Abstract
Thymosin beta 4 (Tβ4) and thymosin beta 10 (Tβ10) are two members of the β-thymosin family, involved in multiple cellular activities in different organs in multiple animal species. Here we report the expression pattern of Tβ4 and Tβ10 in rat tissues, in the gut and in annexed glands. The two peptide were differently expressed: Tβ4 was absent in salivary glands whereas Tβ10 was expressed in parotid and in submandibular glands. Tβ4 was mildly expressed in the tongue and in the esophagus, where Tβ10 was absent. A similar expression was found in the stomach, ileum and colon mucosa. In pancreas Tβ4 reactivity was restricted to the Langerhans islet cells; Tβ4 was also detected in the exocrine cells. Both peptide were not expressed in liver cells. When the rat expression pattern in rat organs was compared to reactivity for Tβ4 and Tβ10 in humans, marked differences were found. Our data clearly indicate a species-specific expression of Tβ4 and Tβ10, characterized by the actual unpredictability of the expression of these peptides in different cells and tissues. The common high expression of Tβ4 in mast cells, both in humans and in rats, represents one of the few similarities between these two species.
Key words: rats, thymosin beta 4, thymosin beta 10, immunohistochemistry, gastrointestinal tract
Introduction
Beta thymosins are a versatile family of small peptides expressed in multiple tissues in mammals, that show many intracellular and extracellular activities.1,2 These peptides are named thymosins after their first isolation in the calf thymus.3 Fifteen highly homologous beta thymosin variants, containing 40 to 44 amino acid residues, have been described.4 Among beta thymosins, thymosin beta 4 (Tβ4)5,6 and thymosin beta 10 (Tβ10)7 are the most abundant in human cells and rat tissues.8 During the years, beta thymosins have been detected inside of cells of different organs,9-14 as well as in human blood,15 in human saliva,16 in tears17 and in wound fluid after abdominal surgery.18 Many physiological properties and cellular functions are connected to Tβ4: Gactin-sequestering,1 promotion of cell migration,8 angiogenesis,9,19 stem cell differentiation,11 modulation of cytokines and chemokines.20 This peptide is also involved in lesion-induced neuroplasticity through microglia upregulation and it participates in the growth of neuronal processes.21 Therefore, the mRNA encoding for Tβ4 is expressed in mouse embryonic stem cells and in mesodermal-like cells (cardiac and skeletal muscle).22 Moreover, recent studies demonstrated a role of Tβ4 in inducing the expression of the vascular endothelial growth factor (VEGF) in colon cancer cells in experimental models.19 Tβ4 also partecipates to the modulation of human colonic immune system23 probably through degranulation of mucosal mast cells.24 Contrasting results have been published on the role of Tβ10 in tumour progression. On one hand, Tβ10 diminishes tumor growth, angiogenesis and proliferation25 and Tβ10 over-expression has been related to the increase of apoptosis in human ovarian cancer cells.25 On the other hand, Tβ10 has been associated to the progression of papillary thyroid carcinoma26 and over-expression of the peptide has been observed in non-small cell lung cancer27 and in pancreatic cancer. 28 The recent report by our group of a strong expression for Tβ10 in the human salivary glands during development,29 induced us to better analyse the protein expression pattern for Tβ10 in the oro-gastro-intestinal tract in adult rats, in order to show if Tβ10 is expressed in the gastrointestinal tract in adulthood, and to compare the expression of this peptide with that reported in the human digestive tract and in annexed glands.24,30,31 Moreover, rat tissues were immunostained for Tβ4, with the aim of verifying the reciprocal interactions of Tβ4 and Tβ10 in the oro-gastrointestinal tract.
