Abstract
Cyclic shedding of the endometrium is unique to menstruating species. The status of the decidua in mouse menstrual-like models seems to differ from that of the predecidua in humans before endometrial breakdown. The aim of this study was to determine how this difference in decidual status is related to endometrial breakdown. A mouse menstruallike model was generated by pharmacological progesterone withdrawal. Histomorphological analysis and reticular fiber staining were used to evaluate endometrial status. In situ zymography was used to determine the localization of active collagenase and gelatinase. The functional endometrial layer containing the mature decidual-like zone (MDZ) and predecidual-like zone (PZ) underwent breakdown. The reticular fibers underwent disruption and fragmentation and became loose or disappeared at 12 h in the PZ, where active collagenase and gelatinase were limited. The reticular fibers were visibly reduced at 24 h in the MDZ, where active collagenase was detected. A few reticular fibers remained; however, the functional layer had sloughed into the lumen of the uterus. The results showed that reticular fibers of the PZ are actively degraded during endometrial shedding.
Key words: mouse menstrual-like model, predecidual-like zone, reticular fiber, gelatinase, collagenase
Introduction
During each menstrual cycle, human endometrium undergoes extensive breakdown and remodeling of the functional layer, regulated by estrogen and progesterone. Menstruation is unique to humans; the mechanism underlying menstruation has not yet been completely characterized, partly due to the lack of a suitable model.
The mouse menstrual-like model was first developed in 1984.1 Ovariectomized mice were prepared by sequential administration of estrogen and progesterone, and oil was injected into the uterine lumen to artificially induce decidualization. Subsequently, the process of progesterone withdrawal was mimicked, and the endometrium underwent breakdown and shedding. The model was optimized nearly 30 years later by Salamonsen.2 We have previously established a mouse menstrual-like model by pharmacological progesterone withdrawal3 with mifepristone administration and the mouse menstrual-like models have been used to study the mechanism underlying menstruation.1-6 The decidua is the foundation of menstrual induction in mice. In the mouse model, oil is injected into the uterus lumen to induce stromal decidualization. Specifically, the stroma cells undergo decidualization and predecidualization at the histomorphologic level. However, in humans, the stroma only undergoes predecidualization in the late secretory phase. The status of stroma cells before menstruation differs between mice and humans. It is unclear why the different status of decidua in mouse and human could both lead to endometrium breakdown. In the extracellular matrix (ECM) of the human endometrium, the reticular fibers may indirectly reflect endometrial status. Matrix metalloproteinases degrade ECM components. Understanding the process of endometrial breakdown might explain the differences in decidual status.
In this study, we observed the process of endometrial breakdown in a mouse menstruallike model and sought to understand the active role of the predecidual-like zone (PZ) in endometrial breakdown by assessing changes in the organization of reticular fibers and the localization of active collagenase and gelatinase in the process.
Materials and Methods
Animals
Female virgin C57 mice (age 8-12 weeks) were obtained from the Animal Services of the National Research Institute for Family Planning in China. The mice were kept under controlled lighting (light on from 06:00 to 18:00 h) and temperature (21±1°C) conditions and allowed free access to food and water. Experimental and surgical procedures were approved by the institute’s Animal Ethics Committee (approval ID: 20100318).
Mouse menstrual-like model
Animal manipulations were performed as described by Wang et al.3 Briefly, animals were ovariectomized under anesthesia and allowed to recover for 1 week (Figure 1). On days 1, 2, and 3 after recovery, all mice were subcutaneously (s.c.) injected with 100 ng 17 β-estradiol (E2; Alfa Aesar Inc., Heysham, UK) in arachis oil at 09:30 h. On day 7 after recovery, progesterone implants were inserted s.c. into the back of each mouse at 09:30 h, with simultaneous subcutaneous injections of 50 ng progesterone (Sigma-Aldrich, St. Louis, MO, USA) and 5 ng 17 β-E2 in arachis oil. On days 8 and 9, 5 ng 17 β-E2 in arachis oil was injected s.c. at 09:30 h. On day 9, at 11:30 h, 20 μL arachis oil was injected into the lumen of the left uterine horn of each mouse through a dorsal incision in order to induce decidualization. The right uterine horn of each mouse was untreated and served as a negative control. On day 11 (48 h later), 120 mg/kg mifepristone (Beijing Zizhu Pharmaceutical Co. Ltd., Beijing, China) was given to each mouse by intragastric administration at 11:30 h (regarded as 0 h). Mice were sacrificed by cervical dislocation at 0, 8, 12, 16, and 24 h (n=7/time point) after mifepristone administration, and the uterine horns were harvested at each time point. The mice were not treated by mifepristone and served as the positive control and were sacrificed at 24, 32 and 72 h. Any mouse in which the treated uterine horn had not decidualized, as assessed by gross morphological observation, was excluded.
