Abstract
In this work we analysed, by immunohistochemistry, a series of brain tumors to detect the levels and cellular distribution of Hsp60 and Hsp70. We found that Hsp60 levels were significantly higher than those of Hsp70 in neuroepithelial tumors, while levels of both molecules were not significantly different from each other in meningeal neoplasms. In particular, Hsp60 immunopositivity was present mainly at the cytoplasmic level, while Hsp70 immunopositivity was found both in the cytoplasm and in the nucleus of tumor cells. The levels of these molecules in healthy control cells were always very low. Finally, Hsp60 and Hsp70 levels did not correlate with the different types (WHO grade) of neoplasm. Our results are partially in agreement with previous studies and suggest that Hsp60 is not increased by a passive phenomenon (e.g., due to the stress caused by the peritumor environment on cancer cells) but may be actively implicated in tumor progression, e.g. inhibiting tumor cell death or antitumor immune system response, as already postulated in vitro. We also briefly discuss the most recent publications on the extramitochondrial localization of Hsp60 in tumor cells and its role in tumor progression.
Key words: Hsp60, Hsp70, astrocytoma, glioblastoma multiformae, medulloblastoma, meningioma
Introduction
Primary brain tumors account for 2% of human neoplasms in adults and for 20% in pediatric patients.1 These tumors consist of a varied group of neoplasms which arise in the brain and its surrounding structures. The Word Health Organization (WHO) established a uniform terminology and grading system for brain tumors.2 Neuroepithelial tumors, the most prevalent group, include glioblastoma multiforme (GBM), medulloblastoma and astrocytoma, while tumors of the meninges include meningioma.
Astrocytomas are classified into four grades. Pylocitic astrocytoma (grade I) usually occurs in children or young adults, while astocytomas of other grades are typically diagnosed in adults.1,3 GBM is the most common and deadliest malignant primary brain tumor in adults, classified as grade IV astrocytoma. Medulloblastoma is the most common embryonal tumor (these are tumors that arise from embryonal or immature cells at the earliest stage of their development);3 it occurs in children and young adults. Meningiomas are tumors of meningothelial cells; they appear in childhood or adolescence, but most are diagnosed in middle or later adult life. Atypical meningiomas (grade II) show more cellularity, mitotic activity and foci of necrosis than grade I meningiomas.1,3
Heat shock proteins (Hsps) are a class of molecules highly conserved during evolution with crucial roles in tissue homeostasis.4 Due to their importance for cell survival and function, it has been postulated that they may be involved in the development of a number of human diseases, now called chaperonopathies.4 Some forms of tumors are now considered chaperonopathies by collaboration, since chaperones favor the tumor (e.g., by inhibiting tumor cell apoptosis and/or antitumor immune response) rather than the host organism.5-8 The presence and levels of various Hsps in human brain tumors have not been extensively investigated to date. We conducted a study on a subset of human brain neoplasms to compare the levels of two Hsps considered among the most important players during carcinogenesis.
Materials and Methods
Sixty brain tumors, including both neuroepithelial and meningeal neoplasms, classified accordingly to the WHO parameters, were retrospectively collected from files of the Pathologic Anatomy Unit of the Civico Hospital, Palermo, Italy. In particular, we randomly selected ten pilocytic astrocytomas [female:male (F:M) ratio = 1:4; age range: 4-17 yrs], ten grade II astrocytomas (F:M ratio = 1:1; age range: 27-84 yrs), ten glioblastoma multiformae (F:M ratio = 4:1; age range: 44-73 yrs), ten medulloblastomas (F:M ratio = 2:3; age range: 8-23 yrs), ten grade I meningiomas (F:M ratio = 4:1; age range: 41-76 yrs), and ten grade II meningiomas (F:M ratio = 1:1; age range: 59-77 yrs). Moreover, we selected five normal brain samples (F:M ratio = 1:1; age range: 4-85 yrs) from our files, and obtained slides showing normal glial cells, neurons and meningeal (pial) cells to be used as normal controls. All the specimens had been previously formalin-fixed and paraffin-embedded.
We obtained sections of a thickness of 4-5 µm from all specimens for histological re-evaluation and immunohistochemical testing. The histological re-evaluation was performed on haematoxylin-eosin stained slides. Immunostaining and statistic analysis was performed as already described.9 The primary antibodies used were anti-Hsp60 (mouse monoclonal antibody, dilution 1:400, Cat. No. H4149, Sigma Co., Saint Louis, MO, USA), and anti-Hsp70 (mouse monoclonal antibody, dilution 1:200, Cat. No. SC-24, Santa Cruz Biotechnology, Dallas, TX, USA). Appropriate positive controls, as well as non-immune serum for negative controls, were run concurrently. Immunostaining evaluations were performed by two blinded expert pathologists (FR, NS) and the percentage of positive tumor cells was calculated in ten random high power fields at a magnification of 400x.