Materials and Methods
In order to test Tβ4 and Tβ10 immunoreactivity in animals, 20 male wistar rats were the object of our study, divided into 10 male and 10 female. Tissues were obtained from each animal including samples from tongue, esophagus, stomach, ileum, colon, parotid gland, sub-mandibular gland, sublingual gland, liver and pancreas. All samples were fixed in 10% formalin, paraffin-embedded and routinely processed. Paraffin sections were immunostained with anti-Tβ4 and anti-Tβ10 antibodies, using the labeled streptavidin-biotin complex system (LSAB2, Dako, Cytomation, Carpinteria, CA, USA) in a Dako Autostainer (Dako). Briefly, samples were deparaffinized, rehydrated, and endogenous peroxidase activity was quenced (30 min) by 0.3% hydrogen peroxide in methanol. Slides were then subjected to heat-induced antigen retrieval by steaming unstained sections in a Target Retrieval Solution (Dako TRS pH 6.1) for 30 min. Slides were then incubated with 10% normal goat serum in phosphate-buffered saline (PBS) for 60 min to block non-specific binding, followed by incubation (60 min at room temperature) with a monoclonal anti-Thymosin Beta 4 antibody (Bachem-Peninsula Lab, San Carlos, CA, USA) and with a monoclonal anti-Thymosin Beta 10, respectively diluted 1:600 and 1:500 in the blocking solution. Slides were extensively washed with PBS containing 0.01% Triton X-100 and incubated with a secondary reagent (En Vision kit) according with the manufacturer instructions (Dako, Glostrup, Denmark). Diaminobenzidine (DAB) was used as chromogen. After additional washes, colour was developed using the AEC reagent (Dako), sections were counterstained with Mayer’s hematoxylin and mounted. Sections of reactive lymph nodes with Tβ4-immunoreactive histiocytes were utilized as a positive control. As a negative control, the same procedure was applied omitting the primary antibody.
Results
Parotid
Tβ4 immunoreactivity was completely absent both in acini and in ducts (Figure 1a). Tβ10 was expressed both in acinar serous cells, showing a granular pattern, as well as in ductal cells, in which a homogeneous cytoplasmic staining was detected (Figure 1b).
Figure 1.
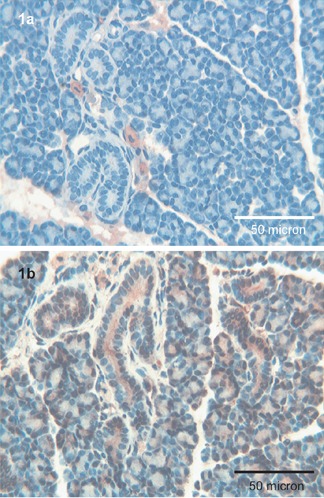
Parotid. a) No immunoreactivity for Tβ4 is detected in the parotid gland; scattered mast cells are strongly immunoreactive for the peptide; OM 250x. b) A granular and diffuse positivity for Tβ10 is observed in all the acinar structures; ductal cells show an apical and homogeneous reactivity for the peptide; OM 400x.
Submandibular gland
Fine Tβ4-immunoreactive granules were observed in the periglandular stroma and in scattered mast cells, in the absence of any reactivity inside the salivary gland cells (Figure 2a). A strong reactivity for Tβ10 was observed in acinar serous cells, appearing as coarse granules and in mucous cells appearing as fine granules. The ducts show a homogeneous cytoplasmic staining and intraluminal granular deposits (Figure 2b).
Figure 2.
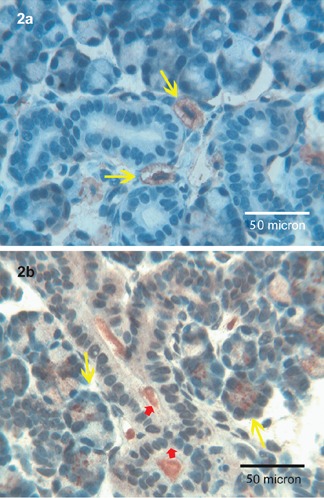
Submandibular glands. a) Tβ4 is not expressed in the structures of the submandibular glands; only a fine positivity could be detectable in the surrounding stroma; mast cells show a strong granular cytoplasmic positivity (yellow arrows); OM 400x. b) Coarse granules of Tβ10 are observed in the acini of the sub-mandibular gland (yellow arrows); ductal cells present a fine cytoplasmic immunoreactivity for the peptide mainly localized in the lumen (red arrows); OM 400x.
Sublingual glands
No reactivity was detected for Tβ4 and for Tβ10.
