Abstract
This is a case of eosinophilic cystitis in a 56-year-old indigenous Australian woman who presented with urosepsis on the background of a urinary tract infection unresponsive to oral antibiotics. After resolution of the urosepsis, she had persisting urinary retention and a cystoscopy/bladder biopsy suggested eosinophilic cystitis. After 1 month of intravesical hydrocortisone and oral loratadine, repeat cystoscopy showed vast improvement in the bladder lesions. This case further strengthens the use of intravesical steroids and oral antihistamines for the management of eosinophilic cystitis.
Background
First described in 1960 by Brown,1 eosinophilic cystitis is a histological diagnosis characterised by eosinophil infiltration of all layers of the bladder, mucosal fibrosis and muscle necrosis.2 The histopathological features of eosinophilic cystitis have been classified as being in the ‘acute or ‘chronic’ phase.2 It is this biphasic nature of morphology that provides a clue to the diagnosis.3 Yamada and Taguchi have observed that cases of eosinophilic cystitis have more than 20 eosinophils/five 200× fields in the acute phase of the condition. The presence of Charcot-Leyden crystals may help in the diagnosis in the acute phase. In the chronic phase, eosinophilia is not significant but rather chronic inflammation is present with scarring.3–5 Clinically, patients may present with dysuria, frequency, haematuria, supra-pubic pain, urinary retention, impaired renal function or asymptomatically.2 6 7 All age groups are affected and there is equal distribution among the adult sexes.2 Eosinophilic cystitis is a rare condition, with a pooled analysis in 2000 identifying only 135 cases reported in international literature.2 Its aetiology is not clear, however allergies or bladder wall injury appear to be present in most cases.2 6 Treatment modalities reported include avoidance of the suspected causative antigen, a wide range of intravenous, oral and intravesical agents and a variety of surgical interventions.2 6 7
Case presentation
A 56-year-old Australian Indigenous woman presented with urosepsis after being treated initially with a course of oral antibiotics for a urinary tract infection that grew Escherichia coli and Providencia species. She was clinically and biochemically in renal failure being acidotic, drowsy, hyperkalaemic and hypotensive at the time of presentation. Her medical history included type 2 diabetes (managed with oral hypoglycaemic agents) and previous urinary tract infections. She was also a heavy cigarette smoker.
She responded well to resuscitation measures and was started on intravenous meropenem, with a change to oral ciprofloxacin after a few days of intravenous antibiotics.
Frank haematuria was noted and she received bladder washouts for the clots, with eventual clearing of the heavy haematuria from her urine. It was unclear initially whether the clot retention was the reason for the acute renal failure and the subsequent urosepsis or whether it was just a contributing factor. After further investigations, the clot retention was found to be a part of contributing clinical picture.
After the acute urosepsis settled, the indwelling catheter was removed, but her trial of void was unsuccessful, with residuals in excess of 500 mL shown on the bladder scan. The Urologists recommended that she would most likely benefit from supervised self-intermittent catheterisation four times a day.
Investigations
Haemoproteinuria was found in the urinalysis and prompted a renal tract ultrasound scan to be performed. The scan revealed a moderate bilateral hydronephrosis with a diffusely thick-walled bladder—no obstructing lesions seen. The urologists were consulted to review her who then proceeded with a cystoscopy/bilateral retrograde pyelogram and a bladder biopsy. No obvious filling defects were seen on the retrograde pyelogram. During the cystoscopy, multiple irregular tan coloured lesions were seen scattered throughout the bladder as well as some clots and biopsies were obtained. Histology showed a thickened and oedematous urothelium with mild intraepithelial inflammation (see figure 1). The lamina propria was oedematous with a moderate mixed inflammatory cell infiltrate of lymphocytes, plasma cells, scattered eosinophils and neutrophils. Inflammation in the muscularis propria, however, consisted of numerous eosinophils admixed with lymphocytes, separating smooth muscle fibres and associated focally with fibrosis. There were scattered lymphoid aggregates. There was no necrosis and no evidence of a vasculitic process. Parasitic organisms were not identified. As such, a diagnosis of eosinophilic cystitis was suggested. Her blood eosinophil count was within normal limits.
Figure 1.
