Abstract
Anaplastic lymphoma kinase (ALK) rearranged non-small-cell lung cancer (NSCLC) is highly responsive to crizotinib, an oral ATP-competitive selective inhibitor of ALK. However, crizotinib exhibits extremely poor blood–brain barrier penetration; therefore, it is considered to play a limited role in the treatment of brain metastases. We present a case of a 50-year-old man with a diagnosis of ALK-rearranged NSCLC with brain metastasis and malignant pleural effusion. Despite the several systemic chemotherapy regimens and whole brain radiotherapy, brain metastasis was refractory; therefore, crizotinib was initiated. A CT scan showed a slight reduction in the brain metastasis and no change in intrathoracic disease 17 weeks after initiating crizotinib. Moreover, CT obtained 12 months after crizotinib treatment revealed brain metastasis without progression. To our knowledge, the present case is the second report of crizotinib-responsive brain metastases due to echinoderm microtubule-associated protein-like 4-ALK (EML4-ALK)-rearranged NSCLC.
Background
The echinoderm microtubule-associated protein-like 4–anaplastic lymphoma kinase (EML4-ALK) oncogene fusion is identified in 4% patients with non-small-cell lung cancers (NSCLCs).1 2 ALK-rearranged NSCLC is highly responsive to crizotinib, an oral ATP-competitive selective inhibitor of ALK.3 However, crizotinib exhibits extremely poor blood–brain barrier (BBB) penetration; therefore, it is considered to play a limited role in the treatment of brain metastases.4 In this study, we present a case of the brain metastasis due to lung adenocarcinoma showing a long-lasting response to crizotinib.
Case presentation
A 50-year-old man, with a history of smoking 300 pack-year, visited our hospital for dyspnoea on exertion. A chest radiograph obtained on admission revealed a massive left pleural effusion and right mediastinal deviation (figure 1). CT revealed left lung masses with pleural effusion and multiple brain nodules (figure 2). Cytological examination of the pleural effusion revealed neoplastic adenocarcinoma cells. The epidermal growth factor receptor (EGFR) mutation analysis was indicative of wild-type tumour. He was diagnosed with NSCLC (clinical stage IV, T3N2M1a) with brain metastasis and malignant pleural effusion in May 2011.
Figure 1.
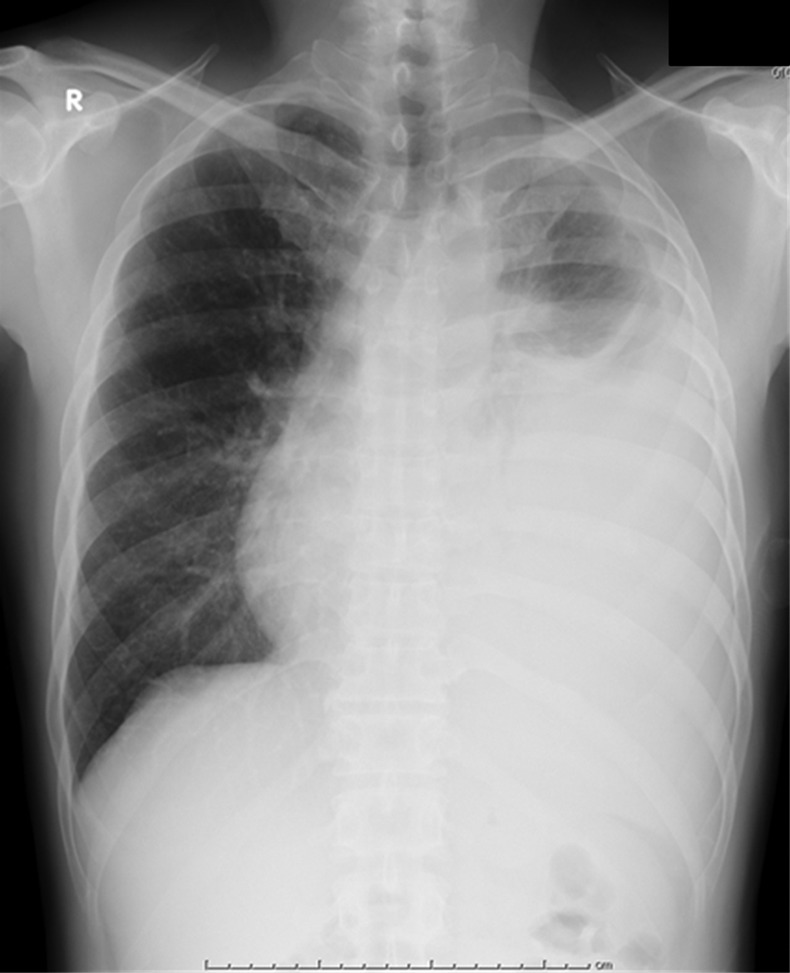
A chest radiograph obtained on admission revealed a massive left pleural effusion and right mediastinal deviation.
