Abstract
Spontaneous obliteration of an arteriovenous malformation (SOAVM) is a rare event that is not completely understood. Less than 100 cases of SOAVMs have been reported in the literature. We present a unique case of a middle-aged patient with spontaneous obliteration of a cerebral arteriovenous malformation (AVM) who developed an ischemic stroke due to thrombosis of the stagnant proximal segment of the inferior branch of the middle cerebral artery feeder. Although the pathophysiology is not well understood, the arterial feeder hemodynamic changes post SOAVM may behave similarly to what occurs in rare cases after surgical resection of AVMs. Our case raises the hypothesis that stagnation of flow in spontaneous AVM obliteration may lead to delayed ischemic stroke in the territory of the feeding artery.
Keywords: Arteriovenous Malformation, Blood Flow, Magnetic Resonance Angiography
Background
Spontaneous obliteration of an arteriovenous malformation (SOAVM) is an unlikely event that occurs in approximately 1% of all intracranial arteriovenous malformations (AVMs).1 The exact pathophysiology of AVM obliteration is not completely understood but several theories have been proposed, including thromboembolism, hereditary coagulopathies, atherosclerosis of the feeding vessels, and compression of the feeding vessels by expanding hemorrhages.2–9 Less than 100 cases of SOAVMs have been reported in the literature. Presentations of SOAVM can be variable depending on the primary site of occlusion (arterial feeders, nidus, or draining veins). For many SOAVMs with primarily venous occlusion, a common presentation may be hemorrhage as a result of increased intranidal pressure secondary to venous congestion. In addition, SOAVMs may also present with seizures, neurological deficits, or, rarely, incidentally.5 We present a unique case of a patient with spontaneous obliteration of a cerebral AVM that developed an ischemic stroke due to thrombosis of the stagnant main middle cerebral artery (MCA) feeder.
Case presentation
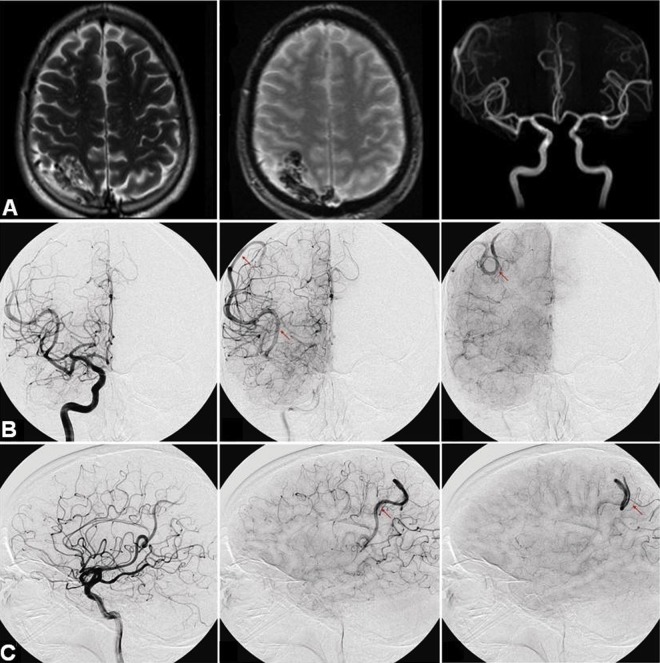
A previously healthy middle-aged patient presented to an outside hospital after a simple partial seizure involving the left upper extremity with secondary generalization. CT and MRI of the brain were performed, revealing a 3.5×2.5×2 cm right parietal AVM (figure 1A).
Figure 1.
(A) MRI of the brain demonstrating a 3.5×2.5×2 cm well circumscribed right posterior parietal lesion with a tangle of vessels suggestive of an arteriovenous malformation (AVM). (B, C) Cerebral angiogram demonstrating stagnation of blood flow and ectatic right inferior M2 feeder in the area of the previously described AVM.
