Abstract
Diaminobenzidine photoconversion is a technique by which a fluorescent dye is transformed into a stably insoluble, brown, electrondense signal, thus enabling examination at both bright field light microscopy and transmission electron microscopy. In this work, a procedure is proposed for combining photoconversion and immunoelectron microscopy: in vitro cell cultures have been first submitted to photoconversion to analyse the intracellular fate of either fluorescent nanoparticles or photosensitizing molecules, then processed for transmission electron microscopy; different fixative solutions and embedding media have been used, and the ultrathin sections were finally submitted to post-embedding immunogold cytochemistry. Under all conditions the photoconversion reaction product and the target antigen were properly detected in the same section; Epon-embedded, osmicated samples required a pre-treatment with sodium metaperiodate to unmask the antigenic sites. This simple and reliable procedure exploits a single sample to simultaneously localise the photoconversion product and a variety of antigens allowing a specific identification of subcellular organelles at the ultrastructural level.
Key words: diaminobenzidine, photoconversion, immunogold cytochemistry, transmission electron microscopy
Introduction
Photoconversion is a procedure by which a fluorescent dye is transformed into a stably stained product, thus enabling a long-lasting analysis of fluorescently-labelled cellular structures at both bright field light microscopy and transmission electron microscopy. It exploits the property of fluorescent dyes to generate reactive oxygen species under intense illumination, and is accomplished by illuminating a fluorescently labelled sample at the excitation wavelength of the fluorochrome in the presence of O2 and of the chromogen 3,3’ diaminobenzidine (DAB). Under these conditions, the dye molecules generate reactive oxygen species which oxidize DAB, thus inducing the formation of insoluble, brown, electrondense precipitates. As DAB is only oxidized in the close proximity of the fluorochrome molecules (due to the very short half-life of the reactive oxygen species), this technique enables to precisely localize at high resolution the fluorescently labelled structures.
This procedure was first used by Maranto1 to convert the intracellularly injected fluorochrome Lucifer Yellow into a stable signal for light and electron microscopic examination. Subsequently, this technique has been extended to a variety of fluorochromes used either as cell tracers or antibody markers,2-4 thus allowing fine investigation of e.g. neuronal networks, 5-9 synaptic vesicle turnover,10-13 endocytosis and exocytosis,14-17 endoplasmic reticulum and Golgi apparatus.18-21
The original photoconversion technique has been improved by intensifying the DAB reaction product using heavy metals such as osmium tetroxide22 or a mixture of osmium tetroxide and potassium ferrocyanide.23 Moreover, modified procedures have been set up to avoid the intrinsic background staining due to peroxisomal enzyme activity.24 The conventional photoconversion method has been also associated with techniques of fluorescence recovery after photobleaching (FRAP)25 or of 3D electron tomography,19,20,26-28 or adapted for confocal laser scanning microscopy.29 The specificity of the fluorochrome (or the fluorochrome-conjugated probes) for a molecular target is the prerequisite for its accurate localization at the ultrastructural level by means of the photoconversion procedures. However, investigating the dynamic interaction of cellular organelles with different molecular factors involved in cell mechanisms often requires the simultaneous detection in situ of more than one molecule.
To this aim, in the present work we combined DAB photoconversion and post-embedding immunocytochemistry to simultaneously assess the distribution of photoconverted nanoparticles30,31 or photosensitizing molecules32 and gold-labelled antigens allowing a specific identification of subcellular organelles at transmission electron microscopy.
Materials and Methods
Cell cultures and treatments
Human HeLa cells and rat neuronal B50 were grown in 25 cm2 flasks in Dulbecco Modified Eagles Medium (DMEM) supplemented with 10% foetal bovine serum, 1% glutamine, 100U of penicillin and streptomycin (Celbio s.r.l., Milan, Italy), in a humidified air atmosphere containing 5% CO2.