Figure 2.
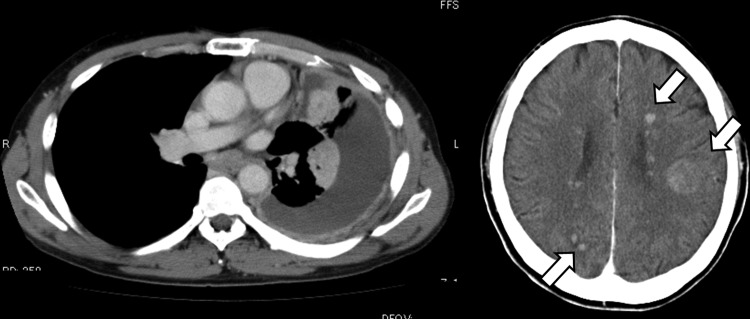
CT revealed left lung nodules with pleural effusion, and multiple brain nodules (arrow).
Outcome and follow-up
Despite administration of four cycles of combination chemotherapy with pemetrexed and carboplatin, the disease progressed and erlotinib was administered as the second line of treatment. Moreover, daily fractions of whole brain radiotherapy to a total of 30 Gy were administered in November 2011, but the brain metastasis was refractory. Eventually, docetaxel was administered as a third-line treatment to chemotherapy, even though the brain metastasis was progressive.
Because the disease progressed despite multiple chemotherapy regimens, we performed a transbronchial tumour biopsy, which revealed a positive EML4-ALK rearrangement (break-apart fluorescent in situ hybridisation). Therefore, crizotinib (250 mg twice daily) was initiated in August 2012 as the fourth line of treatment. CT scan showed a slight reduction in the brain metastasis and no change in intrathoracic disease 17 weeks after initiating crizotinib (figure 3). Moreover, CT obtained 12 months after the crizotinib treatment revealed brain metastasis without progression. The patient is currently being followed up as an outpatient.
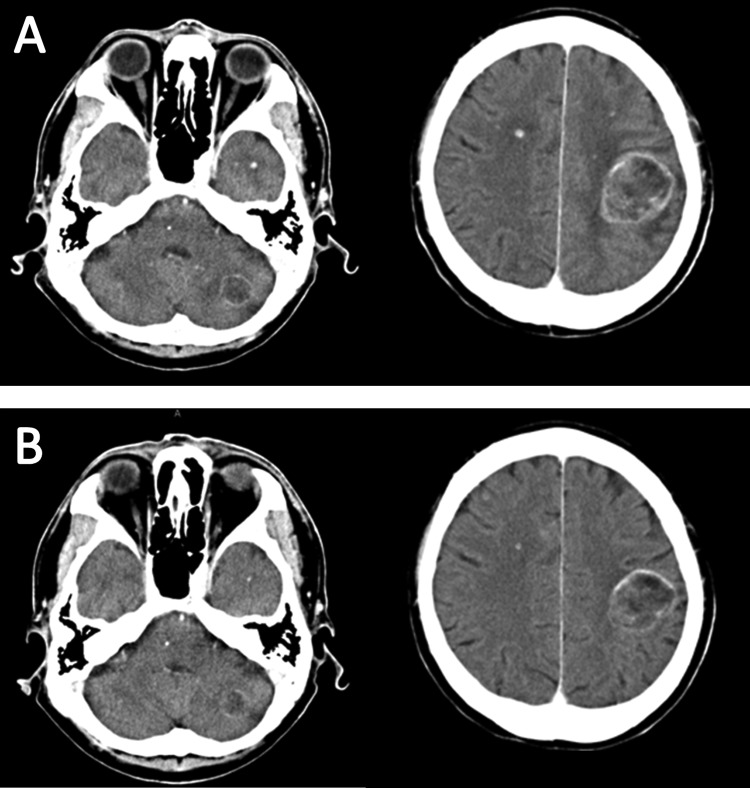
Figure 3.
(A) Contrast-enhanced brain CT before initiating crizotinib showing masses with ring enhancement in left frontal lobe and cerebellar hemisphere, and multiple tiny nodules. (B) Contrast-enhanced brain CT obtained on 17 weeks after initiating crizotinib showing all metastatic lesions decreasing in size.
