Abstract
A 33-year-old woman was referred for an ultrasound of the abdomen because of biliary colic. The symptoms had started 2 months after giving birth to her first child. The ultrasound showed gallstones, but it also revealed multiple focal liver lesions that were initially thought to be malignant. The examination was supplemented with a CT scan, contrast-enhanced ultrasound (CEUS) and MRI. The lesions were suspected to be peliosis hepatis—a rare morphological entity characterised by multiple blood-filled cavities in the liver. Because of uncertainty as to the aetiology of the lesions demonstrated at CEUS and MRI, the diagnosis was definitively confirmed by large-size needle biopsies. Regular size biopsies were initially insufficient for diagnosis. The use of oral contraceptives for several years or the recent pregnancy may have been the cause of peliosis hepatis in this patient.
Background
Peliosis is a rare disease showing usually multiple blood-filled parenchymal cysts. Although, it is most often observed in the liver, it has been described in other organs such as the spleen and lungs.1 Although the pathogenesis of peliosis is unclear, it has been associated with numerous possible causes. While, early descriptions were reported peliosis with a range of chronic wasting conditions such as tuberculosis, the lesion is now more usually described together with a variety of drugs (particularly oral contraceptives and anabolic steroids), organ transplantation and malignancy.2 It has also been reported in immunocompromised patients with HIV usually in association with bacterial infection with Bartonella species.3 There have been a few case reports of infants with peliosis hepatitis (PH) suggesting that the lesion may also be congenital.4 5 Reports show that PH may be lethal as a result of haemoperitoneum following either spontaneous or iatrogenic rupture of the cysts.2 6
Our case shows that in the course of diagnosis of a common disease such as gallstones, a rare condition such as PH may be discovered incidentally. In addition, we show the importance of a multidisciplinary approach involving clinical, radiological and histopathological examination in order to establish the correct diagnosis of PH.
Case presentation
An otherwise healthy 33-year-old woman presented with a period of severe intermittent epigastric pain associated with nausea and vomiting, subsiding within half an hour. Two months prior to the onset of symptoms she had given birth to her first child. During pregnancy, the patient had hypertension, fatigue and experienced nightly sweating, but had no history of weight loss. There was no history of viral or bacterial infections and no known exposure to chemicals at work or at home, besides normal household cleaning products. She had a family history of hypertension, but no other vascular diseases or cancer. She had not received drugs as steroids, but she had been using oral contraceptives for 12 years. She had taken the same third generation combined oral contraceptive for the entire period.
