Abstract
The normal ANK protein has a strong influence on anti-calcification. It is known that TGF-β1 is also able to induce extracellular inorganic pyrophosphate (ePPi) elaboration via the TGF-β1-induced ank gene expression and the mitogen-activated protein kinase (MAPK) signaling acts as a downstream effector of TGF-β1. We hypothesized that the expression of the ank gene is regulated by mechanics through TGF-β1-p38 pathway. In this study, we investigated the mechanism of short-time mechanical tension-induced ank gene expression. We found that the continuous cyclic mechanical tension (CCMT) increased the ank gene expression in the endplate chondrocytes, and there was an increase in the TGF-β1 expression after CCMT stimulation. The ank gene expression significantly increased when treated by TGF-β1 in a dose-dependent manner and decreased when treated by SB431542 (ALK inhibitor) in a dose-dependent manner. Our study results indicate that CCMT-induced ank gene expressions may be regulated by TGF-β1 and p38 MAPK pathway.
Key words: Continuous cyclic mechanical tension (CCMT), Endplate chondrocytes, ank, TGF-β1, p38
Introduction
Intervertebral disc degeneration is the most common causes of low back pain. It manifestes as loss of the disc space and loss of signal intensity on Magnetic Resonance Imaging (MRI).1 The endplate cartilage is a layer of hyaline cartilage, approximately 0.6-mm thick, lying between the vertebral body and the intervertebral disc. Endplate cartilage calcification plays an important role in the process of disc degeneration. The intervertebral disc is the largest avascular tissue in the body. One of the main pathways for nutrients to reach the avascular nucleus pulposus is by diffusion from the blood supply of the vertebral body through this endplate cartilage. Endplate calcification could impede the passage of nutrients from the blood to the intervertebral disc leading to alterations in the mechanical material properties of the disc, which can result in failure to maintain the nucleus pulposus.2-4
The normal ANK protein has a strong function on anti-calcification. ANK is a transporter able to export intracellular inorganic pyrophosphate (iPPi) from cells and is known to be upregulated in osteoarthritis.5 Abnormal extracellular inorganic pyrophosphate (ePPi) metabolism has been implicated in abnormal calcification, decreased concentrations leading to basic calcium phosphate deposition in the articular tissues. Inorganic pyrophosphate (PPi) is also a substrate for alkaline phosphatase which generates the inorganic phosphate (Pi) needed for mineralization. In ank -/- mice, loss of ANK activity results in diminished ePPi levels and consequent basic calcium phosphate (BCP) calcification in joints.6,7
Mechanical cervical traction is an intervention that is often recommended for the treatment of patients with neck pain.8 However, intermittent cyclic mechanical tension (ICMT) could induce the calcification of endplate chondrocytes and long-term mechanical stimulation decreased the ank gene expression as was evident from our previous study.9 In this study, we investigated the mechanism of shorttime mechanical tension-induced ank gene expression. First we focused on the ank gene expression by applying continuous cyclic mechanical tension (CCMT). This was different from the previous mechanical loading used in our earlier study, the CCMT applied continuous stimulation throughout the study period, using short-term mechanical stimulation in vitro. Transforming growth factor-beta-1 (TGF-β1) plays a significant role in regulating crystal deposition in endplate cartilage. TGF-β1 is a potent regulator of cell proliferation and a modulator of interactions of cell with their extracellular matrix (ECM).10 TGF-β1 is also able to induce ePPi elaboration via TGF-β1-induced ank gene expression.11 Besides, our study on calcification of endplate chondrocytes induced by ICMT indirectly demonstrated that TGF-β1 is a key factor to regulate the calcification process. The p38-MAPK pathway is important in mechanotransduction and signaling of ank expression.11-13 However, there is no evidence that CCMT regulates the expression of ank through TGF-β1-p38 pathway. So in the endplate chondrocytes, was it possible that CCMT could regulate the expression of ank through the TGF-β1 and p38 pathway. The role of TGF-β1 in regulating expression of ank was examined by measuring the expression upon pretreatment with TGF-β1 and SB431542, a selective activin receptor-like kinase (ALK) receptor inhibitor. The role of p38 in regulating expression of ank upon CCMT stimulation was investigated by studying the expression of phosphorylated p38 with pre-treatment of SB203580, a specific inhibitor of p38 kinase, and prior to CCMT stimulation.
Materials and Methods
Chondrocytes isolation and culture
Primary chondrocytes were isolated from the lumbar spine endplate cartilage of Sprague-Dawley rats (160-180g). Cartilage of the L1-L5 endplates was carefully removed from the vertebrae and minced into small pieces (<0.03mm3). The isolation method and culture conditions of chondrocytes were used in Musumeci’s and our papers. 9,14 The second passage cells were used for experiments.15 The study was carried out in strict accordance with the recommendation of the Guide for the Care and Use of Medical Laboratory Animals (Ministry of Health, P.R. China). This study protocol was approved by the Medical Laboratory Animals Care and Use Committee of Anhui Province and the Ethics Committee of Yijishan Hospital of Wannan Medical College and in accordance with the guideline for the Chinese ethical conduct in care and use of animals.