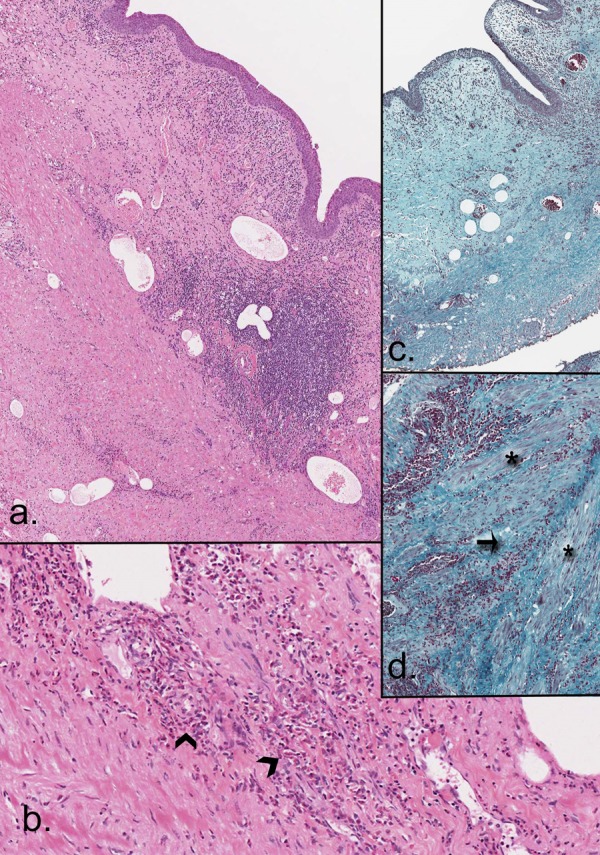
(A) H&E demonstrating inflammation in the lamina propria and between layers of the muscularis; (B) H&E showing high numbers of eosinophils (arrowheads); (C and D) Masson's Trichrome stains showing fibrosis and inflammation (arrow) separating smooth muscle fibres (asterisk) of the muscularis propria.
An investigation of note, the strongyloides serology, returned positive. She denied ever being previously diagnosed with this and was not immunocompromised.
Treatment
Upon the advice of the urologist she was started on 100 mg of intravesical hydrocortisone daily in addition to 10 mg of oral loratadine, a prospective management plan as seen in the Van den Ouden review. The hydrocortisone was injected in the bladder through the urinary catheter.
The positive strongyloides serology was treated with a 1-week course of ivermectin. A repeat culture of the urine grew a multiresistant E coli and a further 7-day course of meropenem was completed. She continued with steroid and antihistamine therapy for 4 weeks.
Outcome and follow-up
Functionally in the first few weeks of starting treatment her bladder appeared not to be functioning, with bladder scans showing regularly in excess of 400 mL. Despite few weeks of treatment, thoughts were that her bladder function may remain atonic and that she may have to self-catheterise indefinitely. However, given that her diabetes was not poorly controlled and still on oral hypoglycaemic agents, it was unlikely that she had an atonic bladder secondary to diabetic autoneuropathy. It was decided to persist with the intravesical steroid and antihistamine for an additional 2 weeks, with the hope that her bladder function would eventually return. Within 2 weeks, her bladder function returned, with postvoid residual volumes of less than 20 mL. The intravesical steroid and the antihistamine were ceased, and the patient was subsequently discharged.
A flexible cystoscopy was repeated 4 weeks before the start of the treatment, to observe for response to treatment. The bladder mucosa appeared normal with a vast improvement in comparison to the initial cystoscopy though no repeat biopsies were taken. Upon the recommendation of the urologist, she was to receive a further 2 weeks of intravesical hydrocortisone and oral loratadine.
Her strongyloides serology was unfortunately not repeated after the start of the ivermectin to assess response to treatment.
Repeat ultrasound of the renal tract at an outpatient appointment 6 months later showed resolution of the bilateral hydronephrosis, with a much thinner bladder wall.
However, within a year following her discharge back home, her symptoms had returned. She was admitted to hospital with acute on chronic renal failure and with bladder residual volumes again in excess of 200 mL. She was seen by the urologists and it appeared that her cystitis had not completely resolved. There was some persisting thickening of the bladder wall on ultrasound but not as bad as previously seen.
It appears that the eosinophilic cystitis was initially treated with successful functional outcome (not requiring intermittent self-catheterisation) but within a year, similar symptoms reoccurred. The biopsy showed a mild chronic cystitis with scattered eosinophils, without histologically diagnostic features of eosinophilic cystitis. She subsequently was restarted on intermittent self-catheterisation and remains on it for the time being. On her representation, she was not treated with intravesical steroids and an antihistamine.
This raises the question whether prior to her initial presentation she had pre-existing chronic cystitis and developed a superseding eosinophilic infection. It appeared that the supposed eosinophilic cystitis responded to the intravesical steroid and oral antihistamine treatment, as shown by the minimal presence of eosinophils on subsequent biopsy 1 year later. However, her ongoing chronic cystitis and urinary retention remains a topic of discussion among the clinicians.
Discussion
The diagnosis of eosinophilic cystitis is a histological one and requires strong clinical suspicion.
The aetiology of eosinophilic cystitis is uncertain. Of 83 cases reviewed by Itano and Malek6 in 2001, 29% had no underlying cause identified. Of those with putative causative factors, 25% had confirmed transitional cell carcinoma (TCC) of the bladder and 16% had allergic respiratory disease (either asthma or allergic rhinitis). The remaining had either bladder outlet obstruction, autoimmune disease, non-urological parasitosis or eosinophilic enteritis. Van den Ouden's pooled analysis of 135 cases found peripheral eosinophilia and allergies present in 38%, bladder injury in the form of surgery, recurrent UTI or intravesical mitomycin C in 36.5% and the medication tranilast, an antiasthmatic used only in Japan and South Korea, in 7.5%.2 In this case, the presence of strongyloides was the most likely causative factor. In Van den Ouden's review, four cases of parasitic infections were suggested as potential aetiological factors for the development of eosinophilic cystitis, though strongyloides itself was not specifically mentioned as the causative parasite. No other parasite infections and their geographical locations were described in this review.