HeLa cells were seeded onto glass coverslips in six-multiwell plates (1×105 per well) 24 h before being incubated for 60 min in the dark with 10–6 M Hypocrellin B acetate (HypB-Ac) (as reported by Croce et al.33). HypB was purchased from Axxora.com (Vinci-Biochem, Florence, Italy) and HypB-Ac was prepared according to Croce et al.33 The addition of two acetate groups to the native HypB facilitates cell internalization compared to the native molecule, and makes HypB-Ac to act as a fluorogenic substrate: the modified compound cannot serve, per se, as a photosensitizer but once inside a cell the bound groups are enzymatically removed by esterases, and the photophysical properties of the native molecules are restored. HypB-Ac was diluted at the final concentration in serum-free culture medium to prevent the hydrolysis of acetate groups by serum esterases. At the end of the incubation times, slides were removed from the medium, rinsed with PBS, and processed for transmission electron microscopy (see below). To avoid unwanted photobleaching and/or photoactive effects, these operations were performed under low light illumination.
Rat neuronal B50 were trypsinized when subconfluent, and seeded on glass coverslips in twelve-multiwell dishes (1×103 per well). Two days after seeding, the initial medium was removed and the cells were incubated for 24 h with 450 μL of fresh medium plus 50 μL of suspension of fluorescein 5(6)-isothiocyanate (FITC)-containing chitosan nanoparticles (as reported by Malatesta et al.30).
DAB photoconversion and sample processing for transmission electron microscopy
HypB-Ac-loaded HeLa cells and nanoparticle-loaded B50 cells on coverslips were fixed with 2.5% (v/v) glutaraldehyde and 2% (w/v) paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, at 4°C for 1h, washed and incubated with DAB (20 mg/10 mL in Tris HCl 0.05 M, pH 7.6) under simultaneous irradiation with two 8W Osram Blacklite 350 lamps for 2 h at room temperature (these lamps have emission peaks of high intensity at 430-450, 550 and 580 nm, thus being suitable for FITC and HypB excitation). In order to avoid cell damage due to heating effect of irradiation, the multiwell dishes were maintained on ice.
After irradiation, the cells were post-fixed with 1% OsO4 and 1.5% potassium ferrocyanide at room temperature for 1 h, dehydrated with acetone and embedded in Epon resin. Some B50 cell samples were fixed with 4% (w/v) paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, at 4°C for 1h, incubated with DAB under light irradiation, treated with 0.5 M NH4Cl in PBS at 4°C for 30 min to block free aldehydes, dehydrated with ethanol and embedded in LRWhite resin polymerized for 24 h at 60°C. As controls, some samples were processed as described above but omitting both DAB incubation and exposure to light.
Post-embedding immunocytochemistry
Ultrathin (70-90 nm thick) sections of HeLa or B50 cell samples were collected on 200 mesh nickel grids coated with a formvar-carbon layer. Before starting the immunocytochemical procedure, Epon-embedded samples were pretreated with a 0.2 M aqueous solution of sodium metaperiodate for 60 min at room temperature in order to improve antibody binding.34
Both LRWhite- and Epon-embedded samples were then processed for immunocytochemistry by using human autoimmune sera recognizing either the lysosomal/endosomal proteins corresponding to the autophagy marker LC3B35 or the mitochondrial 70 kDa E2 subunit of the pyruvate dehydrogenase complex.36 Sections were floated on 0.1% ovalbumin (Fluka, St. Louis, MO, USA) in PBS for 3 min, and then incubated for 17 h at 4°C with the primary antibody diluted in PBS containing 0.1% bovine serum albumin (Fluka) and 0.05% Tween 20 (the anti-lysosome/endosome serum was diluted 1:10, the anti-mitochondria serum 1:50). After rinsing in PBS-Tween and in PBS, the sections were floated on ovalbumin, and then reacted for 45 min at room temperature with 12 nm-gold-conjugated protein A (British Biocell International, Cardiff, UK) diluted 1:20 in PBS. Finally, the sections were extensively rinsed in PBS and water, and air-dried. As controls, the primary antibodies were omitted from test sections.
Ultrathin sections were weakly stained with 4.7% aqueous solution of uranyl acetate for 2 min for better visualization of the photoconversion reaction product. All samples were observed in a Philips Morgagni transmission electron microscope (FEI Company Italia S.r.l., Milan, Italy) operating at 80kV and equipped with an Olympus Megaview II camera for digital image acquisition.