Application of cyclic strain
Endplate chondrocytes were plated at the density of 1×105 cells/cm2 in 2 mL of medium on six-well flexible silicone rubber BioFlex™ plates coated with collagen type I (Flexcell International Corporation, Hillsborough, NC, USA).16 The cells were cultured for 48 h to allow them to attach and reach 80-90% confluency, at which time the growth medium was replaced, and then mechanical strain was applied. A continuous cyclic mechanical strain at 0.5 Hz sinusoidal curve at 3%, 6%, or 9% elongation was applied using an FX-4000T™ Flexercell® Tension Plus™ unit (Flexcell International Corporation) according to the method elaborated in our previous study.9 The cultures were incubated in a humidified atmosphere at 37°C and 5% CO2 while stretching. Cells were harvested immediately after CCMT stimulation was applied. NC group means no mechanical strain was applied.
Live/Dead cell viability assay
A LIVE/DEAD viability/cytotoxicity kit (Invitrogen, Carlsbad, CA, USA) was used after the application of CCMT and removal of supernatant to confirm that the NC group and the CCMT group endplate chondrocytes remained viable and adherent. The kit was used following manufacturer specifications.
Real time PCR
Total RNA was isolated using Tirol reagent (Invitrogen) according to the manufacturer’s instructions. After reverse transcription reaction, real time PCR (RT-PCR) was performed by a Roche Light Cycler 480 system using SYBR®Premix Ex TaqTM (Takara, Dalian, China) according to the manufacturer’s instructions. The sequences of genes are shown in Table 1. All RT-PCR data were normalized to the GAPDH gene for quantitative comparisons.
Table 1.
Sequences of primers used in the real time PCR.
| Genes | Forward primer | Reverse primer | Accession number | Product length(bp) |
|---|---|---|---|---|
| TGF-β1 | CATCCATGACATGAACCGACCCTT | ACAGAAGTTGGCATGGTAGCCCTT | NM_021578.2 | 220 |
| Ank | CAAGAGAGACAGGGCCAAAG | AAGGCAGCGAGATACAGGAA | NM_053714.1 | 177 |
| GAPDH | CTCAACTACATGGTCTACATGTTCCA | CTTCCCATTCTCAGCCTTGACT | NM_017008.3 | 81 |
ELISA
The NC and CCMT groups’ chondrocytes supernatant were collected. ELISA (Bender Med Systems, Vienna, Austria) for TGF-β1 was performed using standard protocols.
CCMT-induced ank expression leads to ePPi generation
Rat endplate chondrocytes were transfected with siRNA (ank) 24 h before CCMT stimulation, and then stimulated for 20 min of 3% CCMT. Total RNA was extracted from rat endplate chondrocytes and real-time PCR analysis was performed.
Western blotting analysis and protein kinase inhibitor assay
Cells were lysed on ice for 30 min in lyses buffer containing 50 mM Tris-HCl, pH7.4, 150 mM NaCl, 1% Nonidet P-40, and 0.1% SDS supplemented with protease inhibitors (10 μg/mL leupeptin, 10 μg/mL pep statin A, and 10 μg/mL apportioning). For western blotting analysis, 15 μg of sample was resolved on 12% SDSPAGE and electro-transferred onto nitrocellulose membranes (Whatman, Piscataway, NJ, USA). The primary antibodies used were anti-ERK1/2 (rabbit monoclonal anti-p44/42 MAPK, Cell Signaling Technology, Inc., Danvers, MA, USA); anti-p38 (rabbit monoclonal anti-p38 MAPK, Cell Signaling Technology); anti-SAPK/JNK (rabbit monoclonal anti-SAPK/JNK, Cell Signaling Technology); anti-Phospho-p38 [rabbit monoclonal anti-phospho-p38 MAPK (Thr180/Tyr182), Cell Signaling Technology]; anti-phospho-ERK1/2 [rabbit monoclonal antiphospho p44/42 MAPK (Thr202/Tyr204), Cell Signaling Technology]; anti-phospho-SAPK/JNK [rabbit monoclonal anti-phospho-SAPK/JNK (Thr183/Tyr185), Cell Signaling Technology] at a dilution of 1:1000; anti-SMAD2/3 (Smad2/3 Antibody, Cell Signaling Technology) at a dilution of 1:1000; anti-PSMAD2 (Phospho-Smad2 (Ser245/250/255) Antibody, Cell Signaling Technology) at a dilution of 1:1000; anti-TGF-β Receptor II [TGF-β Receptor II (K105) Antibody, Cell Signaling Technology] at a dilution of 1:1000; anti-smad7 (ab90085, Abcam, Cambridge, MA, USA) at a dilution of 1:1000; anti-ank (ab58698, Abcam) at a dilution of 1.25:1000. For normalization of protein loading, GAPDH (Cell Signaling Technology) antibody was used at 1:5000 dilutions. Infrared labeled secondary antibodies goat antibodies anti-rabbit IRDye 800 (Li-Cor Biosciences, Lincoln, NE, USA) was added to bind to the primary antibody. The bound complex was detected using the Odyssey Infrared Imaging System (Li-Cor Biosciences). The images were analyzed using the Odyssey Application Software, version1.2 (Li-Cor Biosciences) to obtain the integrated intensities. For the kinase assays, endplate chondrocytes were pretreated with 5-50 μM of the specific inhibitor of p38 kinase (SB203580, InvivoGene, San Diego, CA, USA) and a selective ALK receptor inhibitor (SB431542, Sigma, St. Louis, MO, USA) for 30 min and subsequently exposed to CCMT (0.5HZ, sin3%) for 20 min. The expression levels of ank were analyzed by real time PCR and Western blot.