Where the causative antigen is clearly identified and easily avoided, that is, tranilast, cure rates are excellent (96%) with avoidance of the antigen alone.2 Treatment of underlying infection and/or immune suppression has had variable success.2 6 7 Owing to the rareness of eosinophilic cystitis, gold standard guidelines for its management have not yet been established. Van den Ouden's pooled analysis from 2000 appears to be one of the reviews to date that has provided information on the different management plans implemented by clinicians worldwide. Of the treatment plans reviewed, not one particular method stood above the other in terms of clinical statistical significance. Of the 135 cases examined by Van den Ouden, 54 were treated with oral therapy in the form of corticosteroids alone or in combination with antihistamines and/or antibiotics, non-steroidal anti-inflammatory drugs (NSAIDs), azathioprine or cyclophosphamide. A 66% cure, defined as complete absence of symptoms and long-term seqeulea, was achieved.2 There certainly appears to be a role for the use of intravesical steroids in eosinophilic cystitis as described by Van den Ouden, however, no definitive studies have been conducted. The use of corticosteroids is thought to suppress the inflammatory reaction in the bladder and inhibit the formation of immune complexes.8 Presumably the topical application of steroids would confer the benefit of direct action on the lesions as well as avoiding the need for systemic use of steroids. However, further research is required.
Where symptoms or coexisting disease prompted surgical intervention (33 cases), a 70% cure rate was achieved. The surgical treatments undertaken included transurethral resection of the bladder lesions with or without oral therapy, exploratory laparotomy, partial and/or total cystectomy or augmentation ileocystoplasty.2 9–11
Success rates for the different treatments were variable, with transurethral resection of the bladder lesions combined with corticosteroids; antihistamines or antibiotics (if a concurrent urinary tract infection is present) seemed most successful.
Learning points.
Eosinophilic cystitis is an extremely rare condition with less than 150 cases reported worldwide.
The exact aetiology of eosinophilic cystitis is unknown but parasitic infections should be considered as a possible cause and treat accordingly.
Given the high incidence of transitional cell carcinoma of the bladder in eosinophilic cystitis, its exclusion is imperative.
Many treatment strategies have been implemented including corticosteroids, non-steroidal anti-inflammatory drugs, antihistamines, antibiotics and even surgery.
Consider the use of intravesical steroids in combination with oral antihistamines for the successful treatment of diagnosed eosinophilic cystitis.
Footnotes
Contributors: All authors have contributed significantly towards the preparation of the case report.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Brown EW. Eosinophilic granuloma of the bladder. J Urol 1960;2013:665–8 [DOI] [PubMed] [Google Scholar]
- 2.Van den Ouden D. Diagnosis and management of eosinophilic cystitis: a pooled analysis of 135 cases. Eur Urol 2000;2013:386–94 [DOI] [PubMed] [Google Scholar]
- 3.Popescu OE, Landas SK, Haas GP. The spectrum of eosinophilic cystitis in males. Arch Pathol Lab Med 2009;2013:289–94 [DOI] [PubMed] [Google Scholar]
- 4.Yamada T, Taguchi H. Clinical study of interstitial cystitis: I-(1) the etiological consideration of 4 cases of interstitial cystitis with advanced contracted bladder. Nippon Hinyokika Gakkai Zasshi 1984;2013:638–45 [PubMed] [Google Scholar]
- 5.Yamada T, Taguchi H. Clinical study of interstitial cystitis: I-(1) the etiological consideration of 4 cases of interstitial cystitis with advanced contracted bladder. Hinyokika Gakkai Zasshi 1984;2013:795–801 [PubMed] [Google Scholar]
- 6.Itano NMB, Malek RS. Eosinophilic cystitis in adults. J Urol 2001;2013:805–7 [PubMed] [Google Scholar]
- 7.Van den Ouden D, Van Kaam N, Eland D. Eosinophilic cystitis presenting as urinary retention. Urol Int 2001;2013:22–6 [DOI] [PubMed] [Google Scholar]
- 8.Littleton RH, Farah RN, Cerny JC. Eosinophilic cystitis: an uncommon form of cystitis. J Urol 1982;2013:132–3 [DOI] [PubMed] [Google Scholar]
- 9.Sidh SM, Smith SP, Siber SB, et al. Eosinophilic cystitis: advanced disease requiring surgical intervention. Urology 1980;2013:23–6 [DOI] [PubMed] [Google Scholar]
- 10.Oh SJ, Chi JG, Lee SE. Eosinophilic cystitis caused by vesical sparganosis: a case report. J Urol 1993;2013:581–3 [DOI] [PubMed] [Google Scholar]
- 11.Cardini S, Smulevich E, Salvadori A, et al. Augmentation ileocystoplasty in a case of eosinophilic cystitis. Minerva Urol Nefrol 1997;2013:219–23 [PubMed] [Google Scholar]