Results and Discussion
In all samples, DAB photoconversion gave rise to a finely granular electrondense product; this confirmed the presence of photoactive HypB molecules inside endosomes, multivescicular bodies, late lysosomes and mitochondria (Figure 1 a-c) as well as in the cytosol of HeLa cells (as already reported in a previous paper32), and the occurrence of FITC-containing nanoparticles in the cytosol and inside endosomes and late lysosomes of B50 cells30,31 (Figure 1 d,e).
Figure 1.
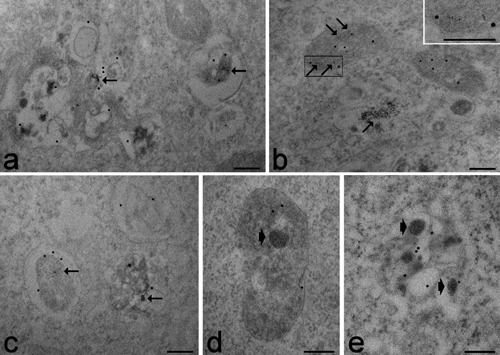
a-c) HeLa cells post-fixed with osmium tetroxide and potassium ferrocyanide and embedded in Epon resin; sections have been pre-treated with sodium metaperiodate and either stained with uranyl acetate (a,b) or observed unstained (c). The photoconversion product (arrows) indicates the presence of photoactive HypB molecules; the inset shows the finely granular product in mitochondria. In (a) and (c) the antibody recognizing anti-lysosome/endosome proteins specifically labels multivesicular bodies and late lysosomes; in b) mitochondria are labelled by the antibody recognizing the pyruvate dehydrogenase complex. d) B50 cells post-fixed with osmium tetroxide and potassium ferrocyanide, and embedded in Epon resin; the sections have been pre-treated with sodium metaperiodate and stained with uranyl acetate; e) B50 cells embedded in LRWhite resin without post-fixation; sections have been stained with uranyl acetate. The photoconversion product (arrowheads) reveals FITC-containing nanoparticles. The anti-lysosome/endosome antibody specifically labels late lysosomes; note that the immunolabelling density is higher in (e) compared to (d). Scale bars, 250 nm.
The electrondense granular precipitate was evident in cells submitted to post-fixation with osmium tetroxide and potassium ferrocyanide, and embedded in Epon resin (Figure 1 a-d) as well as in cells embedded in the LRWhite resin without post-fixation (Figure 1 e). The control samples (where both DAB incubation and light exposure were omitted) did not show any reaction product (not shown). In photoconverted samples embedded either in Epon or in LRWhite resin, the post-embedding immunocytochemical procedure gave positive results, thus demonstrating that the cell molecules preserve the ability to react with specific antibodies after the photoconversion procedure applied in our experiments: the serum recognising lysosomal/endosomal proteins specifically labelled endosomes, multivesicular bodies and late lysosomes (Figure 1 a,c-e), while the probe recognizing the pyruvate dehydrogenase complex specifically labelled mitochondria (Figure 1 b). Notably, the immunogold signal also occurred on organelles devoid of the photoconversion product, thus excluding interference of the DAB precipitates with the antibody binding.
It is known that the fixation and embedding procedures we applied to the Epon-embedded cells are optimal for good morphological preservation, but may cause denaturation of many molecules which could become barely detectable by antibodies. In the Epon-embedded samples, the pre-treatment with sodium metaperiodate (which is able to unmask the antigenic sites on osmicated thin sections without altering their ultrastructural preservation34) successfully improved antibody binding both increasing the specific signal and decreasing background, although the gold labelling was always weaker than in LRWhiteembedded sections. However, it is worth noting that the treatment with oxidizing agents which solubilise osmium molecules leading to their removal from the embedded tissue,34 markedly decreased the electrondensity of the photoconversion product, sometimes making the finest granular deposits hardly visible, especially when photosensitizing molecules occurred in the cytosol or in mitochondria. This limitation should be taken into consideration in selecting the fixation/embedding procedure for photoconverted samples to be processed for immunocytochemistry, particularly when the photoconverting molecules are supposed to be scanty and/or scattered. LRWhite-embedded samples proved to be optimal for the simultaneous detection of photoconversion products and immunolabelled molecules. In LRWhite-embedded cells the photoconversion deposits were well visible: this demonstrates that the morphology and distribution of the DAB precipitates are not altered by the mild fixation with paraformaldehyde (in the absence of osmication) and the embedding in an acrylic hydrophilic resin which is known to improve antibody-antigen interactions while preserving a sufficiently good ultrastructure.