Investigations
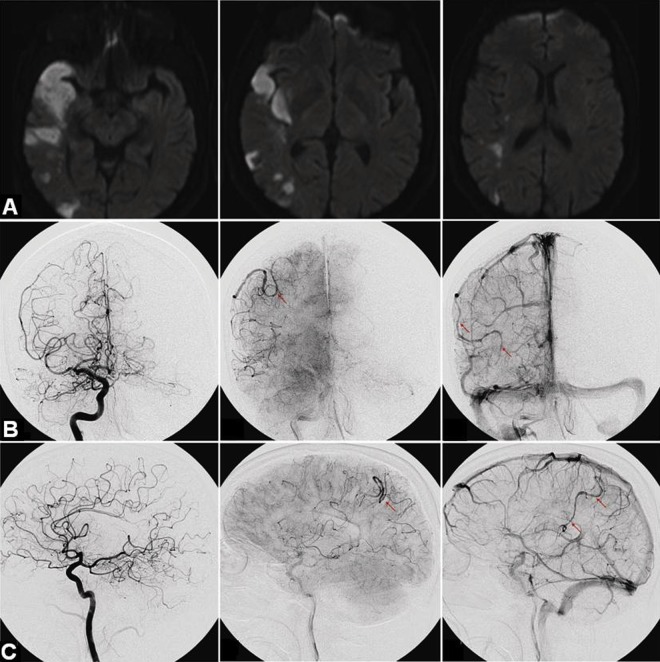
MR angiographic imaging revealed an enlarged right inferior division of the MCA (figure 1A). Subsequently, the patient was referred to our institution for a diagnostic angiogram, which demonstrated an enlarged and ectatic right inferior MCA branch with stagnation of blood flow consistent with a spontaneously thrombosed right parietal AVM previously fed by this branch (figure 1B, C). The patient was lost to follow-up and readmitted 2 years later with left hemiparesis, confusion, and dysarthria. MRI of the brain revealed an acute infarction involving the right temporal and parietal lobes in the distribution of the inferior MCA division (figure 2A). Follow-up catheter angiography revealed complete occlusion of the right inferior MCA division (figure 2B, C). Sluggish retrograde leptomeningeal reconstitution of the distal segment of the artery was noted. The carotid system had no atherosclerosis, dissection, or fibromuscular dysplasia. A stroke work-up, including cardiac telemetry, transthoracic echocardiogram with shunt study, and hypercoagulable panel, revealed no abnormalities.
Figure 2.
(A) MRI of the brain demonstrating large acute infarcts in the right temporal and parietal lobes in the distribution of the right middle cerebral artery. (B, C) Cerebral angiography demonstrating occluded right parietal arteriovenous malformation. There is also evidence of complete proximal occlusion of the right inferior M2 and retrograde filling in this branch via the leptomeningeal collateral.
Outcome and follow-up
The patient was discharged with intact mental status, minimal dysarthria, and no residual motor deficits. In an attempt to maintain patency of the residual retrograde angular artery filling, aspirin was started. No further events had occurred at the 6 month follow-up.
Discussion
SOAVM is a rare occurrence that occurs in approximately 1% of all intracranial AVMs.1 10 Although SOAVMs may have been considered a good prognosticator in the past, as it was thought to prevent hemorrhage, it is now known that SOAVM commonly results in hemorrhage, likely due to thrombosis of venous drainage of the AVM.5 10 Other presentations of SOAVM include seizures, neurological deficits, and headaches; otherwise, spontaneously thrombosed malformations may be incidentally diagnosed. We speculate that our patient presented with a large infarct after delayed thrombosis of the main SOAVM feeding artery. To our knowledge, this represents the first case of an ischemic stroke related to very delayed thrombosis of the main arterial AVM feeder after SOAVM.
SOAVM has been reported to occur more frequently in small AVMs with a single draining vein or single feeding artery.5 9 10 The pathophysiology of SOAVM has been extensively discussed.1 10 11 Possible causes for SOAVM are varied, including atherosclerosis or embolism generating feeding artery obstruction, coagulopathy, and mass effect from intracerebral hemorrhage or other causes.5 10
The arterial feeder hemodynamic changes post SOAVM may behave similarly to what occurs after surgical resection of AVMs. Stagnant arterial flow after resection of an AVM, as evidenced by delayed washout of contrast material on angiogram, is a common phenomenon.12 13 Although it has been suggested that these ‘stagnant arterial feeders’ could lead to ischemia, significant flow stagnation in the arterial feeder post resection has been demonstrated to correlate with higher oxygen saturation by microspectrophotometry in the AVM surrounding brain tissue.13
Several authors have described retrograde thrombosis of feeding arteries post-resection of an AVM.14–16 The presence of ‘stagnating arteries’, low blood flow velocity, and hemodynamic stress in the arterial wall have been associated with the development of retrograde feeder thrombosis.14 However, this phenomenon is likely to occur sooner in comparison with our patient's presentation. Also, in our case, there was thrombosis seen in the proximal segment of the feeding artery that was not adjacent to the AVM nidus. Interestingly, a surgical series described a 14% (five patients) incidence of retrograde thrombosis of the feeding artery post AVM resection.17 The risk factors for thrombosis were older age (mean 46 years) and medium or large AVMs, which is consistent with our case. Neurological manifestations from infarction were observed in three of the five patients with thrombosis, corroborating the fact that stagnating arteries may lead to deleterious consequences.
Thrombosis of AVM arterial feeders following SOAVM has not been previously described. This case raises the hypothesis that stagnation of flow post AVM resection or spontaneous obliteration may increase the risk of ischemic stroke in the primary feeding artery territory.
Learning points.