Tongue
A mild immunoreactivity for Tβ4 was detected in the superficial layers of the stratified epithelium. Moreover, a weak immunostaining for the peptide was observed in muscle cells, mainly localized at the cell membrane (Figure 3). No reactivity for Tβ10 was found.
Figure 3.
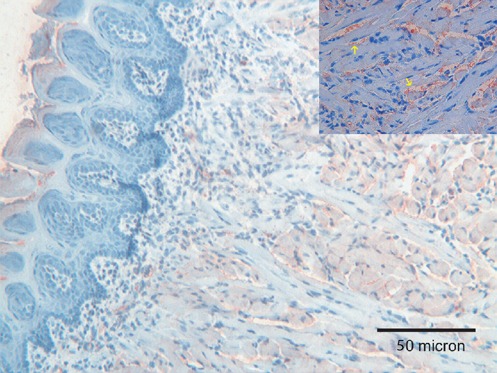
Tongue. A weak immunoreactivity for Tβ4 is observed only in the superficial layers of the tongue’s epithelium; a weak immunostaining for the peptide was observed in cell membranes of muscle cells (see inset, yellow arrows); OM 250x.
Esophagus
Scattered Tβ4-immunoreactive granules were detected inside the esophageal lumen. No reactivity for Tβ10 was observed.
Stomach
Immunoreactivity for Tβ4 was restricted to scattered foveolar cells and to intraluminal granular deposits (Figure 4a). A similar expression pattern was observed for Tβ10 (Figure 4b).
Figure 4.
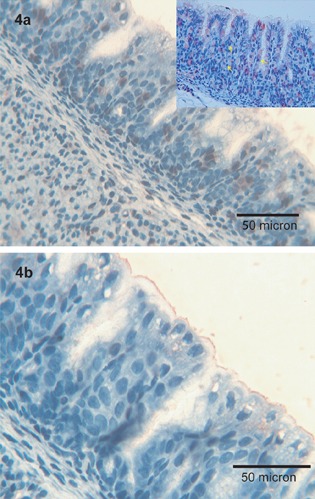
Stomach. a) Immunoreactivity for Tβ4 is expressed in scattered foveolar cells (see inset, yellow arrows) and in the luminal surface of the stomach; OM 400x. b) Tβ10 is mainly localized in the luminal border of the foveolar cells of the stomach; OM 250x.
Ileum
Tβ4 was maily expressed in the cytoplasm of enterocytes covering ileal villi (Figure 5a). A similar pattern characterized immunoreactivity for Tβ10 (Figure 5b).
Figure 5.
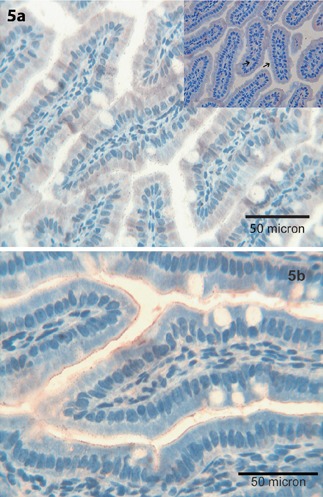
Ileum. a,b) Tβ4 and Tβ10 are detected in the surface epithelium of enterocytes covering the villi of the ileum (see inset, black arrows); OM 400x.
Colon
Tβ4 was maily expressed at the apical pole of enterocytes, and in fine granular deposits inside the intestinal lumen (Figure 6a), paralleling the expression pattern observed for Tβ10 (Figure 6b).
Figure 6.
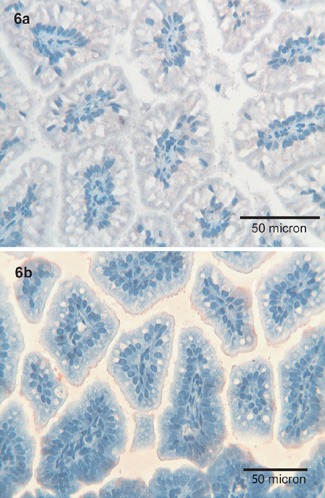
Colon. a,b) A fine granular positivity for Tβ4 is observed in the brush border of the enterocytes in the surface epithelium of the colon and in the intestinal lumen; Tβ10 parallels the immunoreactivity of Tβ4; OM 400x.