Silencing experiments with small interfering RNA
The siRNA sequences (designed by Shanghai GenePharma Co.Ltd) and were ank sense 5’-CUGGCCAACACGAACAACA-3’ and antisense 5’-UGUUGUUCGUGUUGGCCAG-3’ were used at final concentrations of 50 nM. Ank siRNA transfection after CTS stimulation 20 min (0.5HZ, sin3%). Briefly, siRNA and lipofectamin2000 were diluted separately in serum free medium, and then diluted lipofectamin was added to siRNA. After 20 min incubation at room temperature, cells were washed with PBS and incubated for 4 h with siRNA-lipofectamin mix. Then, after disposal of the mix, a medium containing 10% FBS was added to that culture dish. Two days later, Ank mRNA expression was used as a negative control to check for the specificity of siRNA effects.
Radiometric assay for extracellular inorganic pyrophosphate
The ePPi levels were measured using the differential adsorption of UDP-(6-3H) glucose (GE Healthcare), and its reaction product 6-phospho-(6-3H) gluconate on activated charcoal, as previously described. The standard concentrations, ranging from 10 to 400 pmol of PPi, were included in each assay. After adsorption of the reaction mixture on charcoal, and centrifugation at 14,000 rpm for 10 min, 100 µL of the supernatant was removed carefully and counted for radioactivity in 5 mL of Bio-Safe II (Research Products International Corp, Mt. Prospect, IL, USA). Results were expressed as picomole of ePPi per microgram of total cell proteins.
Transfection of SMAD7 expression plasmid
Cells were exposed to each biochemical agent for 1 h before 3% CCMT stimulation. SMAD7/pcDNA3.1(+) expression plasmid, which contained rat SMAD7 coding sequence was transiently transfected into end-plate chondrocytes by lipofectamine2000 (Invitrogen). After 24 h post-transfection, cells were exposed to mechanical loading.
Statistical analysis
As to the data presented for Figures 1-5, statistical comparisons were made by performing Student’s t-test to check differences between non-loading (NC) and CCMT groups. Statistical comparisons were made by performing ANOVA with SPSS (SPSS, Inc., Chicago, IL, USA version 10.0), followed by a Student-Newman-Keuls post-test in Figures 1 and 3-5. A P value <0.05 was considered as statistically significant.
Figure 1.
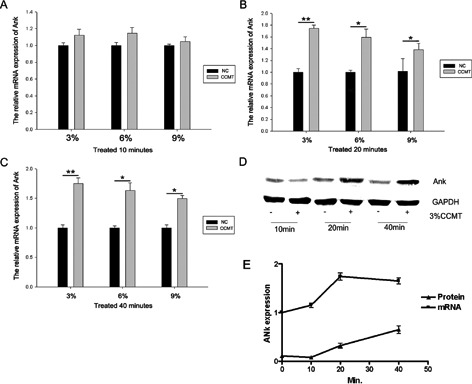
CCMT increased the ank gene expression in rat endplate chondrocytes. Chondrocytes were treated with CCMT at 3%, 6%, and 9% elongation for 10 min (A), 20 min (B) or 40 min (C) and the mRNA expression of ank was then measured. The protein expressions of ank were also assessed (D). The expression phase of ANK mRNA and protein after 3% CCMT. Statistically significant differences were indicated in (E). GAPDH expression was used as an internal control. GAPDH protein level acted as a loading control for Western blotting. All experiments were repeated at least three times. The columns represent the mean ± SE. *P<0.05, **P<0.01 vs nonloading.
Figure 2.