Figure 1.
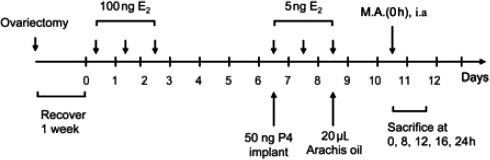
Treatment schedule. Mifepristone administration is regarded as 0 h. M.A., mifepristone administration; E2, estradiol; P4, progesterone
Each uterine horn was stored at alcohol fixative (1:3 v/v methanol:ethanol) for histomorphological examination, reticular fiber staining and in situ zymography. In situ zymography was performed as described by Gawlak et al., with modifications.7 Briefly, the specimens were immersed in alcohol fixative (1:3 v/v methanol:ethanol), stored at 4°C for 6 h, and then stored at -20°C overnight. The uteri were soaked in 90% and 100% ethanol at 4°C for 1 and 2 h, respectively, and then treated to several mixtures of ethanol and polyester wax (Science Services, Munich, Germany) with increasing concentrations of wax and by progressively increasing the temperature to 42°C (in pure wax). Then, the specimens were embedded in 100% polyester wax. Blocks were cut into 7-μm sections using a rotary microtome (RM 2235; Leica, Wetzlar, Germany).
In situ zymography
The sections (thickness, 7 μm) were gently dewaxed twice in absolute ethanol at room temperature, with each dewaxing step lasting 1 min. After dewaxing, the alcohol was removed and the sections were hydrated with phosphate-buffered saline (PBS), pH 7.4, at room temperature. We employed the in situ zymography approach described by Gawlak et al., together with modifications in order to detect the localization of ECM proteolytic activity (including gelatinase and collagenase) in endometrial sections.7 First, the sections were preincubated in PBS. Then, the slides were washed 3 times in PBS and overlaid with the fluorogenic substrate DQ gelatin (component A of the EnzChek gelatinase/collagenase assay kit; Invitrogen/Molecular Probes, Eugene, OR, USA) or DQ collagen type I (Invitrogen/Molecular Probes) diluted 1:25 in reaction buffer supplied by the manufacturer, for 40 min at 37°C, protected from light. All the slides were washed 3 times in PBS at room temperature and fixed in 4% paraformaldehyde for 10 min at room temperature. Next, the slides were mounted directly with Vectashield HardSet Mounting Medium (Vector, Burlingame, CA, USA) and observed under an LMD 6000 fluorescence microscope (Leica, Wetzlar, Germany). As specificity controls, we pretreated samples with 100 mM 1,10-phenanthroline (a general metalloproteinase inhibitor; component C of the EnzChek Gelatinase/Collagenase Assay Kit; Invitrogen/ Molecular Probes) in order to inactivate endogenous enzymes. To check for non-specific effects, the reactions were also performed in the presence of solvent alone.
Reticular fiber staining
The Gordon and Sweets’ staining method was used to observe the reticular structure of the endometrium.