In order to optimise the observation of both photoconversion and immunogold signals, the Epon-embedded and LRWhite-embedded cells were weakly stained with uranyl acetate to increase sample contrast without masking the sometimes minutely dispersed photoconversion precipitates. Uranyl acetate was preferred to lead citrate, which often gives rise to a tinily granular staining37 that could closely resemble the photoconversion product. The interference of uranyl acetate staining in the visualization of the reaction product was excluded by observing unstained samples after DAB photoconversion and immunogold labeling (Figure 1c). Photoconversion and immunohistochemistry have seldom been combined in previous studies, but pre-embedding techniques were only used to label photoconverted neurons.38,39
The simple and reliable procedure of the present work allows to exploit a photoconverted sample to detect a variety of antigens in different ultrathin sections; it will also be possible to simultaneously localise with high precision and sensitivity several antigens on a single section of a photoconverted sample by using probes conjugated with gold grains of different sizes.
Acknowledgments
This work and B. Cisterna fellowship were supported by the “Fondazione Cariverona”, project Verona Nanomedicine Initiative. M. Costanzo is a PhD student in receipt of fellowships from the Dottorato di Ricerca in Imaging multimodale in Biomedicina (University of Verona). Thanks are due to Dr. Claudia Alpini (IRCCS Policlinico San Matteo, Pavia) for the kind gift of human autoimmune sera and to Prof. Elisa Fasani (Department of Chemistry, University of Pavia) for preparation of Hypocrellin B acetate.
References
- 1.Maranto AR. Neuronal mapping: a photooxidation reaction makes lucifer yellow useful for electron microscopy. Science 1982;217:953-5 [DOI] [PubMed] [Google Scholar]
- 2.Sandell JH, Masland RH. Photoconversion of some fluorescent markers to a diaminobenzidine product. J Histochem Cytochem 1988;36:555-9 [DOI] [PubMed] [Google Scholar]
- 3.Lubke J. Photoconversion of different fluorescent substances for light and electron microscopy. Neurosci Protocols 1993;50:601-13 [Google Scholar]
- 4.Singleton CD, Casagrande VA. A reliable and sensitive method for fluorescent photoconversion. J Neurosci Methods 1996;64:47-54 [DOI] [PubMed] [Google Scholar]
- 5.Balercia G, Chen S, Bentivoglio M. Electron microscopic analysis of fluorescent neuronal labeling after photoconversion. J Neurosci Methods 1992;45:87-98 [DOI] [PubMed] [Google Scholar]
- 6.Buhl EH. Intracellular injection in fixed slices in combination with neuroanatomical tracing techniques and electron microscopy to determine multisynaptic pathways in the brain. Microsc Res Tech 1993;24:15-30 [DOI] [PubMed] [Google Scholar]
- 7.Gan WB, Bishop DL, Turney SG, Lichtman JW. Vital imaging and ultrastructural analysis of individual axon terminals labeled by iontophoretic application of lipophilic dye. J Neurosci Methods 1999;93:13-20 [DOI] [PubMed] [Google Scholar]
- 8.Hanani M, Belzer V, Rich A, Faussone-Pellegrini SM. Visualization of interstitial cells of Cajal in living, intact tissues. Microsc Res Tech 1999;47:336-43 [DOI] [PubMed] [Google Scholar]
- 9.LoGiudice L, Sterling P, Matthews G. Vesicle recycling at ribbon synapses in the finely branched axon terminals of mouse retinal bipolar neurons. Neurosci 2009;164:1546-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harata N, Pyle JL, Aravanis AM, Mozhayeva M, Kavalali ET, Tsien RW. Limited numbers of recycling vesicles in small CNS nerve terminals: implications for neural signaling and vesicular cycling. Trends Neurosci 2001;24:637-43 [DOI] [PubMed] [Google Scholar]