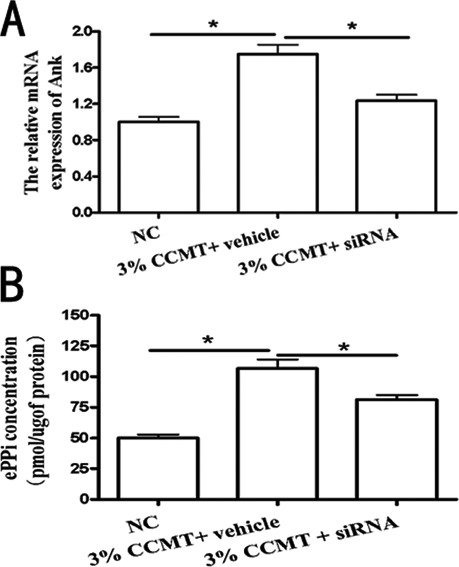
CCMT-increased ank expression leads to ePPi generation. Contribution of ank to transforming CCMT-induced increase in extracellular inorganic pyrophosphate (ePPi) production. The effect of small interfering RNA (siRNA) on Ank mRNA levels (A). The effect of ank on ePPi levels (B). Data showed siRNA (ank) down-regulated CCMT-induced ePPi expression (B). All experiments were repeated at least three times. The columns represent the mean ± SE. *P<0.05, **P<0.01 vs nonloading.
Figure 3.
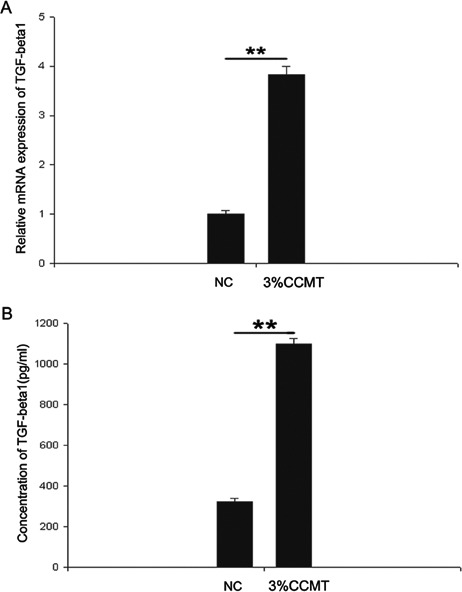
CCMT-increased TGF-β1 expression. Endplate chondrocytes were treated with or without CCMT for 20 min. Afterwards the supernatant was harvested. Real time PCR (A) and ELISA (B) showing up regulation of TGF-β1 expression; GAPDH mRNA expression was used as an internal control for real time PCR. GAPDH protein level acted as a loading control for Western blotting. All experiments were repeated at least three times. The columns represent the mean ± SE. *P<0.05, **P<0.01 vs nonloading.
Figure 4.
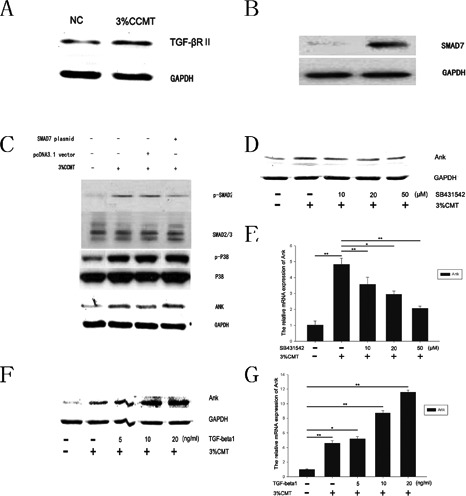
CCMT increased ank expression via the TGF-β1 pathway in endplate chondrocytes. Western blot (A) analysis showed that the expression levels of TGF-βRII up-regulated after 3%CCMT stimulation. Endplate chondrocytes were transfected with SMAD7/pcDNA3.1(+) or pcDNA3.1 vector. Western blot (B) analysis showed that the expression levels of SMAD7 up-regulated in endplate chondrocytes trasfected with SMAD7/pcDNA3.1(+). After 1 day transfection, cells were exposed to 3%CCMT, cells trasfected with SMAD7 showed lower p-SMAD2 expression than vector transfected group after 3%CCMT stimulation, but the p-P38 and Ank expression had no significant effect. Endplate chondrocytes cultured in serum-free medium were pretreated with SB203580 or TGF-β1 for 30 min then stimulated with CCMT for 20 min. Western blot (D, F) and Real time PCR (E, G) analysis showed that the expression levels of ank were attenuated or increased by SB203580 or TGF-β1 in a dose-dependent manner. GAPDH mRNA expression was used as an internal control for real time PCR. GAPDH protein level acted as a loading control for Western blotting. All experiments were repeated at least three times. The columns represent the mean ± SE. *P<0.05, **P<0.01 vs nonloading.
Figure 5.
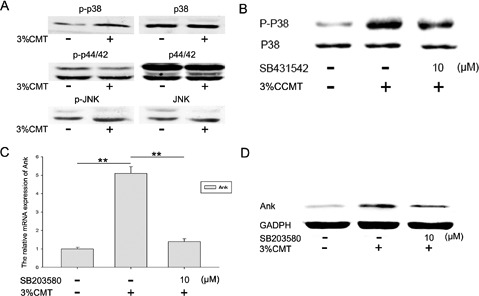
P38 functioned as a downstream effector of TGF-β1 and was involved in regulation of ank after CCMT stimulation. Western blotting (A) shows the protein phosphorylation of p38, but not of p44/42 and JNK, in endplate chondrocytes after CCMT for 20 min. All experiments were repeated at least three times. SB431542 (10 μm) down-regulated CCMT-induced p-p38 expression (B); SB203580 (10 μm) down-regulated CCMT-induced ANK expressions both in mRNA (C) and protein (D) levels. GAPDH mRNA expression was used as an internal control for real time PCR. GAPDH protein level acted as a loading control for Western blotting. The columns represent the mean ± SE. *P<0.05; **P<0.01 vs nonloading.