Statistical analyses were performed as already described.10 Briefly, we used the Mann-Whitney U-test for comparison between groups and the Kruskal-Wallis test for multiple comparisons. Probability values of P<0.05 were considered significant.
Results and Discussion
Statistical analyses of the immunohistochemical experiments showed that Hsp60 levels were significantly higher P<0.01) than those of Hsp70 in neuroepithelial tumors (Figure 1), while levels of both molecules did not show any significant difference from each other in meningeal neoplasms (P>0.05). In particular, Hsp60 immunopositivity was present mainly at the cytoplasmic level, while Hsp70 immunopositivity was found both in the cytoplasm and nucleus of tumor cells (Figures 2 and 3). The levels of these molecules in the normal control cells were always very low (Figures 2 and 3, insets). Finally, the levels of Hsp60 and Hsp70 did not correlate with the different types (WHO grade) of neoplasm (data not shown).
Figure 1.
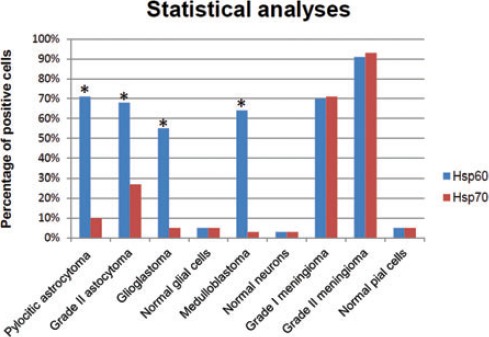
Statistical analysis of the immunohistochemistry results for Hsp60 and Hsp70 in brain tumors. The immunopositivity of Hsp60 was elevated in all brain tumor types studied, while Hsp70 immunopositivity was found to be very low in neuroepithelial tumors. In the latter, Hsp60 levels were significantly higher than those of Hsp70. In contrast, in meningiomas, the expression of both Hsp60 and Hsp70 was elevated, without significant differences between them. Finally, the expression of these proteins in healthy control cells was very low. Asterisks indicate statistical significant difference between Hsp60 and Hsp70 (P<0.01).
Figure 2.
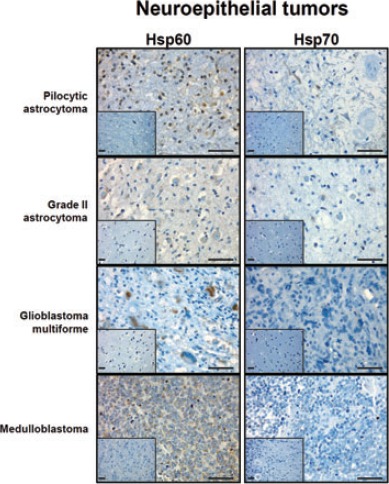
Representative pictures of immunohistochemical analyses for Hsp60 and Hsp70 in brain neuroepithelial tumours. Insets show normal control cell positivity. Scale bar: 50 µm.
Figure 3.
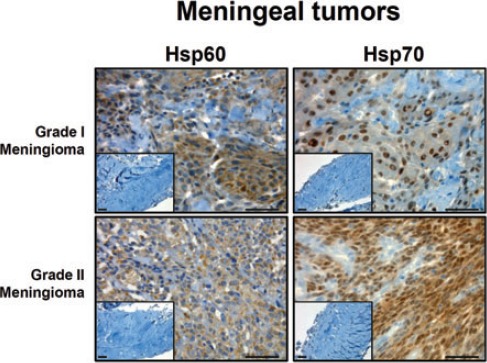
Representative pictures of immunohistochemical analyses for Hsp60 and Hsp70 in brain meningeal tumours. Insets show normal control cell positivity. Scale bar: 50 µm.
Our results are partially in agreement with previous studies;11-13 in particular, Kato and colleagues11 found Hsp60 immunopositivity in a number of brain tumors, while no significant immunohistochemical reactions were seen in sections of normal brain tissue. Batistatou et al.12 found Hsp70 presence in glial tumors, mainly in the cytosol, and described a statistically significant association of the cytosolic Hsp70 expression with a nuclear expression of the BCL2-associated Athano-Gene 1 (BAG1) protein. Finally, Hauser et al.13 described Hsp70, Hsp27 and Hsp90 positivity in medulloblastomas, with no association with tumor prognosis, indicating these proteins as a putative molecular target for anticancer therapy.
