Abstract
A previously well 66-year-old woman presented with a recurrent transudative right-sided pleural effusion. A nodular liver with coarse echotexture was demonstrated on ultrasound and subsequent MRI found hepatocellular carcinoma. In the absence of cardiopulmonary disease and significant protein uria, the recurrent pleural effusion was presumed to be hepatic hydrothorax despite the absence of ascites or other clinical features of chronic liver disease. The patient is currently awaiting liver transplantation.
Background
Hepatic hydrothorax is an uncommon differential diagnosis of a transudative pleural effusion. It most frequently occurs in the context of ascites and other features of portal hypertension due to decompensated liver disease. More rarely it may be the index presentation for chronic liver disease and must prompt appropriate workup for cirrhosis and causes of decompensation including hepatocellular carcinoma (HCC). In this unusual case, hepatic hydrothorax was the only manifestation of decompensated chronic liver disease, a scenario that has scantly been described in medical literature. We therefore propose that this important differential diagnosis for pleural effusion warrants careful investigation and referral to a gastroenterologist (or hepatologist) for further workup and appropriate treatment.
Case presentation
A 66-year-old Caucasian woman was admitted with progressive dyspnoea, right-sided chest discomfort and significantly reduced exercise tolerance.
At presentation she was tachypnoeaic with oxygen saturations of 95% in air and afebrile. An examination of the chest revealed reduced expansion, stony dullness to percussion and reduced breath sounds on the right, consistent with unilateral pleural effusion. She was obese (body mass index 40) and was noted to have at least eight spiders naive her on chest wall. However, there were no other peripheral stigmata of chronic liver disease and examination of the cardiovascular, gastrointestinal and neurological systems was otherwise unremarkable.
Six weeks previously she had undergone bilateral salpingo-oophrectomy for symptomatic benign ovarian cysts and required admission shortly after to drain a pleural effusion in the context of ipsilateral pneumonia. A total of 2 L of effusion was drained on this admission.
She was a foster-career, normally fit and well and had never smoked and consumed alcohol. She did not take regular medication.
Investigations
On admission, she had a moderately raised C reactive protein and mildly deranged liver function tests with a hepatitic pattern (albumin 30, aspartate aminotransferase 62, γ-glutamyl transpeptidase 129, bilirubin 22, international normalised ratio 1.1). A basic liver screen (hepatitis A, B and C viruses, antinuclear antibody, antismooth muscle, antimitochondrial antibody, antiliver–kidney microsomal, immunoglobulins) was unremarkable but α-feto protein was moderately raised (5.9 units).
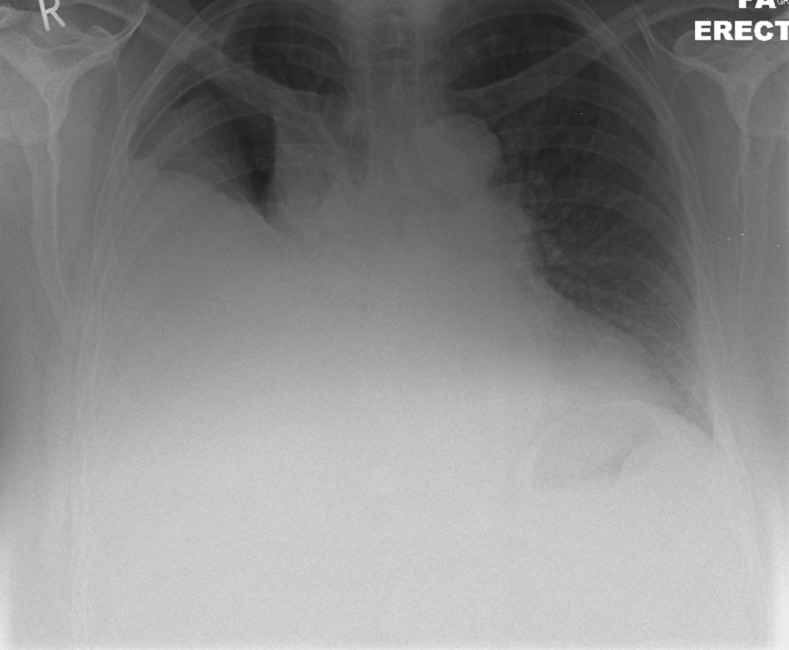
The chest X-ray demonstrated a reacculumation of the right-sided pleural effusion (figure 1). Three litres of clear pleural fluid was drained on this admission. Pleural fluid protein and lactate dehydrogenase (LDH) was consistent with a transudate via Light's criteria, serum protein was 68, pleural 23 and LDH 601; cytology demonstrated no malignant cells and there was no growth on microbiological culture.