Pancreas
Tβ4 immunoreactivity was restricted to Langherans islets, in the absence of any significant immunostaining in the esocrine pancreas (Figure 7a). On the contrary, Tβ10 was detected both in the exocrine and in the endocrine pancreas. Endocrine cells of the Langherans islets showed a strong cytoplasmic reactivity, whereas in acinar cells Tβ10 was mainly detected in granular deposits. No immunostaining was found in ductal cells nor inside the tubular lumen (Figure 7b).
Figure 7.
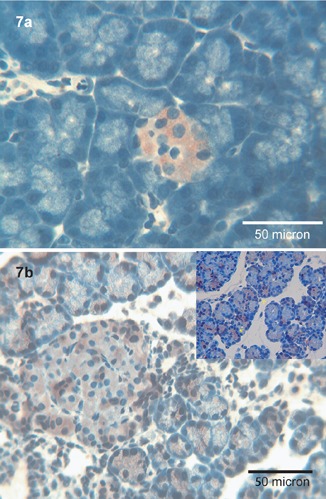
Pancreas. a) Tβ4 is diffusely immunoexpressed in the islets of Langherans; no immunoreactivity is observed in the rest of pancreatic parenchima; OM 400x. b) Immunoreactivity for Tβ10 is localized both in islets of Langherans and in the acini (see inset); the latest show a fine granular positivity in the absence of ductal reactivity; OM 400x.
Liver
No reactivity for Tβ4 and Tβ10 was observed in the liver samples. Data regarding immunoreactivity for both thymosins in the different rat organs are summarized in Table 1.
Table 1.
Immunoreactivity for thymosin beta 4 (Tβ4) and thymosin beta 10 (Tβ10) in rat tissues and humans.
| Organ | Tβ4 Rat | Tβ10 Rat | Tβ4 Human | Tβ10 Human |
|---|---|---|---|---|
| Parothyd | - | +++ | + | + |
| Submandibular gland | - | +++ | + | + |
| Sublingual gland | - | - | + | + |
| Tongue | + | - | + | ++ |
| Oesofagus | - | - | + | - |
| Stomach | + | + | + | - |
| Ileum | + | + | + | - |
| Colon | + | + | ++ | - |
| Pancreas | + | ++ | ++ | + |
| Liver | - | - | +++ | +++ |
Discussion
The role of Tβ4 and Tβ10, the beta-thymosins expressed virtually in all mammalian tissues and cells, has not been completely clarified yet. Previous studies on their expression in the rat central nervous system evidenced that temporal and cellular patterns of their expression are different, suggesting that each beta-thymosin could play a specific physiological function during development and in adulthood.32 Our study confirms the existence of marked differences in the distribution of Tβ4 and Tβ10 in the oro-gastro-intestinal tract of the adult rat. The most striking differences were found in the parotid and submandibular glands, in which Tβ4 was absent whereas immunostaining for Tβ10 was strong and diffuse. On the contrary, no reactivity for both beta-thymosins was detected in sublingual glands. These differences in beta-thymosin expression between different salivary glands confirm that each beta-thymosin probably plays a specific role in each salivary gland, irrespectively of their common embryogenesis.
No significant difference in Tβ4 and Tβ10 expression was present in the gastrointestinal tract: the absence of reactivity for both beta-thymosin in the oesofagus contrasts with the presence of both peptides in the enterocytes of the remaining gastrointestinal tract, confirming that a patchy distribution of these peptides should be expected, even in different parts of the same system.
