Abstract
Amyloid-beta peptide (Aβ) is implicated in the pathogenesis of Alzheimer's disease (AD), a neurodegenerative disorder. This study was designed to determine the effect of four medicinal plants used to treat neurodegenerative diseases on Aβ-induced cell death. Cytotoxicity of the ethanol extracts of the plants was determined against SH-SY5Y (human neuroblastoma) cells which were untreated, as well as toxically induced with Aβ, using the MTT and neutral red uptake assays. Cell viability was reduced to 16% when exposed to 20 µM Aβ25–35 for 72 h. The methanol extract of the roots of Ziziphus mucronata Willd., Lannea schweinfurthii (Engl.) Engl. and Terminalia sericea Burch. ex DC., were the least toxic to the SH-SY5Ycells at the highest concentration tested (100 µg/ml). All four plants tested were observed to reduce the effects of Aβ-induced neuronal cell death, indicating that they may contain compounds which may be relevant in the prevention of AD progression.
Keywords: Amyloid-beta peptide, medicinal plants, neurodegenerative disorders, neurotoxicity, SH-SY5Y cells
Introduction
Neurodegenerative disease is a term applied to a variety of conditions which result from a chronic breakdown and deterioration of neurons, particularly those of the central nervous system (CNS). These neurons may accumulate aggregated proteins which cause dysfunction (Houghton and Howes, 2005). Alzheimer's disease (AD) is a neurodegenerative disorder and the most common cause of age-related intellectual impairment occurring after the age of sixty (Silva et al., 2004). Patients with AD suffer from marked reduction of cholinergic neuronal function in those areas of the brain responsible for higher mental functions, which partially account for the impairments in activities of daily life (Coyle and Kershaw, 2001). One of the major pathological features of AD is the abundant presence of amyloid plaques in the brain tissues of affected individuals (Pereira et al., 2005). These plaques are made up of amyloid-β proteins (Aβ), which are neurotoxic and are actively involved in the neuronal degeneration that occurs in AD (Ji et al., 2006). As a result, modulation of its toxicity has been identified to be an important therapeutic approach to control the onset of AD (Findeis, 2000; Kawahara and Kuroda, 2000; Ji et al., 2006). In the in vitro model of AD, Aβ has been used to initiate neurotoxicity in various types of cultured cells (Puttfarcken et al., 1996; Boyd-Kimball et al., 2004; Martin et al., 2004; Limpeanchob et al., 2008).
In spite of this mechanistic understanding of the pathophysiology of AD, current medication for AD is very limited and the available ones have several side effects including gastrointestinal disturbances and problems associated with bioavailability (Melzer, 1998, Schulz, 2003). Natural products have provided an alternative strategy for AD therapy as they are usually safer and have fewer adverse effects than chemically synthesised drugs (Kang et al, 2011). Recent findings have shown that natural products have the potential not only to prevent Aβ toxicity, but also to prevent the production of Aβ (Yu et al., 2005). For example, resveratrol (derived from red grape), curcumin (derived from spice turmeric) and (−)-epigallocatechin-3-gallate (derived from green tea) have been reported to reduce the effect of Aβ in the cerebral cortex; curcumin is reported to have the ability to bind small Aβ peptides to block Aβ aggregation as well as fibril and oligomer Aβ formation (Yu et al., 2005; Kang et al., 2011).
In southern Africa, approximately 3500 species of higher plants are used as traditional medicines (Gericke, 2002). These plants contain chemical substances with interesting pharmacological effects and several of these plants are used to treat neurological and age-related disorders (Gericke, 2002). In a previous study, a number of plants, including Ziziphus mucronata Willd. (Rhamnaceae) (roots), Lannea schweinfurthii (Engl.) Engl. (Anacardiaceae) (roots), Terminalia sericea Burch. ex DC. (Combretaceae) (roots) and Crinum bulbispermum (Burm.f.) Milne-Redh. & Schweick. (Amaryllidaceae) (roots and bulbs), were shown to possess the ability to inhibit acetylcholinesterase and to contain antioxidant capacity (Adewusi et al., 2011), indicating their potential for use in treatment of neurodegenerative diseases. The aim of this study was to determine whether extracts from these plants are able to reduce neuronal cell death in SH-SY5Y (human neuroblastoma) cells treated with Aβ peptide.