Figure 1.
Chest X-ray demonstrating right-sided pleural effusion.
Liver ultrasound demonstrated features in keeping with cirrhosis and a focal liver lesion consistent sonographically with a simple cyst. Subsequent contrast-enhanced CT of the chest/abdomen/pelvis confirmed a simple pleural effusion but demonstrated no additional features to suggest malignant disease, and there was no ascites.
The urinary protein:creatine ratio was within normal limits and transthoracic ECG showed a preserved LV function with no features of pulmonary hypertension.
Differential diagnosis
With a transudative pleural effusion in the absence of other cardiopulmonary or renal disease, hepatic hydrothorax secondary to liver disease was felt to be the most probable cause. The cause of the liver disease was uncertain. Given the patient's body habitus and normal liver screen, non-alcoholic fatty liver disease seemed likely. Whereas a minority (approximately 5%) of malignant effusions is transudates, the lack of any pleural thickening/nodularity and any other intrapulmonary abnormalities, alongside the liver investigations, excluded the need for more invasive chest examinations (eg, thoracoscopy).
Treatment
As this was the second presentation with pleural effusion, talc pleurodesis was attempted but there was recurrence of effusion within 1 week of removal of the pleural drain. The oral diuretic spironolactone was started and uptitrated to a dose of 200 mg once daily which significantly improved the pleural effusion while the patient underwent further assessment.
Management of hepatic hydrothorax involves management of underlying decompensated chronic liver disease and specific management for the hydrothorax.
The management of decompensated chronic liver disease is well described by multiple international guidelines.1 Disease-specific interventions should be employed where appropriate, after screening of liver under the care of a gastroenterologist/hepatologist. Patients should be assessed for liver transplantation where appropriate.
Specific management of the hydrothorax is similar to that of ascites. In the first instance a combination of low sodium, high protein diet, sodium restriction (sodium intake of 70–90 mmol/day2) and diuretics. Aldosterone antagonists and loop diuretics can be used in isolation of combination although a rational approach would be to begin with a single agent and uptitrate to clinical response as would be appropriate to manage ascites.3
Symptomatic hydrothorax may require initial therapeutic thoracocentesis. Options for recurrent reaccumulation of pleural fluid include formal chest drainage with pleurodesis which has been described.4 5 However, rapid reaccumulation of fluid from increased portal pressures6 7 is common. There have been reports suggesting that continuous airway pressure with the use of tetracycline alongside pleurodesis may lower the peritoneal gradient pressure and thus making pleurodesis more successful.8 Although data regarding this are limited and apart from a widely cited abstract, there has been no other evidence to support this. Indwelling pleural catheters (with intermittent domiciliary drainage guided by symptoms) are being increasingly used in the management of malignant pleural effusions and in theory represent a management option for patients with hepatic hydrothorax who are unfit for specific hepatic intervention. There are risks of precipitating hepato-renal syndrome but in selected patients (largely with poorer-prognosis), these can be employed relatively successfully.9