Results
Histomorphological analysis and reticular fiber staining
Histomorphological analysis and reticular fiber staining clearly showed changes in the endometrium after progesterone withdrawal. Histomorphological analysis at 0 h (Figure 2 A1, B1), that is, at 49 h after arachis oil infusion, showed that the stromal cells in the endometrium decidualized well and formed different decidual zones. The decidual cells has large, round nuclei and abundant cytoplasm and were closely arranged in the subluminal stroma. In the decidualizing endometrium, vascularity is enhanced in the mature decidual-like zone (MDZ). The spindle-like or round stromal cells close to the MDZ were lined up closely and had mostly round nuclei containing large nucleoli. High vascularity was observed in the PZ. These zones formed the functional layer. The stromal cells between the PZ and muscle layer of the uterus were spindle shaped, closely arranged, and oriented parallel to the smooth muscle layer. No differentiation of stromal cells into decidual cells was observed in this zone, which was named the nondecidualized zone (NZ) and formed the basal layer. Reticular fibers were abundant and distributed throughout the decidual area spanning the PZ to the MDZ. They consisted of a dense mesh of regular, smooth, thick, and dark fibrils, which surrounded the decidual stromal cells (Figure 2C1). The reticular fibers in the MDZ and PZ differed in structure; in the MDZ, the reticular fibers appeared thick, while in the PZ, thick reticular fibers were interlaced with the fine ones (Figure 2 D1, E1, F1).
Figure 2.
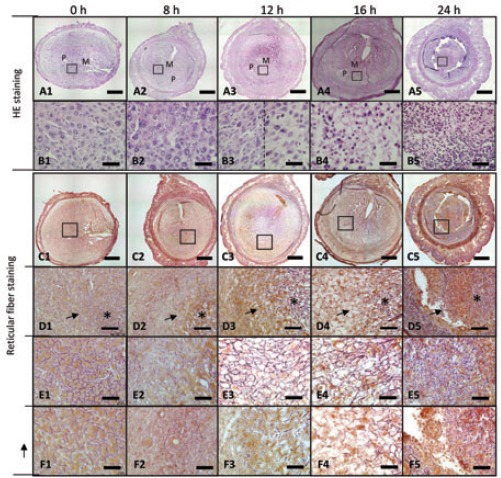
Histological observations of reticular fibers during endometrial breakdown in the mouse menstrual-like model. A1-A5) Representative histomorphological features at different time points during mouse endometrial breakdown; M, mature decidual-like zone (MDZ); P, predecidual-like zone; scale bars: 400 μm. B1-B5) higher magnification of the area indicated by the small blank boxes in A1-A5; scale bars: 50 μm. C1-C5) Reticular fiber staining; the small blank boxes indicates the PZ and MDZ, also shown at higher magnification, scale bars: 400 μm. D1-D5) The symbols (stars and arrows) represent the MDZ and PZ, respectively, and the indicated areas are shown at higher magnification (E1-E5, F1-F5); scale bars: 100 μm. E1-E5) Higher magnification of the areas indicated by the symbol * in D1-D5, showing the MDZ; scale bars: 50 μm. F1-F5) Higher magnification of the areas indicated by arrows in D1-D5, showing the PZ; scale bars: 50 μm.
Histomorphological analysis at 8 h (Figure 2 A2, B2) showed that decidualization occurred progressively, with the MDZ and PZ further expanding towards the NZ, and the boundary between the PZ and NZ becoming clear. In the MDZ, the stromal cells near the uterine lumen were large in size, with more brightly eosinophilic cytoplasm and round clear nuclei. No obvious changes were observed in the stromal cells of the PZ, with the exception of the appearance of some expanded blood capillaries with increased numbers of red blood cells. At 8 h, the reticular fibers showed characteristics similar to those seen at 0 h (Figure 2 C2, D2, E2, F2).
Histomorphological analysis at 12 h (Figure 2 A3, B3) showed that most of the stromal cells in the MDZ were small in size and tend to have condensed cytoplasm. The number of reticular fibers was lower in comparison to the numbers seen at 0 and 8 h, and a few irregular, black, short, linear structures were observed in the shedding tissue. The boundary between the PZ and NZ was clear. In the PZ, the structure appeared loosened, with increased intercellular spaces. Most stromal cells with decreased cytoplasm and pyknotic nuclei underwent regressive changes. Between 8 and 12 h, the dense reticular fiber network changed to a loose structure. At 12 h, the reticular fibers were less robust than at 0 and 8 h, indicating that they underwent disruption and fragmentation, and then began to loosen or disappear (Figure 2 C3, D3, E3, F3).