Clinical examination was normal, apart from the finding of slight discomfort on abdominal palpation. Hepatomegaly was not found.
Investigations
The patient had elevated levels of alkaline phosphatase (367 U/L (reference interval 35–105 U/L)), γ-glutamyl transpeptidase (390 U/L (reference interval 10–75 U/L)) and alanine aminotransferase (152 U/L (reference interval 10–45 U/L)).
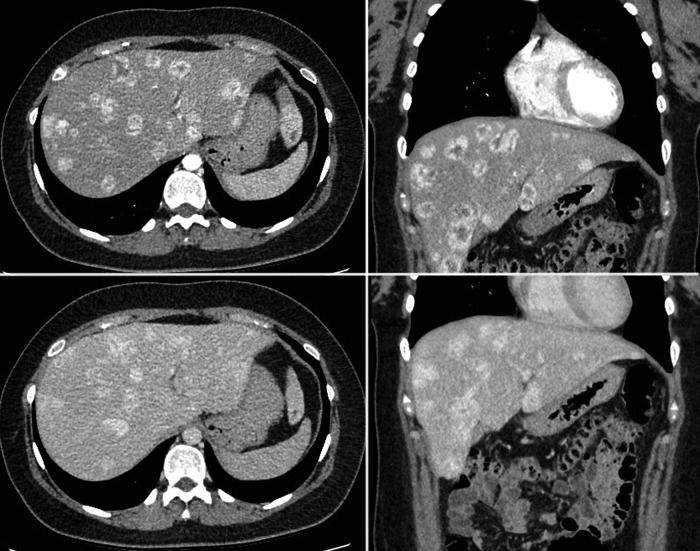
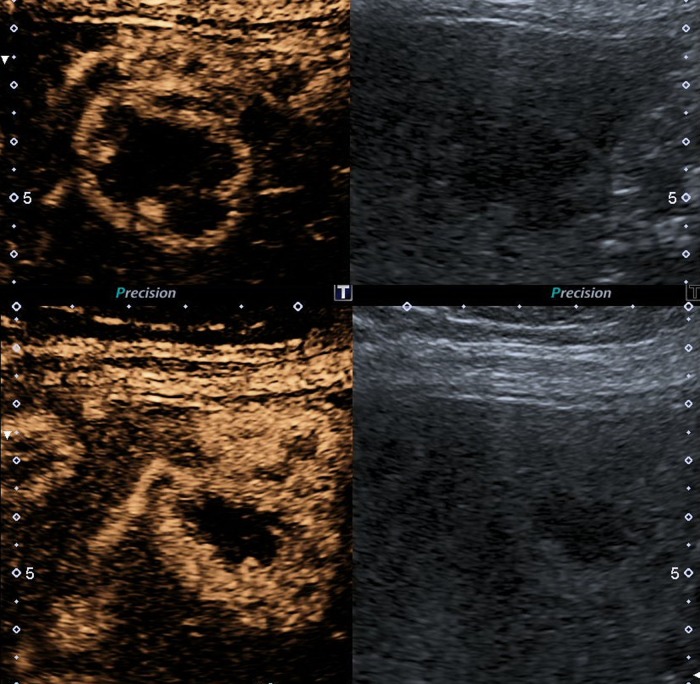
An abdominal ultrasound was performed, confirming multiple gallstones in the gallbladder. In addition, it revealed multiple hypoechogenic lesions of varying sizes throughout the liver (figure 1). A subsequent abdominal CT scan, confirmed over 50 liver lesions with early peripheral hyperattenuation in the arterial phase and centripetal homogenous hyperattenuation in the portal phase (time delay 50 s) for all but the three largest lesions, which showed incomplete filling (figure 2). A contrast-enhanced ultrasound (CEUS) was performed (Applio500, Toshiba, Japan) with SonoVue (Bracco, Italy), showing peripheral ring enhancement in the early arterial phase with centripetal filling at a constant modest speed regardless of lesion size (figure 3). Homogenous hyperenhancement was, thereby, first achieved for small lesions already in the arterial phase and for larger lesions in the (late) parenchymal phase (figure 4). One lesion showed a slight central washout after 6 min. These findings were suggestive of PH because of the early ring enhancement of the blood-filled cavities and non-globular fill-in. Later, a MR cholangiopancreatography (MRCP) was performed and showed increased intensity in all lesions in T2-weighted images (figure 5). PH was suspected, but in order to exclude malignancy, a biopsy was performed.
Figure 1.
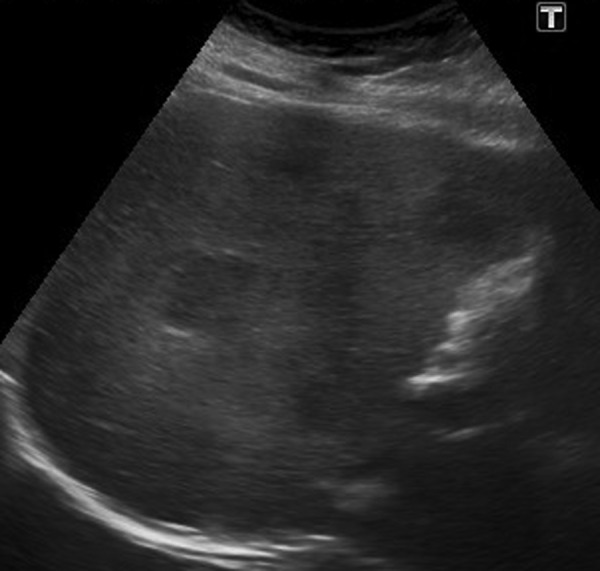
B-mode ultrasound. Several poorly defined hypoechogenic lesions are observed in the right liver lobe.
Figure 2.
CT scan in arterial phase, upper panel and venous phase, lover panel in axial left panel and coronal, right panel, scan plane. Multiple lesions in left and right liver lobe. The enhancement pattern shows in arterial phase that small lesions are homogeneous hyperattenuated while larger lesions have a peripheral ring of hyperattenation, with central slightly hypoattenuation compared with normal liver tissue. In portal venous phase almost all lesions show homogeneously hypoattenuation or isoattenuation indicating a centripetal enhancement pattern without washout.
Figure 3.
Contrast-enhanced ultrasound (CEUS) dual screen display on CEUS showing the baseline grey scale image on the right and contrast image on the left: early arterial phase of CEUS (15 s at top panel and 19 s at the bottom panel). Arterial blood supply and sinusoidal anastomosis gives a centripetal ring hyperenhancement. Small lesions are already homogenously hyperenhanced after 19 s.
Figure 4.