Transjugular intrahepatic portosystemic shunt (TIPSS) is warranted in refractory hydrothorax and in patients who do not tolerate diuretics.10 TIPSS, acts to reduce portal hypertension which drives formation of ascites. Studies have shown TIPSS to be largely successful with reported response rates ranging between 70% and 80% but usually act as a temporising measure prior to liver transplantation.11 12
Outcome and follow-up
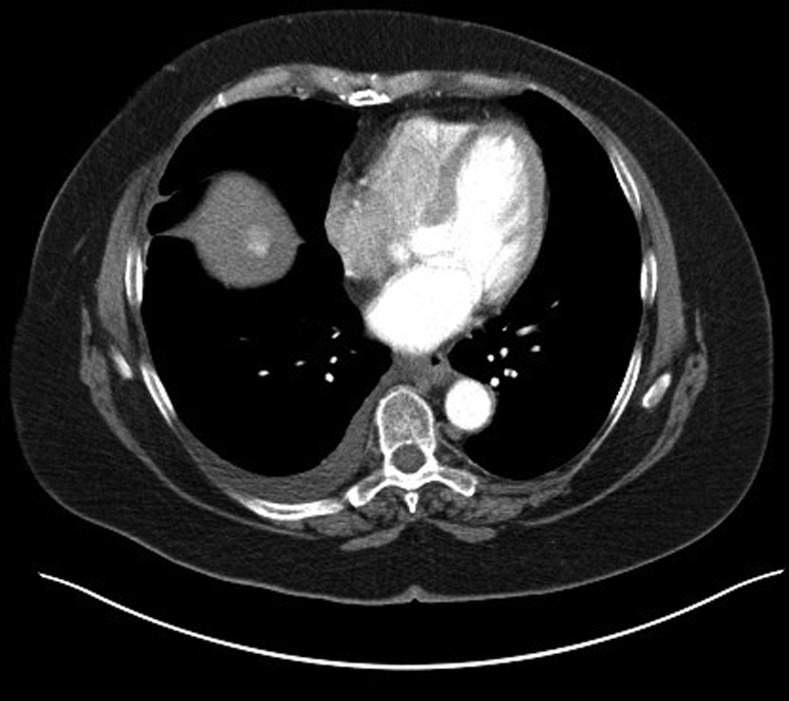
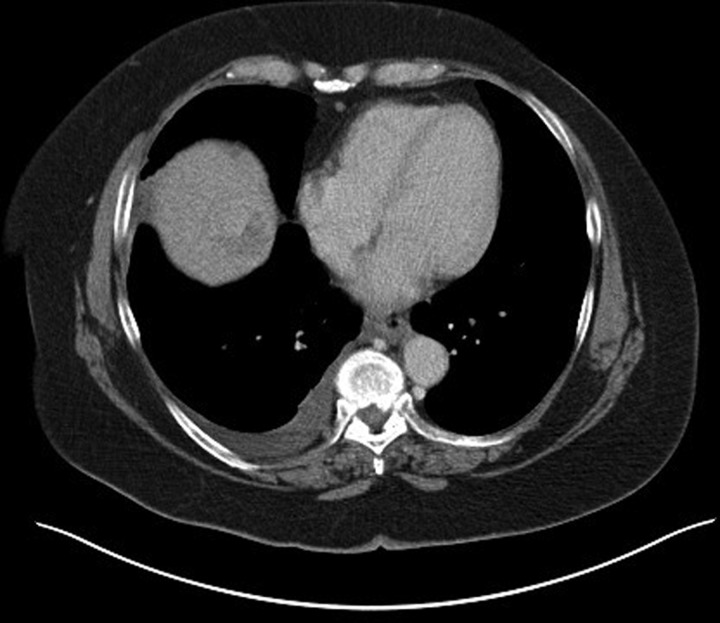
The patient was referred to a specialist hepatology unit to consider TIPSS as a definitive treatment for hepatic hydrothorax. As part of her workup, MRI of the liver raised the suspicion of isolated HCC, and a contrast-enhanced CT. The first CT slice (figure 2) is in arterial phase and demonstrates a 2.7 cm hypervascular lesion with the second image (figure 3), PV phase, shows that the lesion demonstrates washout. This is characteristic of HCC in a cirrhotic liver. The right pleural effusion is also demonstrated. Owing to her comorbidity, liver transplantation was thought to be unsuitable. At the time of writing she had been referred for transarterial chemoembolisation.
Figure 2.
Contrast-enhanced CT demonstrating hypervascular lesion in the liver.
Figure 3.
Contrast-enhanced CT demonstrating lesion washout in the liver.
Discussion
Hepatic hydrothorax has been defined as a pleural effusion, typically more than 500 mL, in patients with liver cirrhosis without coexisting cardiopulmonary disease.13 Although this definition is not entirely satisfactory it highlights the requirement to exclude competing aetiologies for the effusion. It occurs in 5–10% of end-stage cirrhotic liver disease patients.14
The exact mechanisms involved have not been well-defined. However, it is thought that ascites that form as a consequence of portal hypertension, crosses from the peritoneal to the pleural cavity through small diaphragmatic defects (usually <1 cm).15 The negative intrathoracic pressure favours the transfer of fluid across the defects. In total, 85% of hepatic hydrothorax develops on the right, 13% left and 2% bilaterally.
It is thought that hydrothorax can occur in isolation owing to the superior absorptive capacity of the peritoneum as compared to the pleura. In this rare occurrence ascites production exceeds the capacity of the pleura to resorb ascites generated but not that of the peritoneum.16 Given that hepatic hydrothorax in the absence of ascites is rare (but has been reported17); it follows with the circumstances that the physiological parameters required are specific as well as seldom coexist.
Learning points.
Hepatic hydrothorax is a rare complication in patients with decompensated liver disease and may be the index presentation for cirrhosis.
Management is similar to that for ascites involving sodium restriction, and diuretics.
Chest drain insertion +/− pleurodesis may be necessitated by clinical situation but recurrence is common.