Materials and methods
Plant collection and extract preparation
The plants investigated include the following: Ziziphus mucronata Willd. (Rhamnaceae) (roots), Lannea schweinfurthii (Engl.) Engl. (Anacardiaceae) (roots), Terminalia sericea Burch. ex DC. (Combretaceae) (roots) and Crinum bulbispermum (Burm.f.) Milne-Redh. & Schweick. (Amaryllidaceae) (roots and bulbs). Z. mucronata (voucher number, NH 1909) and T. sericea (voucher number, NH 1808) were deposited at Soutpanbergensis Herbarium; L. schweinfurthii (voucher number, LT 19) was deposited at Venda, Limpopo, and C. bulbispermum was obtained as a gift from the South African National Biodiversity Institute (SANBI), Tshwane. The plant materials were cut into small pieces, air-dried at room temperature, pulverised and stored at ambient temperature till use. Six grams of the pulverised plant material were extracted with 60 ml of ethanol, while shaking for 24 h. The extracts were filtered and concentrated using a rotary vacuum evaporator and then further dried. All extracts were stored at −20°C prior to analysis. The dried extracts were re-dissolved in Dimethyl sulfoxide (DMSO) to the desired test concentrations.
Cell culture
SH-SY5Y cells (ATCC CRL-2266, Rockville, MD, USA) were cultured in Ham's F-12 supplemented with 2% heat-inactivated foetal bovine serum, penicillin (100 U/ml) and streptomycin (100 µg/ml) at 37°C in a humidified incubator at 95% air and 5% CO2. Confluent cells were seeded into 96-well plates at a density of 1 × 105 cells/well.
Cell viability
MTT assay
The 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT) assay as described by Mossmann (1983) was used to measure cell viability. The plated cells were allowed to adhere for 1 h at 37°C after which 20 µl of various concentrations (100, 50, 25, 12.5, 6.25, 3.13, 1.56 and 0.78 µg/ml) of the plant extracts were added. After 72 h of incubation, 20 µl of MTT solution (5 mg/ml) was added to the wells and further incubated for 3 h. 50 µl of solution containing 30% (w/v) N, N-dimethylformamide and 20% SDS in water were then added to breach the cells and dissolve the formazan crystals. The plates were maintained overnight in the incubator at 37°C and absorbance was measured at 570–630 nm using a microtiter plate reader (Labsystems Multiscan EX type 355 plate reader). Wells without cells were used as blanks and were subtracted as background from each sample. Cell viability was expressed as a percentage of the control values.
Neutral red assay
The neutral red uptake assay as described by Borenfreund and Puerner (1984) was also used to assess cell viability. This method is based on the determination of the accumulation of the neutral red dye in the lysosomes of viable, uninjured cells. After treatment and incubation of the cells (as described above for the MTT assay), 150 µl of neutral red dye (100 µg/ml) dissolved in serum free medium (pH 6.4) was added to the culture medium and incubated for 3 h at 37°C. Cells were washed with Phosphate Buffered Saline (PBS), and 150 µl of elution medium (EtOH/AcCOOH, 50%/1%) was added followed by gentle shaking for 60 min, so that complete dissolution could be achieved. Absorbance was recorded at 540–630 nm using a microtiter plate reader. Cell viability was expressed as a percentage of the control values.
Treatment with Aβ25–35
Aβ25–35 was reconstituted in sterile water to a concentration of 1mM. Aliquots were incubated at 37°C for 72 h to form aggregated amyloid. During the experiment, Aβ25–35 was directly added to culture medium to achieve a final concentration of 20 µM. Three concentrations of each of the plant extracts that presented low toxicity (as determined from the tests above) were selected to assess their possible protective effects. The cells were plated as described above and pre-treated with the plant extracts for 2 h before adding Aβ25–35 and then incubated for 72 h. The viability of the cells was determined by the MTT and neutral red assays as described above. Cell viability was expressed as a percentage of the control values.
Statistical analysis
All determinations were carried out on three occasions in triplicate. The results are reported as mean ± standard deviation (S.D.). Calculation of IC50 values was done using Microsoft Excel 2009 Version for Windows. A p < 0.05 value was considered to be significant.