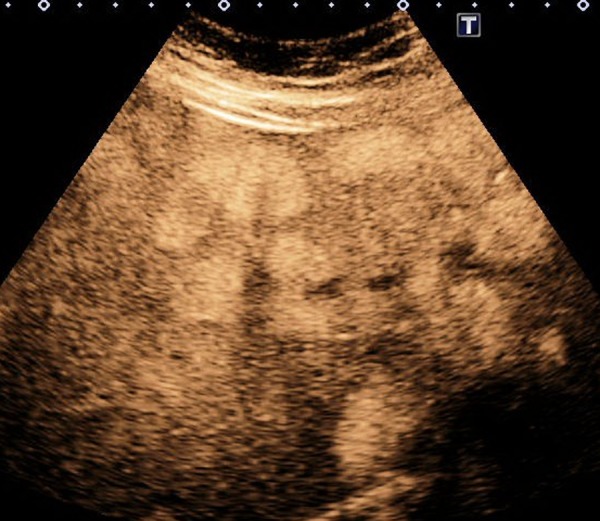
Contrast-enhanced ultrasound at late phase multiple homogenous hyperenhanced lesions with no sign of washout after 4:30 min.
Figure 5.
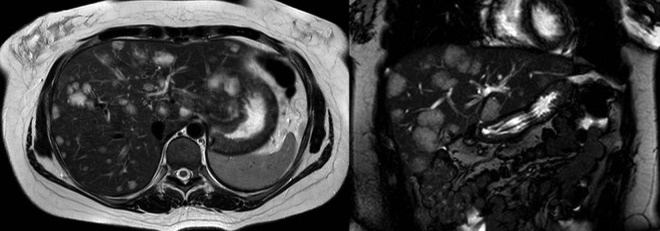
MR cholangiopancreatography scan in axial, left panel and coronal scan plane, right panel. Multiple lesions in both liver lobes all bright/hyperintense in T2-weighted sequences.
Initially, an 18 gauge, 22 mm liver biopsy was performed. Although this showed sinusoidal dilation and small blood-filled cavities, the size of the biopsy did not allow an unequivocal diagnosis of PH. In particular, it was not possible to exclude vascular proliferations such as haemangioma. The liver biopsy was then repeated using a 16 gauge, 22 mm needle, revealing extensive sinusoidal dilatation with multiple microcystic vascular cavities with partial endothelial lining, without fibrous stroma, consistent with PH (figure 6).
Figure 6.
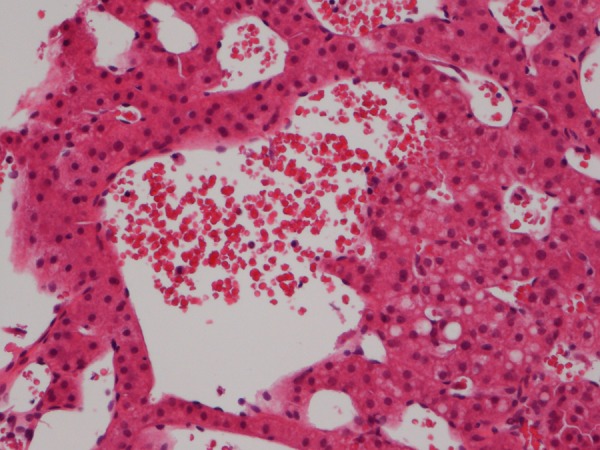
Histological section of liver biopsy showing sinusoidal dilatation together with a central blood-filled microcyst. The biopsy contained numerous similar vascular lesions indicating peliosis hepatis (H&E).
There was no histopathological evidence to suggest bacteria-associated PH. Subsequently, the patient was found to be negative for Bartonella antibodies. In addition, she was shown to be HIVnegative with normal immunoglobulin levels. There was no clinical evidence of immunodeficiency; there were no signs of chronic viral hepatitis B or C.
Because of the abnormal liver function tests and the elevated risk of bleeding, cholecystectomy was postponed until diagnosis of the liver disease was established.
Differential diagnosis
The initial ultrasound showed multiple focal lesions, suspicious for possible malignancy. CT and particularly CEUS and MRI suggested benign lesions with PH as the most likely cause. The initial liver biopsy raised the possible differential diagnoses of liver haemangioma and PH.
This patient presented with common symptoms of biliary colic, but diagnosis and treatment was complicated by the possibility of a rare vascular disease.
Outcome and follow-up
No treatment of PH was initiated and once the diagnosis was established the patient was referred to a tertiary referral centre for hepatic follow-up and long-term observation.