Discussion
Crizotinib has shown marked clinical response to ALK-rearranged NSCLC, but it has been regarded as highly ineffective in brain metastasis because of its extremely poor BBB penetration.3 4 Similar to crizotinib, gefitinib has poor BBB penetration, and cerebrospinal fluid (CSF) drug levels that are sufficient to inhibit tumour growth cannot be achieved by standard-dose gefitinib administration.5 However, as many as 32% patients with brain metastasis due to NSCLC have been reported to be responsive to gefitinib (overall response rate).6 It is suggested that this unexpected effectiveness of gefitinib is partly due to disrupted BBB in most patients with macroscopic metastases.7 In contrast, the CSF-to-plasma ratio of crizotinib concentration is reported to be only 0.0026, even in a patient with brain metastasis,4 which is not similar to concentrations that exert antitumour effects. Therefore, besides the theory of BBB destruction due to cancer cells, the effectiveness of crizotinib against brain metastasis may depend on other factors.
We suggest that administering ionising radiotherapy before crizotinib treatment may play an important role in the effectiveness of crizotinib because brain radiotherapy is known to disrupt the BBB. Yuan et al8 reported that fractionated brain irradiation increases BBB permeability in a murine model by shortening tight junctions between adjacent endothelial cells and increasing the number of vesicles in endothelial cells that provide transport pathways across the BBB. Including our case, only two cases of crizotinib-responsive brain metastases in ALK-rearranged NSCLC have been reported in the literature.9 In both cases, ionising radiotherapy was performed before crizotinib treatment. Therefore, we suggest that brain ionising radiotherapy may affect CSF drug levels.
Interestingly, in the present case, crizotinib seemed to be more effective in brain metastasis than the primary tumour. This treatment effect could not be explained only by the increased drug penetration of BBB, and was not observed in the first report of crizotinib-responsive brain metastasis due to ALK-rearranged NSCLC.9 Moreover, similar antitumour effect was also observed among patients with brain metastasis due to EGFR mutation-positive NSCLC.10 As a possible explanation, it is suggested that exposure to multiple chemotherapeutic agents or ionising radiotherapy may result in some cell lines becoming more sensitive to EGFR inhibitors.11 12 The same mechanism may also occur in ALK-rearranged NSCLC; therefore, cases showing similar treatment effects are required to further clarify these observations.
Learning points.
Brain metastasis due to anaplastic lymphoma kinase-rearranged non-small-cell lung cancer (NSCLC) is usually resistant to crizotinib because of poor blood–brain barrier penetration.
To our knowledge, the present case is the second report that brain metastasis due to NSCLC was responsive to crizotinib.
We suggest that administering ionising radiotherapy before crizotinib treatment may play an important role in the effectiveness of crizotinib.
Footnotes
Contributors: All authors were involved in formulating the treatment plan and writing of the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;2013:561–6 [DOI] [PubMed] [Google Scholar]
- 2.Takeuchi K, Choi YL, Soda M, et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res 2008;2013:6618–24 [DOI] [PubMed] [Google Scholar]
- 3.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;2013:1693–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011;2013:e443–5 [DOI] [PubMed] [Google Scholar]
- 5.Jackman DM, Holmes AJ, Lindeman N, et al. Response and resistance in a non-small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol 2006;2013:4517–20 [DOI] [PubMed] [Google Scholar]
- 6.Wu C, Li YL, Wang ZM, et al. Gefitinib as palliative therapy for lung adenocarcinoma metastatic to the brain. Lung Cancer 2007;2013:359–64 [DOI] [PubMed] [Google Scholar]
- 7.Fidler IJ, Yano S, Zhang RD, et al. The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol 2002;2013:53–7 [DOI] [PubMed] [Google Scholar]
- 8.Yuan H, Gaber MW, Boyd K, et al. Effects of fractionated radiation on the brain vasculature in a murine model: blood-brain barrier permeability, astrocyte proliferation, and ultrastructural changes. Int J Radiat Oncol Biol Phys 2006;2013:860–6 [DOI] [PubMed] [Google Scholar]
- 9.Kaneda H, Okamoto I, Nakagawa K. Rapid response of brain metastasis to crizotinib in a patient with ALK rearrangement-positive non-small-cell lung cancer. J Thorac Oncol 2013;2013:e32–3 [DOI] [PubMed] [Google Scholar]
- 10.Porta R, Sánchez-Torres JM, Paz-Ares L, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J 2011;2013:624–31 [DOI] [PubMed] [Google Scholar]
- 11.Ceresoli GL, Cappuzzo F, Gregorc V, et al. Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann Oncol 2004;2013:1042–7 [DOI] [PubMed] [Google Scholar]
- 12.Dai Q, Ling YH, Lia M, et al. Enhanced sensitivity to the HER1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib hydrochloride in chemotherapy-resistant tumor cell lines. Clin Cancer Res 2005;2013:1572–8 [DOI] [PubMed] [Google Scholar]