images (A and B) showing two lobulated lesions with high signal intensity in the left centrum semiovale and in the right temporal lobe. T2* gradient echo axial images (C and D) showing numerous tiny lesions with very low signal intensity, representing haemosiderin in radiation-induced cavernomas (arrows).
Surgical resection of the extra-axial tumour was performed, and histopathological analysis revealed a typical meningioma (meningothelial, transitional and fibroblastic type, grade I, according to WHO), with no evidence of atypia or malignancy. Seven months after the resection, our patient is symptom free.
The criteria for classifying a tumour as radiation-induced are (1) tumour development in the radiation field, (2) existence of a sufficient latency period, (3) different histological findings between the primary irradiated tumour and the radiation-induced neoplasm and (4) absence of familial predisposition.4 5 The meningioma and cavernomas fulfilled the criteria for classification as radiation-induced lesions. Furthermore, the age and sex of our patient (young man) were not typically compatible with idiopathic meningiomas.
Discussion
It is well known that patients who receive prophylactic or therapeutic brain irradiation can develop radiation-induced intracranial lesions, which can include white matter necrosis, gliosis and demyelination leading to cerebral atrophy, as well as neoplasm formation and vascular proliferative lesions (capillary telangiectasia and cavernous angiomas).6 The most common radiation-induced neoplasms are meningiomas, gliomas and sarcomas. However, vascular proliferative lesions, such as capillary telangiectasia and cavernous angiomas, are also described with increasing incidence. Synchronous radiation-induced tumours/vascular lesions are a rare occurrence. We described a case of RIM with the synchronous presence of 15 asymptomatic RIC. To our knowledge, this has never been described before in the literature.
Compared with their ‘idiopathic’ counterparts, RIM tend to appear in younger patients, and tend to be multiple and more aggressive or atypical. Histopathological examination of our patient's RIM showed no evidence of atypia or malignancy. RIMs develop with a variable latency period, which is generally shorter in patients who received radiotherapy at under 5 years of age.7 8 It has been suggested that mutations and chromosomal lesions play a role in RIM development.9 10
RIC are believed to result from vasculopathy caused by radiation injury, leading to hyalinisation and necrosis of vascular walls, occlusion and neoangiogenesis. Mutations have also been blamed for cavernoma formation.3 11 Their latency period varies, and their incidence reportedly increases over time.12 Poussaint et al reported a series of 20 pediatric patients with intracranial neoplasia in whom delayed intracranial haemorrhage developed after cranial irradiation or radiation combined with systemic or intrathecal chemotherapy. The onset of haemorrhage after treatment occurred with a mean of 8.1 years.13 Young age and larger radiation doses increase the risk of RIC formation.3 14 Many RICs are asymptomatic, follow a benign course and do not require surgical or interventional treatment.6 7 However, haemorrhage reportedly occurs with an incidence of 40%14; thus, MRI should always include a gradient-echo T2* sequence.11 In the present case, 2 of the 15 cavernomas showed high signal intensity on T1W images, compatible with acute/subacute haemorrhage, while the others showed only haemosiderin deposition with very low signal intensity on gradient-echo T2* sequence. No specific symptoms were related to the cavernomas.
The tumorigenic effects of the brain irradiation are well known and meningioma is one of the most common radiation-induced neoplasms. Synchronous development of RICs has been described in the literature but, to our knowledge, the concomitant existence of 15 RIC has not previously been reported. Systematic follow-up with MRI and T2* gradient-echo images is indicated in long-term survivors of brain tumours who have received brain irradiation.
Learning points.
The most common radiation-induced neoplasm is meningioma.
Radiation-induced cavernomas are believed to result from vasculopathy caused by radiation injury.
Synchronous radiation-induced tumours/vascular lesions are a rare occurrence.
Footnotes
Contributors: DC was involved in study concept, DC and EP were involved in study design. EP and AK were involved in literature research. EP and DC were involved in manuscript preparation. DC and AD were involved in manuscript review. All authors read and approved the final version of the manuscript; and manuscript English language editing was by EP and DC.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Baheti AD, Mahore AS, Zade BP, et al. Meningioma and cavernous angioma following childhood radiotheraphy. Cancer Res Ther 2010;2013:333–5 [DOI] [PubMed] [Google Scholar]
- 2.Kamide T, Nakada M, Hayashi Y, et al. Radiation-induced cerebellar high-grade glioma accompanied by meningioma and cavernoma 29 years after the treatment of medulloblastoma: a case report. Neurooncol 2010;2013:299–303 [DOI] [PubMed] [Google Scholar]
- 3.Paramanathan N, Ooi KG, Reeves D, et al. Synchronous radiation induced orbital meningioma and multiple cavernomas. Lin Experiment Ophthalmol 2010;2013:414–17 [DOI] [PubMed] [Google Scholar]
- 4.Kado H, Ogawa T, Hatazawa J, et al. Radiation-induced meningioma evaluated with positron emission tomography with fludeoxyglucose F18. AJNR 1996;2013:937–8 [PMC free article] [PubMed] [Google Scholar]
- 5.Myong NH, Park BJ. Malignant glioma arising at the site of an excised cerebellar hemangioblastoma after irradiation in a von Hippel-Lindau disease patient. Yonsei Med J 2009;2013:576–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain R, Robertson PL, Gandhi D, et al. Radiation-induced cavernomas of the brain. AJNR 2005;2013:1158–62 [PMC free article] [PubMed] [Google Scholar]
- 7.Sadetzki S, Flint-Richter P, Ben-Tal T, et al. Radiation-induced meningioma: a descriptive study of 253 cases. Neurosurg 2002;2013:1078–82 [DOI] [PubMed] [Google Scholar]
- 8.Strojan P, Popovic M, Jereb B. Secondary intracranial meningiomas after high-dose cranial irradiation: report of five cases and review of the literature. Int J Radiat Oncol Biol Phys 2000;2013:65–73 [DOI] [PubMed] [Google Scholar]
- 9.Brassesco MS, Valera ET, Neder L, et al. Childhood radiation-associated atypical meningioma with novel complex rearrangements involving chromosomes 1 and 12. Neuropathology 2009;2013:585–90 [DOI] [PubMed] [Google Scholar]
- 10.Pagni CA, Canavero S, Fiocchi F, et al. Chromosome 22 monosomy in a radiation-induced meningioma. Ital J Neurol Sci 1993;2013:377–9 [DOI] [PubMed] [Google Scholar]
- 11.Noel L, Christmann D, Jacques C, et al. Les angiomes caverneux cerebraux induits par la radiotherapie. J Neuroradiol 2002;2013:49–56 [PubMed] [Google Scholar]
- 12.Lew SM, Morgan JN, Psaty E, et al. Cumulative incidence of radiation-induced cavernomas in long-term survivors of medulloblastoma. Neurosurg 2006;2013(2 Suppl):103–7 [DOI] [PubMed] [Google Scholar]
- 13.Poussaint TY, Siffert J, Barnes PD, et al. Hemorrhagic vasculopathy after treatment of central nervous system neoplasia in childhood: diagnosis and follow-up. AJNR 1995;2013:693–9 [PMC free article] [PubMed] [Google Scholar]
- 14.Keezer MR, Del Maestro R. Radiation-induced cavernous hemangiomas: case report and literature review. Can J Neurol Sci 2009;2013:303–10 [DOI] [PubMed] [Google Scholar]