The peculiar pattern for beta thymosins detected in pancreas deserves some considerations: Tβ10 was strongly expressed both in the exocrine and in the endocrine cells, whereas Tβ4 reactivity was restricted to the Langerhans islet cells. These findings taken together clearly indicate the presence of a complex modulation in the expression of beta-thymosins inside the same organ, each thymosin playing different functions in different cell types, confirming the β-thymosin enigma.33
When data obtained in rat tissues were compared with immunoreactivity for Tβ4 and Tβ10 in human tissues, significant differences were evidenced, supporting the hypothesis that beta-thymosin expression in cells and tissues is species-specific. The most striking differences were found in liver specimens, characterized by the complete absence of both thymosins in rat, contrasting with the strong and diffuse reactivity for Tβ4 and Tβ10 previously reported in humans.34 However, which is the role of Tβ4 and Tβ10 in adult rat tissues? The detailed mechanisms of the action of Tβ4 and Tβ10 in different mammalian cells and tissues are not fully understood, as well as the similarities and differences between these isoforms.35 Previous studies evidenced that activities of these two beta-thymosins are paradoxically different, Tβ4 promoting cell migration and angiogenesis, and Tβ10 inhibiting angiogenesis.35 Opposing effects on angiogenesis are likely to be mediated via Tβ4 stimulation and Tβ10 inhibition of VEGF production.36
Moreover, in contrast to Tβ4, Tβ10 has been shown to be a negative regulator of tumor development and progression.37 In short, Tβ4 might promote cell survival by blocking apoptosis,38 whereas Tβ10 might exert a proapoptotic activity, by accelerating apoptotic cell death.39 According with these data, we may speculate that the complex and pleiotropic expression of Tβ4 and Tβ10 here reported in different tissues and cell types might reflect different direct and indirect effects on the actin cytoskeleton, as well as modulation of signaling pathways that impact on different cellular functions. In particular, over-expression of Tβ4 could be related to prevention of apoptosis by blocking early apoptotic signals,40 whereas overexpression of Tβ10 might be related to its pro-apoptotic activity and to a down-regulation of cell growth35 and of angiogenesis.36
Finally, this study, one of the few in which the immunohistochemical expression pattern of Tβ4 and Tβ10 has been paralleled in the same tissues, evidenced that detailed mechanisms of the action of beta-thymosins in different cells and tissues are not fully understood, and show that our lack of knowledge is particularly evident regarding mature adult tissues, the vast majority of studies on the role of beta-thymosins having been carried out in fetal or tumoral tissues. Further studies exploring the molecular events that are associated with Tβ4 and Tβ10 overexpression or down-regulation are required, in order to give a solution to the thymosin enigma.
Acknowledgments
This work has been supported by “Fondazione Banco di Sardegna”. The authors would like to thank Mr. Ignazio Ferru for the secretarial assistance. The authors also gratefully acknowledge the Sardinia Regional Government for the financial support (P.O.R. Sardegna F.S.E. Operational Programme of the Autonomous Region of Sardinia, European Social Fund 2007-2013 - Axis IV Human Resources, Objective l.3, Line of Activity l.3.1 Avviso di chiamata per il finanziamento di Assegni di Ricerca).
References
- 1.Hannappel E. β-Thymosins. Ann NY Acad Sci 2007;1112:21-37 [DOI] [PubMed] [Google Scholar]
- 2.Mannherz HG, Hannappel E. The β-thymosins: intracellular and extracellular activities of a versatile actin binding protein family. Cell Motil Cytoskeleton 2009;66:839-51 [DOI] [PubMed] [Google Scholar]