- 11.Meunier FA, Nguyen TH, Colasante C, Luo F, Sullivan RKP, Lavidis NA, et al. Sustained synaptic-vesicle recycling by bulk endocytosis contributes to the maintenance of high-rate neurotransmitter release stimulated by glycerotoxin. J Cell Sci 2010;123:1131-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welzel O, Henkel AW, Stroebel AM, Jung J, Tischbirek CH, Ebert K, et al. Systematic heterogeneity of fractional vesicle pool sizes and release rates of hippocampal synapses. Biophys J 2011;100:593-601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoopmann P, Rizzoli SO, Betz WJ. FM dye photoconversion for visualizing synaptic vesicles by electron microscopy. Cold Spring Harb Protoc doi:10.1101/ pdb. prot067611 [DOI] [PubMed] [Google Scholar]
- 14.Fomina AF, Deerinck TJ, Ellisman MH, Cahalana MD. Regulation of membrane trafficking and subcellular organization of endocytic compartments revealed with FM1-43 in resting and activated human T cells. Exp Cell Res 2003;291:150-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishimoto T, Liu TT, Hatakeyama H, Nemoto T, Takahashi N, Kasai H. Sequential compound exocytosis of large dense-core vesicles in PC12 cells studied with TEPIQ (two-photon extracellular polar-tracer imaging-based quantification) analysis. J Physiol 2005;568.3:905-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu TT, Kishimoto T, Hatakeyama H, Nemoto T, Takahashi N, Kasai H. Exocytosis and endocytosis of small vesicles in PC12 cells studied with TEPIQ (two-photon extracellular polar-tracer imaging-based quantification) analysis. J Physiol 2005;568.3:917-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schikorski T. Monitoring rapid endocytosis in the electron microscope via photoconversion of vesicles fluorescently labeled with FM1-43. Methods Mol Biol 2010;657:329-46 [DOI] [PubMed] [Google Scholar]
- 18.Pagano RE, Sepanski MA, Martin OC. Molecular trapping of a fluorescent ceramide analogue at the Golgi apparatus of fixed cells: interaction with endogenous lipids provides a trans-Golgi marker for both light and electron microscopy. J Cell Biol 1989;109:2067-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ladinsky MS, Kremer JR, Furcinitti PS, McIntosh JR, Howell KE. HVEM tomography of the trans-Golgi network: structural insights and identification of a lace-like vesicle coat. J Cell Biol 1994;127:29-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meisslitzer-Ruppitsch C, Vetterlein M, Stangl H, Maier S, Neumüller J, Freissmuth M, et al. Electron microscopic visualization of fluorescent signals in cellular compartments and organelles by means of DAB-photoconversion. Histochem Cell Biol 2008;130:407-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Röhrl C, Meisslitzer-Ruppitsch C, Bittman R, Li Z, Pabst G, Prassl R, et al. Combined light and electron microscopy using diaminobenzidine photooxidation to monitor trafficking of lipids derived from lipoprotein particles. Curr Pharm Biotechnol 2012;13:331-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson O, Backman J. Enhancement of immunoperoxidase staining using osmium tetroxide. J Neurosci Methods 1983;7:185-93 [DOI] [PubMed] [Google Scholar]
- 23.Lascano EF, Berria MI. PAP labeling enhancement by osmium tetroxide-potassium ferrocyanide treatment. J Histochem Cytochem 1988;36:697-9 [DOI] [PubMed] [Google Scholar]
- 24.Dantuma NP, Pijnenburg MA, Diederen JH, Van der Horst DJ. Electron microscopic visualization of receptor-mediated endocytosis of Dil-labeled lipoproteins by diaminobenzidine photoconversion. J Histochem Cytochem 1998;46:1085-9 [DOI] [PubMed] [Google Scholar]