Results
The CCMT increased the ank gene expression in rat endplate chondrocytes. To confirm that CCMT did not cause cell death of endplate chondrocytes, we investigated the viability of endplate chondrocytes by Live/Dead assay after exposure to CCMT. Our results showed that the NC and CCMT group endplate chondrocytes remained adherent, with no change in viability following the application of CCMT (Figure 6). We applied three levels of elongation of CCMT to test whether different levels had the same effect on the ank gene expression of endplate chondrocytes. RTPCR showed the up-regulation mRNA level expression of ank in 3%, 6%, and 9% groups after loading for 10 min (Figure 1A), 20 min (Figure 1B) and 40 min (Figure 1C). As the 3% elongation group showed the largest increase in the expression of ank mRNA, it was used in the subsequent experiments. Western blot (Figure 1D) showed the up-regulation of ANK protein expression in 20 min and 40 min groups. At last, we analyzed the expression phase of ANK mRNA and protein after 3% CCMT. As shown in Figure 1E, after 3% CCMT treatment, ANK mRNA expression increased at 10 min, which peaked at 20 min, and then decreased at 40 min. Besides, 3% CCMT treatment upregulated ANK protein at 20 min, and maintained at a high level until 4 0min. Because both ANK mRNA and protein increased to the same level, so we chose the 3% elongation and 20 min group in the subsequent experiments.
Figure 6.
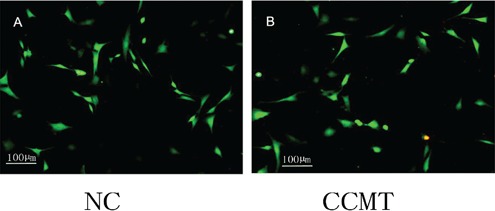
Cell viability assay. LIVE/DEAD assay showed that the NC and CCMT group endplate chondrocytes remained adherent, with no change in viability after the application of CCMT. NC, nonloading; CCMT, continuous cyclic mechanical tension.
CCMT-induced ank expression leads to ePPi generation
The effect of ANK on ePPi levels was assayed in the rat endplate chondrocytes transfected with ank siRNA after CCMT stimulation. Data showed that ANK siRNA could effectively knockdown ANK expression (Figure 2A) and down-regulate CCMT-induced ePPi expression (Figure 2B).
CCMT increased TGF-β1 expression
To examine if TGF-β1 was involved in the regulation of ank upon CCMT stimulation, we examined the expression of TGF-β1. Our results showed that there was a significant increase in the TGF-β1 expression of both mRNA (Figure 3A) and protein (Figure 3B) levels in the CCMT group compared to those in NC group.
CCMT increased ank expression via the TGF-β1 pathway
Western blot analysis showed that the expression levels of TGF-βR up-regulated after 3% CCMT stimulation (Figure 4A). Biochemical studies have shown that SMAD7 blocks signal transduction of TGF-β. To determine whether the regulation of ank by TGF-β1 upon CCMT stimulation was dependent on SMAD2/3, endplate chondrocytes were transfected with SMAD7/pcDNA3.1(+) or pcDNA3.1 vector. Western blot analyses showed that the expression levels of SMAD7 up-regulated in endplate chondrocytes trasfected with SMAD7/pcDNA3.1(+) (Figure 4B). After 1 day’s transfection, cells were exposed to 3%CCMT, cells with SMAD7 trasfection showed lower p-SMAD2 expression than vector transfected group after 3% CCMT stimulation, but the p-P38 and Ank expression had no significant changes (Figure 4C). Endplate chondrocytes were pretreated with TGF-β1 or SB431542 for 30 min prior to CCMT and then were treated by TGF-β1 or SB431542 during CCMT stimulation. Date showed that the ank expression in mRNA (Figure 4 E,G) and protein (Figure 4 D,F) levels significantly increased when treated by TGF-β1 in a dosedependent manner, and decreased when treated by SB431542 compared with the NC group.
CCMT-stimulated phosphorylation of MAPKs in endplate chondrocytes
Phosphorylation of p38 at 20 min after CCMT increased compared to the NC group (Figure 5A), phosphorylation of p44/42 and JNK did not increase after CCMT. The effects of MAPK inhibitors on proteinase expression were analyzed by RT-PCR (Figure 5C) and protein (Figure 5D). SB203580 (10 μM) downregulated CCMT-induced ank expression; U0126 and JNK inhibitor II did not influence ank induction (data not shown); and SB431542 (10μM) down-regulated CCMT-induced p-p38 expression (Figure 5B). Above data suggest that CCMT may activate the p38 pathways in endplate chondrocytes.