Hsp60 is classically considered a mitochondrial molecule, while Hsp70 is commonly present in the cytosol. The presence of a diffuse immunohistochemical positivity for Hsp60 in the cytoplasm, as well as a nuclear positivity for Hsp70, have already been described in other forms of solid tumors14-16 and have been correlated with tumor progression.15-18 The significant difference between Hsp60 and Hsp70 levels specific to neuroepithelial tumors may suggest that the former is not increased by a passive phenomenon (e.g., due to the stress caused by the peritumor environment on cancer cells) but it may instead be actively implicated in tumor progression, e.g. by inhibiting tumor cell death or favoring tumor progression by inhibiting the antitumor response of the immune system, as already postulated in vitro.5,8,19 Moreover, these data confirm that Hsp60 may have different levels and expression than Hsp70 in different neoplasms, including head and neck cancer,20 salivary gland tumors21 and esophageal adenocarcinomas.22 However, its participation in meningeal tumor pathogenesis needs to be further investigated also with the use other techniques like proteomics.23
Indeed, various studies using different techniques have found Hsp60 expression to be increased, decreased or unchanged in several tumors of different anatomical districts.16,24 Furthermore, an increase in Hsp60 expression may correlate either with a more favorable or a worse prognosis.16,17 From a molecular point of view, when increased Hsp60 accumulate in the cytosol where it may have either a pro- or an anti-apoptotic action, depending on whether it has been released by mitochondria or accumulated in the cytosol without mitochondrial import, respectively.25 For these reasons, Hsp60 has been proposed as the molecular Proteus of carcinogenesis.26 In addition, when accumulated in the cytosol, Hsp60 may transfer to the plasma membrane of tumor cells, in turn becoming internalized by lipid rafts in multivesicular bodies and secreted by exosomes.7,8 Finally, some anticancer drugs cause the release of exosomes loaded with Hsp60, which elicit effective natural killer cell antitumor responses, in vitro.27
In our opinion, we are not far from fully understanding the mechanisms by which Hsp60 participates in tumor progression. Molecular studies concerning post-translational modifications that cytosolic Hsp60 undergoes when accumulating in the cytosol of tumor cells could help to fully characterize this proteiform molecule.
References
- 1.McKeever PE. The brain, spinal cord, and meninges. In: Sternberg SS, Antonioli DA, Carter D, Mills SE, Oberman HA.(eds)Diagnostic surgical pathology. 3rd ed.Philadelphia: Lippincott Williams& Wilkins; 1999, p. 389-480 [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumors of the central nervous system. Acta Neuroplathol 2007;114:97-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenblum MK. Central nervous system. In: Rosai J. (ed)Rosai and Ackerman’s surgical pathology. 9th ed.Edinburgh: Mosby, 2004, p. 2461-622 [Google Scholar]
- 4.Macario AJ, Conway de Macario E. Sick chaperones, cellular stress, and disease. N Engl J Med 2005;353:1489-501 [DOI] [PubMed] [Google Scholar]
- 5.Campanella C, Bucchieri F, Ardizzone NM, Marino Gammazza A, Montalbano A, Ribbene A, et al. Upon oxidative stress, the antiapoptotic Hsp60/procaspase-3 complex persists in mucoepidermoid carcinoma cells. Eur J Histochem 2008;52:221-8 [DOI] [PubMed] [Google Scholar]
- 6.Cappello F, Ribbene A, Campanella C, Czarnecka AM, Anzalone R, Bucchieri F, et al. The value of immunohistochemical research on PCNA, p53 and heat shock proteins in prostate cancer management: a review. Eur J Histochem 2006;50:25-34 [PubMed] [Google Scholar]
- 7.Merendino AM, Bucchieri F, Campanella C, Marcianò V, Ribbene A, David S, et al. Hsp60 is actively secreted by human tumor cells. PLoS One 2010;5:e9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campanella C, Bucchieri F, Merendino AM, Fucarino A, Burgio G, Corona DF, et al. The odyssey of Hsp60 from tumor cells to other destinations includes plasma membraneassociated stages and Golgi and exosomal protein-trafficking modalities. PLoS One 2012;7:e42008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rappa F, Greco A, Podrini C, Cappello F, Foti M, Bourgoin L, et al. Immunopositivity for histone macroH2A1 isoforms marks steatosis-associated hepatocellular carcinoma. PLoS One 2013;8:e54458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cappello F, Di Stefano A, David S, Rappa F, Anzalone R, La Rocca G, et al. Hsp60 and Hsp10 down-regulation predicts bronchial epithelial carcinogenesis in smokers with chronic obstructive pulmonary disease. Cancer 2006;107:2417-24 [DOI] [PubMed] [Google Scholar]