At 16 h, a large area of the stroma in the PZ and MDZ exhibited necrosis with hemorrhage (Figure 2 A4, B4). The boundary between the PZ and NZ was also clear and similar to that at 0, 8, and 12 h. The findings for the features of the reticular fibers in the MDZ were the same as those at 12 h; however, fewer irregular reticular fibers or no reticular fibers were observed in the PZ (Figure 2 C4, D4, E4, F4).
At 24 h, all the stromal cells in the PZ and MDZ were necrotic and the entire decidual zone (including the PZ and MDZ) sloughed into the uterine lumen; this process was accompanied by bleeding (Figure 2 A5, B5). In the shedding endometrium, most of the reticular fibers disappeared, and very few irregular, short, linear reticular fibers were visible. Endometrial breakdown and shedding began in the PZ and continued in the MDZ (Figure 2 C5, D5, E5, F5).
When the mice underwent progesterone maintenance instead of progesterone withdrawal at 24, 32 and 72 h, the MDZ continued to expand outwards, whereas the PZ gradually narrowed and surrounded the outside of the MDZ. The NZ, the outermost layer of the endometrium, and the cells remained round and spindle-shaped (Figure 3). Furthermore, the basal zones gradually became thinner. The reticular fibers were well formed and appeared as black thick bundles surrounding the decidual cells.
Figure 3.
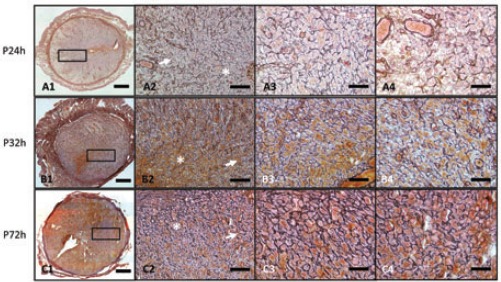
A1-A3) Reticular fibers under progesterone maintenance instead of progesterone withdrawal at 24 h, 32 h, and 72 h shown at low magnification; scale bars: 400 μm. A2, B2, C2) The small boxes indicate the PZ, shown at a higher magnification; scale bars: 100 μm. A3, B3, C3) Stars represent the MDZ, and the indicated areas are also shown at a higher magnification; scale bars: 50 μm. A4, B4, C4) Arrowheads represent PZ, and the indicated areas are also shown at a higher magnification; scale bars: 50 μm. All the sections are representative of the results for at least 3 individuals at the same time point.
In situ zymography
Localization of active collagenase and gelatinase after progesterone withdrawal, shown by green fluorescence in the endometrial sections, was detected by in situ zymography (Figure 4). At 0 h (Figure 4 A1, B1) and 8 h (Figure 4 A2, B2), no active collagenase and gelatinase were detected. With the destruction of the endometrium at 12 h (Figure 4 A3, B3), the green fluorescence of active collagenase and gelatinase was visible and was restricted to the PZ. At 16 and 24 h (Figure 4 A4, B4, 4A5, B5), there was a remarkable shift in the active collagenase signal from the PZ to the entire decidual zone (containing PZ and MDZ), while no active gelatinase was observed.
Figure 4.
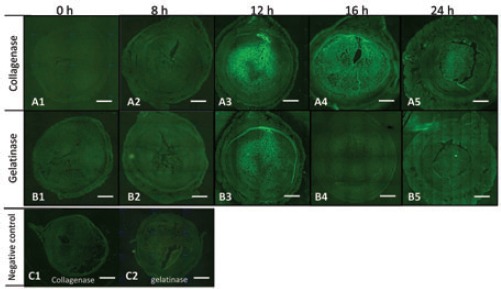
In situ zymography during endometrial breakdown in the mouse menstrual-like model. A1-A5) Localization of active collagenase at each time point. B1-B5) Localization of active gelatinase at each time point. C1-C2) Specificity was verified using 100 mM 1,10-phenanthroline, a general metalloproteinase inhibitor. Scale bars: 400 μm.
Discussion
We used a mouse menstrual-like model, established by pharmacological withdrawal of progesterone, to study the process of endometrial breakdown. To our knowledge, this is the first report of the process of endometrial breakdown in a mouse menstrual-like model in relation with the organization of reticular fibers and localization of active collagenase and gelatinase during this process. Our results highlighted the crucial role of PZ in endometrial breakdown in the mouse model.