Results
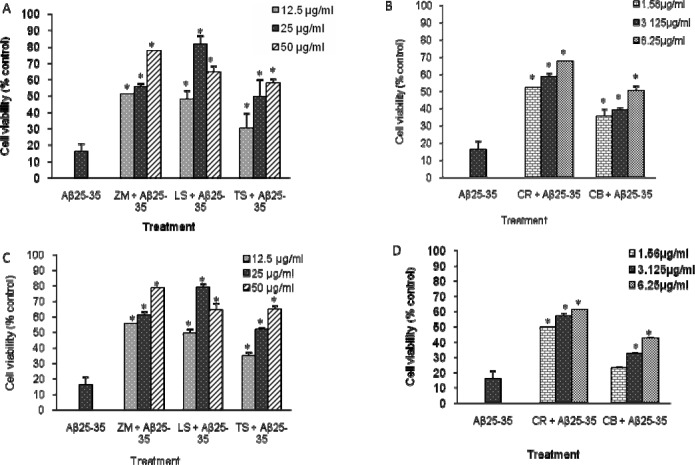
Exposure of the SH-SY5Y cells to Aβ25–35 at 2.5, 5, 10 and 20 µM for 72 h, resulted in a concentration-dependent decrease in cell survival. Cell viability (expressed as the percentage of control measured in the absence of Aβ) was reduced by 84% (Figure 1) when exposed to 20 µM, and this concentration was therefore used for the determination of Aβ induced neuronal cell damage in the present experiment. The same concentration of Aβ25–35′has been used previously in similar experiments (Lin et al., 2006; Liu et al., 2010). The cytotoxic effect of the plant extracts on the viability of the SH-SY5Y cells is presented as IC50 values in Table 1. Values obtained from both the neutral red and MTT assays were comparable. Extracts from the roots of Z. mucronata, L. schweinfurthii and T. sericea were the least toxic with IC50 values exceeding 100 µg/ml, the highest concentration tested, while extracts from the root and bulb of C. bulbispermum showed the highest toxicity as evident from the IC50 values.
Figure 1.
Effect of the plant extracts on Aβ25–35 induced toxicity (20 µM) in SH-SY5Y cells. (A), (B) Neutral red assay. (C), (D) MTT assay. Data expressed as mean ± S.D (n = 9). ZM, Z. mucronata root; LS, L. schweinfurthii root; TS, T. sericea root; CR, C. bulbispermum root; CB, C. bulbispermum bulb. *(p < 0.05) values are significantly different as compared to the positive control.
Table 1.
IC50 values of the methanol extracts of the four plants investigated on untreated SH-SY5Y cells.
| Plant and part | Neutral red assay IC50 (µg/ml) | MTT assay IC50 (µg/ml) |
| Z. mucronata roots | >100 | >100 |
| L. schweinfurthii roots | >100 | >100 |
| T. sericea roots | >100 | 95.14 ± 2.45 |
| C. bulbispermum roots | 10.71 ± 0.26 | 12.53 ± 0.89 |
| C. bulbispermum bulbs | 47.26 ± 0.68 | 48.84 ± 4.62 |
In order to examine the possible neuroprotective effects of the five plant extracts, three concentrations that presented low toxicity from the cytotoxicity assays, were selected to determine if they could reduce cell death induced by Aβ in SH-SY5Y cells. Pretreatment with Z. mucronata and T. sericea methanol extracts reduced the effect of cell death caused by Aβ25–35 (Fig. 1). An improvement in cell viability, relative to the positive control, was observed with Z. mucronata as 77.7% and 78.7% of viable cells were observed with the neutral red and MTT assays respectively, at 50 µg/ml. Pre-treatment with L. schweinfurthii root extract resulted in an optimum dose for inhibition of Aβ25–35 induced cell death at 25 µg/ml, while still maintaining 80% viability (Fig 1). Extracts of the roots and bulbs of C. bulbispermum were found to reduce the effect of Aβ, but were less effective in comparison with the other plants screened.
Discussion
The viability of cells exposed to Aβ25–35 was assessed using the MTT and neutral red uptake assays. These assays are sensitive, quantitative and reliable colorimetric assays for the determination of cell viability (Ban et al., 2006). All four tested plants were observed to reduce the effects of Aβ induced neuronal cell death, indicating that they may contain compounds which may be relevant in the prevention of AD progression.