Discussion
The ANK is a multipass transmembrane protein thought to serve either as an anion channel or as a regulator of such a channel. Previous studies showed that in ank-/- mice, loss of ANK activity results in diminished ePPi levels and consequent basic calcium phosphate (BCP) calcification in joints. The autosomal recessive ank mutation caused spontaneous bony (BCP) ankylosis of peripheral and axial joints, subsequent destructive arthritis, osteophyte formation, and death owing to immobility.6,7 We also found that the change of ank expression is intimately related with endplate chondrocytes calcification in our previous study.9
Previous studies also showed that intermittent mechanical tension promotes osteogenesis of mesenchymal stem cells and osteo-progenitor cells.17,18 Our previous work showed that ICMT could induce the calcification of endplate chondrocytes and long-term mechanical stimulation decreased the ank gene expression.9 Mechanical cervical traction is an intervention that is often recommended for the treatment of patients with neck pain.19 Previous study demonstrates that short-term mechanical cervical traction is beneficial for neck pain. CCMT increases the ank expression as opposed to ICMT stimulation results. It indicates that short-term mechanical cervical traction has a strong function on anti-calcification through up-regulation of ank expression.
TGF-β1 plays an essential role in the regulation of ank gene expression. It has been reported that TGF-β1 increases ank gene expression through Ras/Raf-1/extracellular signal-regulated kinase pathways in chondrocytes and that TGF-β1 is immediately released after mechanical tension stimulation.9,11 A number of mechanisms through which mechanical forces induce proximal biological changes in chondrocytes have been proposed. A recent study found that integrins αvβ3, αvβ5, αvβ6, and αvβ8 appear to change the conformation of the latent TGF-β1 complex by transmitting cell traction forces.20 External mechanical tensile loads transduced by ECM can stimulate NIH3T3 cells through integrins, which then activates multiple downstream signaling pathways, including the TGF-β1 pathway, 21 resulting in cellular changes such as growth, adhesion, migration, ECM synthesis, and proliferation.22,23
Some studies indicate that the dependent variable of human is probably 1000 μ strain in physiological status, and no more than 4000 μ strain in vigorous activity; 1000 μ strain is equal to 1% elongation, and 6% and 9% elongation mechanical strain is unacceptable for human.24 We choose therefore the 3% mechanical strain. Our results showed that 3% CCMT stimulation increases the expression of both ank and TGF-β1. Our data also showed that compared with an NC group, ank mRNA and protein levels significantly increase in a dosedependent manner when cells are treated with TGF-β1 and decrease in a dose-dependent manner when cells are treated with SB431542. These results suggest that during CCMT, TGF-β1 alteration can regulate ank gene expression. Many down-stream signaling molecules are regulated by TGF-β1. Among these molecules is the MAPK family, which is known to regulate ank gene expression25 and is a central conduit through which mechanical forces are transduced into biological responses in cartilage. 26 MAPK pathways are activated by the static and dynamic compression of cartilage, which simultaneously induce intratissue fluid flow, pressure gradients, cell and matrix deformation.27
In the current study, we investigated the inhibitory effects of MAPK on ank expression. CCMT phosphorylated p38 MAPK, which implies that CCMT activates the p38 pathways in endplate chondrocytes. SB203580 could attenuate the induction of both ank mRNA and protein level expression. In the downstream mechanism of TGF-β1-induced ank up-regulation, p38 might play a central role in the response to CCMT. SB431542 could decrease CCMT-induced p-p38 expression. In our experiments, we found that when the p38 inhibitor SB203580 was added, Ank protein expression was higher than that in the control group. However, when the TGFβ receptor inhibitor SB431542 was added, Ank protein expression was still higher than that in the control group, suggesting that the TGFβ and P38 pathways may have a synergetic effect on Ank expression. CCMT-induced ank expression may occur through both the TGF-β1 and p38 signaling pathways. Dynamic shear-activated p38 at later time points and static compression caused transient activation of p38, with maximal phosphorylation occurring at 10 min. ERK1/2 pathway activation was similar to p38 pathway activation, with peak phosphorylation occurring at the earliest time points (10-60 min). Static compression stimulated JNK phosphorylation at the relatively late time point of 1 h;27 p38 may be differentially sensitive to CCMT compared with ERK1/2 and JNK. Our experiments confirmed that 3% CCMT can up-regulate the expression of TGF-β II receptors. The overexpression of Smad7 obviously inhibited Smad2/3, whereas the p-p38 and Ank expression levels were not affected. This finding indicates that p38 activation does not depend on the Smad2/3 pathway and that there may be other pathways that are responsible for these changes.
In this study, ank expression was highly upregulated by CCMT-activated TGF-β1, which subsequently activated p38, at 20 min, but not ERK or JNK. This discrepancy might be partly explained by the different cell types, time course of mechanical stimulation and mechanical strain (tensile or compressive) used in these in vitro experiments.28-32 We found that ICMT decreases ank expression in our previous study; however, CCMT increased ank expression in this study. These contrasting effects of mechanical loads may be due in part to different temporal dynamics and MAPK activation magnitudes, which may have led to load-dependent activation of downstream transcription factors.