Discussion
There has been one case report describing PH in a woman postpartum with Bartonella as the causative agent.7 This resulted in liver failure and coma. The patient was treated with antibiotics and recovered. A second case described PH in a woman postpartum, but with unknown cause.8 The patient was followed with ultrasound examinations, before a liver transplant was performed after 2 years of observation because of continuous hepatic enlargement. It has been speculated that PH may be caused by hormonal changes during pregnancy.1 9
Bartonella henselae is the causative agent of cat scratch disease10 and may cause PH.11 Our patient was fully immunocompetent and had not been in contact with cats during her pregnancy. We found no evidence of Bartonella infection. The patient was previously healthy without any history of infections, with no family history of vascular diseases or cancer, and no known exposure to chemicals or drugs, besides oral contraceptives which she had taken for 12 years. Oral contraceptives have been widely reported as a possible cause for PH.2 PH in our patient may be due to the use of oral contraceptives2 or her recent pregnancy.1 9
On the basis of the initial ultrasound, a malignant liver disease was suspected. As a result, supplementary CT scan, CEUS and MRCP were carried out. A single lesion seen on CEUS was suggestive for PH as were the lesions demonstrated on the MRCP. In order to exclude a malignant diagnosis, liver biopsy was performed. The first biopsy was inconclusive and an additional, larger biopsy was taken to establish the diagnosis. It has previously been described that fine needle aspiration, gauge 18 core biopsy and even wedge biopsy is not always sufficient for correct histological diagnosis of PH and in one case, correct diagnosis was first established after liver transplantation.8 In another case report, a misdiagnosis of simple liver cyst was found. The patient subsequently underwent hepatic lobectomy for other reasons and died as a result of severe haemorrhage from PH.2 Our case demonstrates that larger than normal size biopsy needles may be needed for correct diagnosis of PH. Although, our patient had over 50 lesions, ultrasound guidance is recommended to ensure correct sampling.
According to the literature, liver biopsy remains the most reliable tool in diagnosing PH, but carries the risk of bleeding.12
Although the lesions caused by PH are quite characteristic on MRIs, the diagnosis in our case was not certain. It was decided to risk biopsy-related bleeding in order to confirm the correct diagnosis and, thus, exclude malignancy.
Learning points.
To recognise the imaging characteristics of peliosis hepatis as peliotic lesions may mimic several different types of focal hepatic lesions.
If the lesions observed on MRI are not definitively identified as peliosis hepatis, a liver biopsy should be performed to exclude malignancy, even if there is a risk of bleeding by this procedure.
Larger than normal size biopsy needles may be needed for correct diagnosis of PH.
It is important to establish the diagnosis of PH with certainty before considering biliary surgery.
The correct diagnosis of PH is dependent on a multidisciplinary clinical, radiological and histopathological approach.
Footnotes
Contributors: All the authors have contributed to the diagnostic workup and the preparation of the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Tsokos M, Erbersdobler A. Pathology of peliosis. Forensic Sci Intl 2005;2013:25–33 [DOI] [PubMed] [Google Scholar]
- 2.Atila K, Coker A, Ucar D. A rare clinical entity misdiagnosed as a tumor: peliosis hepatis. Ulus Travma Acil Cerrahi Derg 2007;2013:149–53 [PubMed] [Google Scholar]
- 3.Gazineo JL, Trope BM, Maceira JP, et al. Bacillary angiomatosis: description of 13 cases reported in five reference centers for AIDS treatment in Rio de Janeiro, Brazil. Rev Ins Med Trop São Paulo 2001;2013:1–6 [DOI] [PubMed] [Google Scholar]
- 4.Bracero LA, Gambon TB, Evans R, et al. Ultrasonographic findings in a case of congenital peliosis hepatis. J Ultrasound Med 1995;2013:483–6 [DOI] [PubMed] [Google Scholar]
- 5.Nuernberger SP, Ramos CV. Peliosis hepatis in an infant. J Pediatr 1975;2013:331–40 [DOI] [PubMed] [Google Scholar]
- 6.Lozano F, Corzo JE, León EM, et al. Massive hemoperitoneum: a new manifestation of bacillary peliosis in human immunodeficiency virus infection. Clin Infect Dis 1999;2013:911–12 [DOI] [PubMed] [Google Scholar]
- 7.McCormack G, Fenelon L, Sheehan K, et al. Postpartum coma. Lancet 1998;2013:1700. [DOI] [PubMed] [Google Scholar]
- 8.Muradali D, Wilson SR, Wanless IR, et al. Peliosis Hepatis with Intrahepatic Calcifications. J Ultrasound Med 1996;2013:257–60 [DOI] [PubMed] [Google Scholar]
- 9.Patricot LM, Dumont M, Duvernois JP, et al. A case of hepatic and splenic peliosis occurring in the puerperium after normal pregnancy. J Gynécolo, Obstét Biol Reprod 1986;2013:321–6 [PubMed] [Google Scholar]
- 10.Florin TA, Zaoutis TE, Zaoutis LB. Beyond cat scratch disease: widening spectrum of Bartonella henselae infection. Pediatrics 2008;2013:e1413–25 [DOI] [PubMed] [Google Scholar]
- 11.Maurin M, Birtles R, Raoult D. Current knowledge of Bartonella species. Eur J Clin Microbiol Infect Dis 1997;2013:487–506 [DOI] [PubMed] [Google Scholar]
- 12.Bleibel W, Curry MP. 2013. Peliosis hepatis. http://www.uptodate.com