Spontaneous obliteration of an arteriovenous malformation (SOAVM) is a rare event, in which the pathophysiology is not completely understood.
Most cases of SOAVM present with intracranial hemorrhage.
SOAVMs may behave similarly to what occurs in rare cases after surgical resection of AVMs.
Stagnation of flow after SOAVM may contribute to the development of an ischemic stroke.
Footnotes
Contributors: All authors contributed equally to this manuscript.
Competing interests: None.
Patient consent: Not obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Abdulrauf SI, Malik GM, Awad IA. Spontaneous angiographic obliteration of cerebral arteriovenous malformations. Neurosurgery 1999;2013:280–7 [DOI] [PubMed] [Google Scholar]
- 2.Eisenman JI, Alekoumbides A, Pribram H. Spontaneous thrombosis of vascular malformations of the brain. Acta Radiol 1972;2013:77–85 [DOI] [PubMed] [Google Scholar]
- 3.Kushner J, Alexander E., Jr Partial spontaneous regressive arteriovenous malformation; case report with angiographic evidence. J Neurosurg 1970;2013:360–6 [DOI] [PubMed] [Google Scholar]
- 4.Nukui H, Miyagi O, Tamada J, et al. [Long-term follow-up study by cerebral angiography in cases with arteriovenous malformation of the brain. With special reference to spontaneous disappearance of arteriovenous malformation in cerebral angiography (author's transl)]. Neurol Med Chir 1982;2013:125–32 [DOI] [PubMed] [Google Scholar]
- 5.Panciani PP, Fontanella M, Carlino C, et al. Progressive spontaneous occlusion of a cerebellar AVM: pathogenetic hypothesis and review of literature. Clin Neurol Neurosurg 2008;2013:502–10 [DOI] [PubMed] [Google Scholar]
- 6.Perret G, Nishioka H. Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. Section VI. Arteriovenous malformations. An analysis of 545 cases of cranio-cerebral arteriovenous malformations and fistulae reported to the cooperative study. J Neurosurg 1966;2013:467–90 [DOI] [PubMed] [Google Scholar]
- 7.Perret G, Nishioka H. Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. IV. Cerebral angiography. An analysis of the diagnostic value and complications of carotid and vertebral angiography in 5,484 patients. J Neurosurg 1966;2013:98–114 [DOI] [PubMed] [Google Scholar]
- 8.Taha M, Patel U, Wharton SB, et al. Fatal spontaneous thrombosis of a cerebral arteriovenous malformation in a young patient with a rare heterozygous prothrombin gene mutation. Case report. J Neurosurg 2007;2013(2 Suppl):143–6 [DOI] [PubMed] [Google Scholar]
- 9.Minakawa T, Tanaka R, Koike T, et al. Angiographic follow-up study of cerebral arteriovenous malformations with reference to their enlargement and regression. Neurosurgery 1989;2013:68–74 [DOI] [PubMed] [Google Scholar]
- 10.Patel MC, Hodgson TJ, Kemeny AA, et al. Spontaneous obliteration of pial arteriovenous malformations: a review of 27 cases. AJNR Am J Neuroradiol 2001;2013:531–6 [PMC free article] [PubMed] [Google Scholar]
- 11.Buis DR, Bot JC, Barkhof F, et al. The predictive value of 3D time-of-flight MR angiography in assessment of brain arteriovenous malformation obliteration after radiosurgery. AJNR Am J Neuroradiol 2012;2013:232–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassler W, Gilsbach J, Gaitzsch J. Results and value of immediate postoperative angiography after operations for arteriovenous malformations. Neurochirurgia 1983;2013:146–8 [DOI] [PubMed] [Google Scholar]
- 13.Meyer B, Urbach H, Schaller C, et al. Is stagnating flow in former feeding arteries an indication of cerebral hypoperfusion after resection of arteriovenous malformations? J Neurosurg 2001;2013:36–43 [DOI] [PubMed] [Google Scholar]
- 14.Miyasaka Y, Yada K, Ohwada T, et al. Retrograde thrombosis of feeding arteries after removal of arteriovenous malformations. J Neurosurg 1990;2013:540–5 [DOI] [PubMed] [Google Scholar]
- 15.Solomon RA, Stein BM. Surgical management of arteriovenous malformations that follow the tentorial ring. Neurosurgery 1986;2013:708–15 [DOI] [PubMed] [Google Scholar]
- 16.Hassler W, Steinmetz H. Cerebral hemodynamics in angioma patients: an intraoperative study. J Neurosurg 1987;2013:822–31 [DOI] [PubMed] [Google Scholar]
- 17.Miyasaka Y, Yada K, Ohwada T, et al. Pathophysiologic assessment of stagnating arteries after removal of arteriovenous malformations. AJNR Am J Neuroradiol 1993;2013:15–18 [PMC free article] [PubMed] [Google Scholar]