Study limitations
There were several limitations in the current study. First, in the article, we speculated that CCMT increased the TGF-β1 expression through integrins pathway. Unfortunately, the expression of integrins upon CCMT was not detected. Second, CCMT may regulate the ank gene expression via multiple signaling pathways. However, we only study the TGF-β1-MAPK signaling pathway changes. Third, it is still unknown whether our results with endplate chondrocytes in vitro could represent the biologic behavior of human endplate chondrocytes against mechanical stress in vivo.
In conclusion, the results of the current study demonstrate that CCMT-induced ank gene expression is regulated by the TGF-β1 and p38 MAPK pathways in endplate chondrocytes. Since improving the function of endplate can contribute to postpone the intervertebral disc degeneration process, this information may provide a new approach to treat the intervertebral disc degeneration by appropriate mechanical tension.
Acknowledgments
This work was supported by Chinese national natural sciences fund project (30973025, 81272048), Chinese Anhui Province Education Department Key Fund Project (KJ2010A320).
References
- 1.Hadjipavlou AG, Tzermiadianos MN, Bogduk N, Zindrick MR. The pathophysiology of disc degeneration: a critical review. J Bone Joint Surg Br 2008;90:1261-70 [DOI] [PubMed] [Google Scholar]
- 2.Gruber HE, Gordon B, Norton HJ, Kilburn J, Williams C, Zinchenko N, et al. Analysis of cell death and vertebral end plate bone mineral density in the annulus of the aging sand rat. Spine J 2008;8:475-81 [DOI] [PubMed] [Google Scholar]
- 3.Oda J, Tanaka H, Tsuzuki N. Intervertebral disc changes with aging of human cervical vertebra. From the neonate to the eighties. Spine (Phila Pa 1976) 1988;13:1205-11 [DOI] [PubMed] [Google Scholar]
- 4.Roberts S, Urban JP, Evans H, Eisenstein SM. Transport properties of the human cartilage endplate in relation to its composition and calcification. Spine (Phila Pa 1976) 1996;21:415-20 [DOI] [PubMed] [Google Scholar]
- 5.Hirose J, Ryan LM, Masuda I. Up-regulated expression of cartilage intermediate-layer protein and ANK in articular hyaline cartilage from patients with calcium pyrophosphate dihydrate crystal deposition disease. Arthritis Rheum 2002;46:3218-29 [DOI] [PubMed] [Google Scholar]
- 6.Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science 2000;289:265-70 [DOI] [PubMed] [Google Scholar]
- 7.Masuda I, Hirose J. Animal models of pathologic calcification. Curr Opin Rheuma tol 2002;14:287-91 [DOI] [PubMed] [Google Scholar]
- 8.Cai C, Ming G, Ng LY. Development of a clinical prediction rule to identify patients with neck pain who are likely to benefit from home-based mechanical cervical traction. Eur Spine J 2011;20:912-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu HG, Hu CJ, Wang H, Liu P, Yang XM, Zhang Y, et al. Effects of mechanical strain on ANK, ENPP1 and TGF-beta1 expression in rat endplate chondrocytes in vitro. Mol Med Report 2011;4:831-5 [DOI] [PubMed] [Google Scholar]
- 10.Gruber HE, Norton HJ, Sun Y, Hanley EN., Jr Crystal deposits in the human intervertebral disc: implications for disc degeneration. Spine J 2007;7:444-50 [DOI] [PubMed] [Google Scholar]
- 11.Cailotto F, Bianchi A, Sebillaud S, Venkatesan N, Moulin D, Jouzeau JY, et al. Inorganic pyrophosphate generation by transforming growth factor-beta-1 is mainly dependent on ANK induction by Ras/Raf-1/extracellular signal-regulated kinase pathways in chondrocytes. Arthritis Res Ther 2007;9:R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding L, Heying E, Nicholson N, Stroud NJ, Homandberg GA, Buckwalter JA, et al. Mechanical impact induces cartilage degradation via mitogen activated protein kinases. Osteoarthritis Cartilage 2010;18:1509-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tetsunaga T, Nishida K, Furumatsu T, Naruse K, Hirohata S, Yoshida A, et al. Regulation of mechanical stress-induced MMP-13 and ADAMTS-5 expression by RUNX-2 transcriptional factor in SW1353 chondrocyte-like cells. Osteoarthritis Cartilage 2011;19:222-32 [DOI] [PubMed] [Google Scholar]