Transjugular intrahepatic portosystemic shunt is an efficacious treatment modality for recurrent hepatic hydrothorax.
Thorough assessment is required to identify and treat underlying aetiology of chronic liver disease and cause for decompensation.
Footnotes
Contributors: SD was the gastroenterologist who first examined the patient after she was referred to gastroenterology. KM is the consultant radiologist at the University Hospital Birmingham. He reported on the CT image and contributed to the diagnosis of the patient. AS was the respiratory consultant who treated the patient for her pleural effusion and also provisionally diagnosed her with hepatic hydrothorax.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.European Association for the Study of the Liver EASL clinical guidelines on management of ascites, spontaneous bacterial peritonitis and hepato renal syndrome. J Hepatol 2013:397–417 [DOI] [PubMed] [Google Scholar]
- 2.Cardenas A, Kelleher T, Shopra S. Heptic Hydrothorax. Aliment Pharmacol Ther 2004;2013:271–9 [DOI] [PubMed] [Google Scholar]
- 3.Runyon B. Management of adult patients with ascites due to cirrhosis. Hepatology 2004;2013:841–56 [DOI] [PubMed] [Google Scholar]
- 4.Falchuk KR, Jacoby I, Colucci WS, et al. Tetracycline-induced pleural symphysis for recurrent hydrothorax complicating cirrhosis. Gastroenterology 1977;2013:319–21 [PubMed] [Google Scholar]
- 5.Lin CC, Wu JC, Chang SC, et al. Resolution of refractory hepatic hydrothorax after chemical pleurodesis with minocycline. Zhonghua Yi Xue Za Zhi 2000;2013:704–9 [PubMed] [Google Scholar]
- 6.Ikard RW, Sawyers JL. Persistent hepatic hydrothorax after peritoneo-jugular shunt. Arch Surg 1980;2013:1125–7 [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Panadero F, Antony VB. Pleurodesis: state of the art. Eur Respir J 1997;2013:1648–54 [DOI] [PubMed] [Google Scholar]
- 8.Boiteau R, Tenaillon A, Law Koune JD, et al. Treatment for cirrhotic hydrothorax with CPAP on mask and tetracycline pleural sclerosis [abstract]. Am Rev Respir Dis 1990;2013:A770 [Google Scholar]
- 9.Bhatnagar R, Reid ED, Corcoran JP, et al. The use of indwelling pleural catheter for the management of non-maligniant recurrent pleural effusions. Thorax 2012;67:A115. doi: 10.1136/thoraxjnl-2012-202678.406 [Google Scholar]
- 10.Siegerstetter V, Deibert P, Ochs A, et al. Treatment of refractory hepatic hydrothorax with transjugular intrahepatic portosystemic shunt: long-term results in 40 patients. Eur J Gastroenterol Hepatol 2001;2013:529–34 [DOI] [PubMed] [Google Scholar]
- 11.Gordan FD, Anastopoulos HT, Crenshaw W, et al. The suscessful treatment of symptomatic, refractory hepatic hydrothorax with transjugular intrahepatic portosystemic shunt. Hepatology 1997;2013:1366–9 [DOI] [PubMed] [Google Scholar]
- 12.Siegerstetter V, Deibert P, Ochs A, et al. Treatment of refractory hepatic hydrothorax with transjugular intrahepatic portosystemic shunt: long term results in 40 patients. Eur J Gastroenterol Hepatol 2001;2013:529–34 [DOI] [PubMed] [Google Scholar]
- 13.Strauss R, Boyer T. Hepatic hydrothorax. Semin Liver Dis 1997;2013:227–32 [DOI] [PubMed] [Google Scholar]
- 14.Cardenas A, Kelleher T, Chopra S. Review article: hepatic hydrothorax. Aliment Pharmacol Ther 2004;2013:271–9 [DOI] [PubMed] [Google Scholar]
- 15.Zenda T, Miyamoto S, Murata S, et al. Detection of diaphragmatic defect as the cause of severe hepatic hydrothorax with magnetic resonance imaging. Am J Gastroenterol 1998;2013:2288. [DOI] [PubMed] [Google Scholar]
- 16.Lazaridis K, Frank K, Krowka M, et al. Hepatic hydrothorax: pathogenesis, diagnosis, and management. Am J Med 1999;2013:262–7 [DOI] [PubMed] [Google Scholar]
- 17.John S, Paul M, Murthy U. An unusual presentation of cirrhotic pleural effusion in a patient with no ascites: a case report. Cases J. 2009;2013:6767. [DOI] [PMC free article] [PubMed] [Google Scholar]