Due to the sensitivity and reactivity of the uterus to hormones, it is often difficult to ensure consistency of specimens in endometrial biopsy. Moreover, typically, only a part of the uterus is obtained, leading to contradictory results between studies on the human endometrium and focal observations. Generation of mouse menstrual-like models allows us to overcome these limitations.1-3 Mouse menstrual-like models obtained by physiological and pharmacological progesterone withdrawal allow determination of the time course of endometrial breakdown and regeneration on the whole and observation of real-time endometrial changes in the context of gross and microscopic characteristics.
In mice, decidualization is the process whereby uterine stromal cells proliferate and differentiate into decidual cells in response to blastocyst implantation. The antimesometrial zone is divided into 3 zones, each with specific characteristics. The mature decidual cell zone is a thin and dense cellular zone surrounding the blastocyst. This zone is avascular, while its stromal cells are characterized by polyploid nuclei and a glycogen-and lipid-rich cytoplasm. The predecidual cell zone surrounds the mature decidual zone. In this zone, stromal cells vary between spindle-shaped and round cells, with mostly round nuclei containing large nucleoli. The predecidual zone is well vascularized. The nondecidualized stromal zone surrounds the predecidual cell zone. In the nondecidualized stromal zone, fibroblasts are mostly spindle shaped and oriented in parallel to each other and to the inner smooth muscle layer.8 An artificial decidual response can be induced experimentally by using oil instead of an embryo, initiating similar cytohistological changes in decidualization.9,10 The 3 zones observed in this study were named the MDZ, PZ, and NZ.
Previous studies have observed endometrial breakdown, as seen on reticular fiber staining, in humans and mouse menstrual-like models; therefore, we analyzed reticular fibers in the current study.
We observed abundant reticular fibers distributed throughout the decidual area. The structure of reticular fibers in the PZ changed after progesterone withdrawal, especially between 12 and 16 h, when reticular fibers underwent disruption and fragmentation; the fibers then loosened or disappeared. The structure of reticular fibers in the MDZ varied strikingly at 24 h, when very few irregular, short, linear reticular fibers were observed. These data suggested that reticular fibers in the PZ first undergo disruption and fragmentation and then the entire decidual zone (PZ and MDZ) sloughs into the uterine lumen at 24 h, although the reticular fibers in the MDZ remained partly intact. The succession of events during endometrial breakdown in the mouse menstrual-like model starts with disruption of reticular fibers in the focal zone, followed by shedding of the entire functional layer. This process was similar to that observed in humans, where reticular fibers exhibit focal breakdown, progressing to extensive lysis at menstruation, and the shrunken tissue remnants finally undergo piecemeal shedding.11 In this study, we also observed the active role of the PZ in initiation of endometrial breakdown.
MMPs degraded the endometrial ECM during menstruation in an in vitro human endometrium implant study.11 Moreover, active gelatinase and collagenase were tightly associated with the ECM status, prompting us to analyze active gelatinase and collagenase in addition to reticular fibers in this study.
The amount of gelatinase and collagenase in our mouse model was significantly higher before and during menstruation than at other phases of the cycle. This result was obtained by in situ zymography and is consistent with data obtained from human studies.12 More importantly, active gelatinase and collagenase were confined to the PZ at 12 h, supporting the hypothesis that the PZ is the focal zone where functional layer breakdown starts. Similarly, in humans, active gelatinase and collagenase are confined to discrete foci in the endometrium, where breakdown of endometrium initiates at menstruation.12 Moreover, MMP-1, critical in the initial steps of collagen degradation, was also spatially restricted to the small foci.13 In the current study, localization of enzymatic activity in the PZ at 12 h coincided with the breakdown of reticular fibers. At 16 and 24 h, collagenase activity was detectable throughout the shedding functional layer and degraded reticular fibers in the PZ. By 24 h, the PZ sloughed into the uterine lumen, suggesting that active gelatinase and collagenase first induced the fragmentation or disappearance of reticular fibers in the PZ and then caused the entire functional layer to slough into the uterus. Our findings highlight the role of the PZ in a mouse menstrual-like model. Previous studies have emphasized the importance of the decidua in the establishment of mouse menstrual-like models.1-3 By analyzing the status of reticular fibers and the localization of MMP activity in different decidual zones, we found that it is the PZ, not the MDZ, which is key in endometrial breakdown and shedding in our mouse model. Thus, our study adds to our understanding of the active role of PZ in a mouse menstrual-like model and provides further understanding of the importance of the predecidua in human menstruation.