The low toxicity of Z. mucronata as observed in the present study has been reported in literature (McGaw et al. 2007). The authors tested the hexane, methanol and water extracts of the bark and leaf of the plant and obtained IC50 > 100 µg/ml. In addition, the plant also showed a good effect in reducing Aβ induced cell death. Several compounds including spinosin from Z. spinosa; stepharine, asimilobine, quercetin, kaempferol and phloretin derivatives isolated from Z. jujuba have been reported to have neuroprotective activity (Koetter et al., 2009; Chen, 1998; Qian, 1996; Wing, 2001; Pawlowska et al., 2009; Taati et al., 2011). A possible underlying mechanism of the effect observed with Z. mucronata may be associated with the presence of these compounds.
The ethanol extract of T. sericea roots showed low cytotoxicity with IC50 value of > 90% in both the neutral red and MTT assays. Similar findings have been reported for T. spinosa as the dichloromethane/methanol (1:1) and 80% ethanol extracts of the stem-bark showed IC50 values of 99.5 µg/ml and 75.8 µg/ml respectively in the brine shrimp assay (Mbwambo et al., 2011). In addition, the present study shows T. sericea to reduce cell death induced by Aβ. Tannic acid, chebulagic acid, chebulinic acid and corilagin, all isolated from T. chebula, have been reported to protect neurons from ischemic damage induced by transient cerebral ischemia, and these or similar compounds may be responsible for the good activity observed with T. sericea (Park et al., 2011).
The extracts of the root and bulb of C. bulbispermum were the most toxic with IC50 values <50 µg/ml in both assays. The toxic effects of other Crinum species have been reported. For example, extracts of the whole plants of C. papillosum have been reported to decrease viability in HeLa and HT29 cell lines by 25%. In A431 a 50% reduction in viability was found at 100 µg/ml (Kamuhabwa et al., 2000). However, despite the observed toxicity, the roots and bulbs of C. bulbispermum still reduced the cell death induced by Aβ at less toxic doses. This shows that the plant may contain several compounds with possible neuroprotective activity.
L. schweinfurthii was also observed to show low cytotoxicity; however, no comparative literature could be obtained.
Conclusion
All five extracts tested reduced the effects of Aβ induced neuronal cell death in the present study, indicating that they may contain compounds which may be relevant in the prevention of AD progression. Future work will focus on the isolation and the elucidation of neuroprotective compounds which will be subjected to screening for prevention of Aβ25–35 mediated neuronal degeneration.
Acknowledgements
The authors gratefully acknowledge the financial support by the National Research Foundation (Pretoria) and RESCOM (University of Pretoria).
References
- 1.Adewusi EA, Moodley N, Steenkamp V. In vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from southern Africa. Asian Pac J Trop Med. 2011;4:829–835. doi: 10.1016/S1995-7645(11)60203-4. [DOI] [PubMed] [Google Scholar]
- 2.Ban JY, Cho SO, Koh SB, Song K-S, Bae K, Seong YH. Protection of amyloid β protein (25–35) - induced neurotoxicity by methanol extract of Smila cischinae rhizome in cultured rat cortical neurons. J Ethnopharmacol. 2006;106:230–237. doi: 10.1016/j.jep.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 3.Borenfreund E, Puerner JA. A simple quantitative procedure using monolayer cultures for cytotoxicity assays (HTD/NR-90) J Tissue Culture Meth. 1984;9:7–9. [Google Scholar]
- 4.Boyd-Kimball D, Mohammad Abdul H, Reed T, Sultana R, Butterfield DA. Role of phenylalanine 20 in Alzheimer's amyloid beta-peptide (1–42) - induced oxidative stress and neurotoxicity. Chem Res Toxicol. 2004;17:1743–1749. doi: 10.1021/tx049796w. [DOI] [PubMed] [Google Scholar]