- 25.Darcy KJ, Staras K, Collinson LM, Goda Y. An ultrastructural readout of fluorescence recovery after photobleaching using correlative light and electron microscopy. Nat Protoc 2006;1:988-94 [DOI] [PubMed] [Google Scholar]
- 26.Capani F, Martone ME, Deerinck TJ, Ellisman MH. Selective localization of high concentrations of F-actin in subpopulations of dendritic spines in rat central nervous system: a three-dimensional electron microscopic study. J Comp Neurol 2001;435:156-70 [DOI] [PubMed] [Google Scholar]
- 27.Lichtenstein A, Gaietta GM, Deerinck TJ, Crum J, Sosinsky GE, Beyer EC, et al. The cytoplasmic accumulations of the cataractassociated mutant, Connexin50P88S, are long-lived and form in the endoplasmic reticulum. Exp Eye Res 2009;88:600-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kukulski W, Schorb M, Welsch S, Picco A, Kaksonen M, Briggs JAG. Correlated fluorescence and 3D electron microscopy with high sensitivity and spatial precision. J Cell Biol 2011;192:111-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colello RJ, Tozer J, Henderson SC. Confocal laser scanning microscopic photoconversion: a new method to stabilize fluorescently labeled cellular elements for electron microscopic analysis. Curr Protoc Neurosci 2012;Chapter 2:Unit2.15. doi: 10.1002/0471142301.ns0215s58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malatesta M, Giagnacovo M, Costanzo M, Conti B, Genta I, Dorati R, et al. Diaminobenzidine photoconversion is a suitable tool for tracking the intracellular location of fluorescently labelled nanoparticles at transmission electron microscopy. Eur J Histochem 2012;56:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malatesta M, Costanzo M, Cisterna B, Galimberti V, Biggiogera M, Zancanaro C. Cytochemical methods for tracking nanoparticles and monitoring drug delivery inside the cell. Eur J Histochem 2013;57(Suppl.1):14 [Google Scholar]
- 32.Pellicciari C, Giagnacovo M, Cisterna B, Costanzo M, Croce AC, Bottiroli G, et al. Ultrastructural detection of photosensitizing molecules by fluorescence photoconversion of diaminobenzidine. Histochem Cell Biol 2013;139:863-71 [DOI] [PubMed] [Google Scholar]
- 33.Croce AC, Fasani E, Bottone MG, De Simone U, Santin G, Pellicciari C, et al. Hypocrellin-B acetate as a fluorogenic substrate for enzyme-assisted cell photosensitization. Photochem Photobiol Sci 2011;10:1783-90 [DOI] [PubMed] [Google Scholar]
- 34.Bendayan M, Zollinger M. Ultrastructural localization of antigenic sites on osmiumfixed tissues applying the protein A-gold technique. J Histochem Cytochem 1983;31:101-9 [DOI] [PubMed] [Google Scholar]
- 35.Alpini C, Lotzniker M, Valaperta S, Bottone MG, Malatesta M, Montanelli A, et al. Characterization for anti-cytoplasmic antibodies specificity by morphological and molecular techniques. Autoimmunity Highlights 2012;3:79-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bottone MG, Soldani C, Veneroni P, Avella D, Pisu M, Bernocchi G. Cell proliferation, apoptosis and mitochondrial damage in rat B50 neuronal cells after cisplatin treatment. Cell Prolif 2008;41:506-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mollenhauer HH. Contamination of thin sections: some observations on the cause and elimination of “embedding pepper”. J Electron Microsc Tech 1987;5:59-63 [Google Scholar]
- 38.Papadopoulos GC, Dori I. DiI labeling combined with conventional immunocytochemical techniques for correlated light and electron microscopic studies. J Neurosci Methods 1993;46:251-8 [DOI] [PubMed] [Google Scholar]
- 39.Wegerhoff R, Breidbach O. Intracellular dye injection of previously immunolabeled insect neurons in fixed brain slices. J Neurosci Methods 1994;53:87-93 [DOI] [PubMed] [Google Scholar]