Acknowledgments
The authors would like to thank GM Wilczynski for generously providing technical guidance and polyester wax for the work. This work was supported by the National Nature Science Foundation of China (no. 30901608) and the National Science and Technology Support Program (no. 2012BA I32B05).
References
- 1.Finn CA, Pope M. Vascular and cellular changes in the decidualized endometrium of the ovariectomized mouse following cessation of hormone treatment: a possible model for menstruation. J Endocrinol 1984;100:295-300 [DOI] [PubMed] [Google Scholar]
- 2.Brasted M, White CA, Kennedy TG, Salamonsen LA. Mimicking the events of menstruation in the murine uterus. Biol Reprod 2003;69:1273-80 [DOI] [PubMed] [Google Scholar]
- 3.Xu XB, He B, Wang JD. Menstrual-like changes in mice are provoked through the pharmacologic withdrawal of progesterone using mifepristone following induction of decidualization. Hum Reprod 2007;22:3184-91 [DOI] [PubMed] [Google Scholar]
- 4.Li YF, Xu XB, Chen XH, Wei G, He B, Wang JD. The nuclear factor-kappaB pathway is involved in matrix metalloproteinase-9 expression in RU486-induced endometrium breakdown in mice. Hum Reprod 2012;27:2096-106 [DOI] [PubMed] [Google Scholar]
- 5.Kaitu’u TJ, Shen J, Zhang J, Morison NB, Salamonsen LA. Matrix metalloproteinases in endometrial breakdown and repair: functional significance in a mouse model. Biol Reprod 2005;73:672-80 [DOI] [PubMed] [Google Scholar]
- 6.Fan X, Krieg S, Kuo CJ, Wiegand SJ, Rabinovitch M, Druzin ML, et al. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. FASEB J 2008;22:3571-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gawlak M, Gorkiewicz T, Gorlewicz A, Konopacki FA, Kaczmarek L, Wilczynski GM. High resolution in situ zymography reveals matrix metalloproteinase activity at glutamatergic synapses. Neuroscience 2009;158:167-76 [DOI] [PubMed] [Google Scholar]
- 8.Deb K, Reese J, Paria BC. Methodologies to study implantation in mice. Methods Mol Med 2006;121:9-34 [DOI] [PubMed] [Google Scholar]
- 9.Rachman F, Bernard O, Scheid MP, Bennett D. Immunological studies of mouse decidual cells. II. Studies of cells in artificially induced decidua. J Reprod Immunol 1981;3:41-8 [DOI] [PubMed] [Google Scholar]
- 10.O’Shea JD, Kleinfeld RG, Morrow HA. Ultrastructure of decidualization in the pseudopregnant rat. Am J Anat 1983;166:271-98 [DOI] [PubMed] [Google Scholar]
- 11.Marbaix E, Kokorine I, Moulin P, Donnez J, Eeckhout Y, Courtoy PJ. Menstrual breakdown of human endometrium can be mimicked in vitro and is selectively and reversibly blocked by inhibitors of matrix metalloproteinases. Proc Natl Acad Sci USA 1996;93:9120-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Salamonsen LA. In vivo evidence for active matrix metalloproteinases in human endometrium supports their role in tissue breakdown at menstruation. J Clin Endocrinol Metab 2002;87:2346-51 [DOI] [PubMed] [Google Scholar]
- 13.Kokorine I, Marbaix E, Henriet P, Okada Y, Donnez J, Eeckhout Y, et al. Focal cellular origin and regulation of interstitial collagenase (matrix metalloproteinase-1) are related to menstrual breakdown in the human endometrium. J Cell Sci 1996;109:2151-60 [DOI] [PubMed] [Google Scholar]