- 11.Kato S, Kato M, Hirano A, Takikawa M, Ohama E. The immunohistochemical expression of stress-response protein (srp) 60 in human brain tumours: relationship of srp 60 to the other five srps, proliferating cell nuclear antigen and p53 protein. Histol Histopathol 2001;16:809-20 [DOI] [PubMed] [Google Scholar]
- 12.Batistatou A, Kyzas PA, Goussia A, Arkoumani E, Voulgaris S, Polyzoidis K, et al. Estrogen receptor beta (ERbeta) protein expression correlates with BAG-1 and prognosis in brain glial tumours. J Neurooncol 2006;77:17-23 [DOI] [PubMed] [Google Scholar]
- 13.Hauser P, Hanzély Z, Jakab Z, Oláh L, Szabó E, Jeney A, et al. Expression and prognostic examination of heat shock proteins (HSP 27, HSP 70, and HSP 90) in medulloblastoma. J Pediatr Hematol Oncol 2006;28:461-6 [DOI] [PubMed] [Google Scholar]
- 14.Macario AJL, Cappello F, Conway de Macario E. Chaperonopathies: Diseases in which mortalin and other Hsp-chaperones play a role in etiology and pathogenesis. In: Kaul S., Wadhwa R.(eds)Mortalin biology: life, stress and death. Springer Science, 2012, p. 209-21 [Google Scholar]
- 15.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005;10:86-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rappa F, Farina F, Zummo G, David S, Campanella C, Carini F, et al. HSP-molecular chaperones in cancer biogenesis and tumor therapy: an overview. Anticancer Res 2012;32):5139-50 [PubMed] [Google Scholar]
- 17.Cappello F, Czarnecka AM, La Rocca G, Di Stefano A, Zummo G, Macario AJ. Hsp60 and Hsp10 as antitumor molecular agents. Cancer Biol Ther 2007;6:487-9 [DOI] [PubMed] [Google Scholar]
- 18.Cappello F, David S, Peri G, Farina F, Conway de Macario E, Macario AJ, et al. Hsp60: molecular anatomy and role in colorectal cancer diagnosis and treatment. Front Biosci (Schol Ed) 2011;3:341-51 [DOI] [PubMed] [Google Scholar]
- 19.Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL, et al. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem 2012;287:15874-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiu CC, Lin CY, Lee LY, Chen YJ, Lu YC, Wang HM, et al. Molecular chaperones as a common set of proteins that regulate the invasion phenotype of head and neck cancer. Clin Cancer Res 2011;17:4629-41 [DOI] [PubMed] [Google Scholar]
- 21.Wang G, Gu X, Chen L, Wang Y, Cao B, E Q. Comparison of the expression of 5 heat shock proteins in benign and malignant salivary gland tumor tissues. Oncol Lett 2013;5:1363-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slotta-Huspenina J, Berg D, Bauer K, Wolff C, Malinowsky K, Bauer L, et al. Evidence of prognostic relevant expression profiles of heat-shock proteins and glucose-regulated proteins in oesophageal adenocarcinomas. PLoS One 2012;7:e 41420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen G, Liang S, Xu Z, Zhou L, Xiao S, Xia X, et al. Downregulated expression of HSP27 in human low-grade glioma tissues discovered by a quantitative proteomic analysis. Proteome Sci 2010;8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cappello F, Conway de Macario E, Marasà L, Zummo G, Macario AJ. Hsp60 expression, new locations, functions and perspectives for cancer diagnosis and therapy. Cancer Biol Ther 2008;7:801-9 [DOI] [PubMed] [Google Scholar]
- 25.Chandra D, Choy G, Tang DG. Cytosolic accumulation of HSP60 during apoptosis with or without apparent mitochondrial release: evidence that its pro-apoptotic or pro-survival functions involve differential interactions with caspase-3. J Biol Chem 2007;282:31289-301 [DOI] [PubMed] [Google Scholar]
- 26.Cappello F, Zummo G. HSP60 expression during carcinogenesis: a molecular “proteus” of carcinogenesis? Cell Stress Chaperones 2005;10:263-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL, et al. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem 2012;287:15874-85 [DOI] [PMC free article] [PubMed] [Google Scholar]