- 14.Musumeci G, Loreto C, Carnazza ML, Coppolino F, Cardile V, Leonardi R. Lubricin is expressed in chondrocytes derived from osteoarthritic cartilage encapsulated in poly (ethylene glycol) diacrylate scaffold. Eur J Histochem 2011;55:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veilleux NH, Yannas IV, Spector M. Effect of passage number and collagen type on the proliferative, biosynthetic, and contractile activity of adult canine articular chondrocytes in type I and II collagen-glycosaminoglycan matrices in vitro. Tissue Eng 2004;10:119-27 [DOI] [PubMed] [Google Scholar]
- 16.Kawakita K, Nishiyama T, Fujishiro T, Hayashi S, Kanzaki N, Hashimoto S, et al. Akt phosphorylation in human chondrocytes is regulated by p53R2 in response to mechanical stress. Osteoarthritis Cartilage 2012;20:1603-9 [DOI] [PubMed] [Google Scholar]
- 17.Kanno T, Takahashi T, Tsujisawa T, Ariyoshi W, Nishihara T. Mechanical stress-mediated Runx2 activation is dependent on Ras/ERK1/2 MAPK signaling in osteoblasts. J Cell Biochem 2007;101:1266-77 [DOI] [PubMed] [Google Scholar]
- 18.Khatiwala CB, Peyton SR, Metzke M, Putnam AJ. The regulation of osteogenesis by ECM rigidity in MC3T3-E1 cells requires MAPK activation. J Cell Physiol 2007;211:661-72 [DOI] [PubMed] [Google Scholar]
- 19.Jellad A, Ben Salah Z, Boudokhane S, Migaou H, Bahri I, Rejeb N. The value of intermittent cervical traction in recent cervical radiculopathy. Ann Phys Rehabil Med 2009;52:638-52 [DOI] [PubMed] [Google Scholar]
- 20.Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur J Cell Biol 2008;87:601-15 [DOI] [PubMed] [Google Scholar]
- 21.Katsumi A, Naoe T, Matsushita T, Kaibuchi K, Schwartz MA. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J Biol Chem 2005;280:16546-9 [DOI] [PubMed] [Google Scholar]
- 22.Lee DY, Li YS, Chang SF, Zhou J, Ho HM, Chiu JJ, et al. Oscillatory flow-induced proliferation of osteoblast-like cells is mediated by alphavbeta3 and beta1 integrins through synergistic interactions of focal adhesion kinase and Shc with phosphatidylinositol 3-kinase and the Akt/mTOR/p70S6K pathway. J Biol Chem 2010;285:30-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ngu H, Feng Y, Lu L, Oswald SJ, Longmore GD, Yin FC. Effect of focal adhesion proteins on endothelial cell adhesion, motility and orientation response to cyclic strain. Ann Biomed Eng 2010;38:208-22 [DOI] [PubMed] [Google Scholar]
- 24.Fermor B, Gundle R, Evans M, Emerton M, Pocock A, Murray D. Primary human osteoblast proliferation and prostaglandin E2 release in response to mechanical strain in vitro. Bone 1998;22:637-43 [DOI] [PubMed] [Google Scholar]
- 25.Sohn P, Crowley M, Slattery E, Serra R. Developmental and TGF-beta-mediated regulation of Ank mRNA expression in cartilage and bone. Osteoarthritis Cartilage 2002;10:482-90 [DOI] [PubMed] [Google Scholar]
- 26.Fitzgerald JB, Jin M, Chai DH, Siparsky P, Fanning P, Grodzinsky AJ. Shear- and compression-induced chondrocyte transcription requires MAPK activation in cartilage explants. J Biol Chem 2008;283:6735-43 [DOI] [PubMed] [Google Scholar]
- 27.Fanning PJ, Emkey G, Smith RJ, Grodzinsky AJ, Szasz N, Trippel SB. Mechanical regulation of mitogen-activated protein kinase signaling in articular cartilage. J Biol Chem 2003;278:50940-8 [DOI] [PubMed] [Google Scholar]
- 28.Bougault C, Paumier A, Aubert-Foucher E, Mallein-Gerin F. Molecular analysis of chondrocytes cultured in agarose in response to dynamic compression. BMC Biotechnol 2008;8:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chowdhury TT, Akanji OO, Salter DM, Bader DL, Lee DA. Dynamic compression influences interleukin-1beta-induced nitric oxide and prostaglandin E2 release by articular chondrocytes via alterations in iNOS and COX-2 expression. Biorheology 2008;45:257-74 [PubMed] [Google Scholar]
- 30.Hirano Y, Ishiguro N, Sokabe M, Takigawa M, Naruse K. Effects of tensile and compressive strains on response of a chondrocytic cell line embedded in type I collagen gel. J Biotechnol 2008;133:245-52 [DOI] [PubMed] [Google Scholar]
- 31.Li J, Zhao Z, Yang J, Liu J, Wang J, Li X, et al. p38 MAPK mediated in compressive stress-induced chondrogenesis of rat bone marrow MSCs in 3D alginate scaffolds. J Cell Physiol 2009;221:609-17 [DOI] [PubMed] [Google Scholar]
- 32.Raizman I, De Croos JN, Pilliar R, Kandel RA. Calcium regulates cyclic compressioninduced early changes in chondrocytes during in vitro cartilage tissue formation. Cell Calcium 2010;48:232-42 [DOI] [PubMed] [Google Scholar]