- 3.Klein JJ, Goldstein AL, White A. Enhancement of in vivo incorporation of labeled precursors into DNA and total protein of mouse lymph nodes after administration of thymic extracts. Proc Natl Acad Sci USA 1965;53:812-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hannappel E, Davoust S, Horecker BL. Isolation of peptides from calf thymus. Biochem Biophys Res Commun. 1982;104:266-71 [DOI] [PubMed] [Google Scholar]
- 5.Low TL, Goldstein AL. Chemical characterization of thymosin β4. J Biol Chem 1982;257:1000-6 [PubMed] [Google Scholar]
- 6.Hannappel E, Xu GJ, Morgan J, Hemstead J, Horecker BL. Thymosin β4: a ubiquitous peptide in rat and mouse tissues. Proc Natl Acad Sci USA 1982;79:2172-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ericksson-Viitanen S, Ruggieri S, Natalini P, Horecker BL. Thymosin Beta 10, a new analogue of thymosin beta 4 in mammalian tissues. Arch Biochem Biophys 1983;225:407-13 [DOI] [PubMed] [Google Scholar]
- 8.Yu FX, Lin SC, Morrison-Bogoard M, Atkinson MA, Yin HL. Thymosin beta 10 and thymosin beta 4 both actin sequestering proteins. J Biol Chem 1993;268:502-9 [PubMed] [Google Scholar]
- 9.Philp D, Goldstein AL, Kleinman HK. Thymosin Tβ4 promotes angiogenesis, wound healing, and hair follicle development. Mech Ageing Dev 2004;125:113-5 [DOI] [PubMed] [Google Scholar]
- 10.Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin Tβ4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature 2004;432:466-72 [DOI] [PubMed] [Google Scholar]
- 11.Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, et al. Thymosin Tβ4 induces adult epicardial progenitor mobilization and neovascularization. Nature 2007;445:177-82 [DOI] [PubMed] [Google Scholar]
- 12.Hall AK. Differential expression of thymosin genes in human tumors and in developing human kidney. Int J Cancer 1991;48:672-7 [DOI] [PubMed] [Google Scholar]
- 13.Sun W, Kim H. Neurotrophic roles of the beta-thymosins in the development and regeneration of the nervous system. Ann NY Acad Sci 2007;1112:210-8 [DOI] [PubMed] [Google Scholar]
- 14.Nemolato S, Cabras T, Fanari MU, Cau F, Fanni D, Gerosa C, et al. Immunoreactivity of Thymosin beta 4 in human foetal and adult genitourinary tract. Eur J Histochem 2010;54:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naylor PH, McClure JE, Spangelo BL, Low TL, Goldstein AL. Immunochemical studies on thymosin: radioimmunoassay of thymosin beta 4. Immunopharmacology 1984;7:9-16 [DOI] [PubMed] [Google Scholar]
- 16.Inzitari R, Cabras T, Pisano E, Fanali C, Manconi B, Scarano E, et al. HPLC-ESI-MS analysis of oral human fluid reveals that gingival crevicular fluidi s the main source of oral thymosin Tβ4 and β10. J Sep Sci 2009;32:57-63 [DOI] [PubMed] [Google Scholar]
- 17.Badamchian M, Fagarasan MO, Danner RL, Suffredini AF, Damavandy H, Goldstein AL. Thymosin beta(4) reduces lethality and down regulates inflammatory mediators in endotoxin-induced septic shock. Int Immunopharmacol 2003;3:1225-33 [DOI] [PubMed] [Google Scholar]
- 18.Bodendorf S, Born G, Hannappel E. Determination of thymosin beta4 and protein in human wound fluid after abdominal surgery. Ann NY Acad Sci 2007;1112:418-24 [DOI] [PubMed] [Google Scholar]
- 19.Jo JO, Kim SR, Bae MK, Kang YJ, Ock MS, Kleinman HK, et al. Thymosin β4 induces the expression of vascular endothelial growth factor (VEGF) in a hypoxiainducible factor (HIF)-1 -dependent manner. Biochim Biophys Acta 2010;1803:1244-51 [DOI] [PubMed] [Google Scholar]
- 20.Crockford D, Turjman N, Allan C, Angel J. Thymosin beta4: structure, function, and biological properties supporting current and future clinical applications. Ann NY Acad Sci 2010;1194:179-89 [DOI] [PubMed] [Google Scholar]
- 21.Paulussen M, Landuyt B, Schoofs L, Luyten W, Arckens L. Thymosin beta 4 mRNA and peptide expression in phagocytic cells of different mouse tissues. Peptides 2009;30:1822-32 [DOI] [PubMed] [Google Scholar]
- 22.Gómez-Márquez J, Franco del Amo F, Carpintero P, Anadón R. High levels of mouse thymosin beta4 mRNA in differentiating P19 embryonic cells and during development of cardiovascular tissues. Biochim Biophys Acta 1996;1306:187-93 [DOI] [PubMed] [Google Scholar]
- 23.Elitsur Y, Mutchnick MG, Sakr WA, Luk GD. Thymosin alpha 1 and thymosin beta 4 modulate human colonic lamina propria lymphocyte function. Immunopharmacology 1990;20:89-96 [DOI] [PubMed] [Google Scholar]
- 24.Nemolato S, Cabras T, Fanari MU, Cau F, Fraschini M, Manconi B, et al. Thymosin beta 4 expression in normal skin, colon mucosa and in tumor infiltrating mast cells. Eur J Histochem 2010;54:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim YC, Kim BG, Lee JH. Thymosin β10 expression driven by the human TERT promoter induces ovarian cancer-specific apoptosis through ROS production. PLoS One 2012;7:e35399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fehér LZ, Pocsay G, Krenács L, Zvara A, Bagdi E, Pocsay R, et al. Amplification of thymosin beta 10 and AKAP13 genes in metastatic and aggressive papillary thyroid carcinomas. Pathol Oncol Res 2012;18:449-58 [DOI] [PubMed] [Google Scholar]
- 27.Lee SM, Na YK, Hong HS, Jang EJ, Yoon GS, Park JY, et al. Hypomethylation of the thymosin β(10) gene is not associated with its overexpression in non-small cell lung cancer. Mol Cells 2011;32:343-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M, Zhang Y, Zhai Q, Feurino LW, Fisher WE, Chen C, et al. Thymosin beta-10 is aberrantly expressed in pancreatic cancer and induces JNK activation. Cancer Invest 2009;27:251-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fanni D, Gerosa C, Nemolato S, Locci A, Marinelli V, Cabras T, et al. Thymosin beta 10 expression in developing human salivary glands. Early Hum Dev 2011;87:779-83 [DOI] [PubMed] [Google Scholar]
- 30.Nemolato S, Messana I, Cabras T, Manconi B, Inzitari R, Fanali C, et al. Thymosin beta(4) and beta(10) levels in pre-term newborn oral cavity and foetal salivary glands evidence a switch of secretion during foetal development. PLoS One 2009;4:e5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemolato S, Cabras T, Cau F, Fanari MU, Fanni D, Manconi B, et al. Different thymosin Beta 4 immunoreactivity in foetal and adult gastrointestinal tract. PLoS One 2010;5:e9111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez-Marquez J, Anadon R. The beta-thymosins, small actin-binding peptides widely expressed in the developing and adult cerebellum. Cerebellum 2002;1:95-102 [DOI] [PubMed] [Google Scholar]
- 33.Sun HQ, Yin HL. The β-thymosin enigma. Ann NY Acad Sci 2007;1112:45-55 [DOI] [PubMed] [Google Scholar]
- 34.Nemolato S, Van Eyken P, Cabras T, Cau F, Fanari MU, Locci A, et al. Expression pattern of thymosin beta 4 in the adult human liver. Eur J Histochem 2011;55:e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sribenja S, Li M, Wongkham S, Wongkham C, Yao Q, Chen C. Advances in thymosin beta 10 research: differential expression, molecular mechanisms, and clinical implications in cancer and other conditions. Cancer Invest 2009;27:1016-22 [DOI] [PubMed] [Google Scholar]
- 36.Lee SH, Son MJ, Oh SH, Rho SB, Park K, Kim YJ, et al. Thymosin beta 10 inhibits angiogenesis and tumor growth by interfering with Ras function. Cancer Res 2005;65:137-48 [PubMed] [Google Scholar]
- 37.Freeman KW, Banyard J. Beta-thymosin in cancer: implications for the clinic. Future Oncol 2009;5:755-8 [DOI] [PubMed] [Google Scholar]
- 38.Sosne G, Qui P, Goldstein AL, Wheater M. Biological activities of thymosin beta4 defined by actives sites in short peptides sequences. FASEB J 2010;24:2144-51 [DOI] [PubMed] [Google Scholar]
- 39.Hall AK. Thymosin beta-10 accelerates apoptosis. Cell Mol Biol Res 1995;41:167-80 [PubMed] [Google Scholar]
- 40.Choi SY, Kim DK, Eun B, Kim K, Sun W, Kim H. Anti-apoptotic function of thymosin-beta in developing chick spinal motoneurons. Biochem Biophys Res Commun 2006;346:872-8 [DOI] [PubMed] [Google Scholar]