- 5.Chen Q. The Clinical and pharmacology of the Chinese patent medicine [in Chinese] 1st ed. Beijing: Ren Min Wei Min Chu Ban She; 1998. pp. 342–359. [Google Scholar]
- 6.Coyle J, Kershaw P. Galantamine, a cholinesterase inhibitor that allosterically modulates nicotinic receptors: effects on the course of Alzheimer's disease. Biol Psychiatry. 2001;49:289–299. doi: 10.1016/s0006-3223(00)01101-x. [DOI] [PubMed] [Google Scholar]
- 7.Findeis MA. Approaches to discovery and characterization of inhibitors of amyloid-beta peptide polymerization. Biochim Biophys Acta. 2000;1502:76–84. doi: 10.1016/s0925-4439(00)00034-x. [DOI] [PubMed] [Google Scholar]
- 8.Gericke N. Plants, products and people: Southern African perspectives. In: Iwu MM, Wootton J, editors. Ethnomedicine and Drug Discovery. Advances in Phytomedicine. Vol. 1. Amsterdam: Elsevier; 2002. pp. 155–162. [Google Scholar]
- 9.Houghton PJ, Howes M-J. Natural Products and Derivatives affecting neurotransmission relevant to Alzheimer's and Parkinson's disease. Neurosignals. 2005;14:6–22. doi: 10.1159/000085382. [DOI] [PubMed] [Google Scholar]
- 10.Ji ZN, Dong TTX, Ye WC, Choi RC, Lo CK, Tsim KWK. Ginsenoside Re attenuate β-amyloid and serum-free induced neurotoxicity in PC12 cells. J Ethnopharmacol. 2006;107:48–52. doi: 10.1016/j.jep.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Kamuhabwa A, Nshimo C, de Witte P. Cytotoxicity of some medicinal plant extracts used in Tanzanian traditional medicine. J Ethnopharmacol. 2000;70:143–149. doi: 10.1016/s0378-8741(99)00161-0. [DOI] [PubMed] [Google Scholar]
- 12.Kang I-J, Jeon YE, Yin XF, Nam J-S, You SG, Hong MS, Jang BG, Kim M-J. Butanol extract of Ecklonia cava prevents production and aggregation of beta-amyloid and reduces beta-amyloid mediated neuronal death. Food Chem Toxicol. 2011;49:2252–2259. doi: 10.1016/j.fct.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 13.Kawahara M, Kuroda Y. Molecular mechanism of neurodegeneration induced by Alzheimer's beta-amyloid protein: channel formation and disruption of calcium homeostasis. Brain Res Bull. 2000;53:389–397. doi: 10.1016/s0361-9230(00)00370-1. [DOI] [PubMed] [Google Scholar]
- 14.Koetter U, Barrett M, Lacher S, Abdelrahman A, Dolnick D. Interactions of Magnolia and Ziziphus extracts with selected central nervous system receptors. J Ethnopharmacol. 2009;124:421–425. doi: 10.1016/j.jep.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 15.Limpeanchob N, Jaipan S, Rattanakaruna S, Phrompittayarat W, Ingkaninan K. Neuroprotective effects of Bacopa monnieri on beta-amyloid-induced cell death in primary cortical culture. J Ethnopharmacol. 2008;120:112–117. doi: 10.1016/j.jep.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 16.Lin Y-H, Liu A-H, Wu H-L, Westenbroek C, Song Q-L, Yu H-M, Horst GJT, Li X-J. Salvianolic acid B, an antioxidant from Salvia miltiorrhiza, prevents Aβ25–35-induced reduction in BPRP in PC12 cells. Biochem Biophys Res Commun. 2006;348:593–599. doi: 10.1016/j.bbrc.2006.07.110. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Xu K, Yan M, Wang Y, Zheng X. Protective effects of galantamine against Aβ-induced PC12 cell apoptosis by preventing mitochondrial dysfunction and endoplasmic reticulum stress. Neurochem Int. 2010;57:588–599. doi: 10.1016/j.neuint.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Martin SE, de Fiebre NE, de Fiebre CM. The alpha7 nicotinic acetylcholine receptor-selective antagonist, methylcaconitine, partially protects against beta-amyloid1-42 toxicity in primary neuron-enriched cultures. Brain Res. 2004;1022:254–256. doi: 10.1016/j.brainres.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Mbwambo ZH, Erasto P, Nondo ROS, Innocent E, Kidukuli A. Antibacterial and cytotoxic activities of Terminalia stenostachya and Terminalia spinosa. Tanzan J Health Res. 2011;13:1–8. doi: 10.4314/thrb.v13i2.64759. [DOI] [PubMed] [Google Scholar]
- 20.McGaw LJ, Van der Merwe D, Eloff JN. In vitro antihelmintic, antibacterial and cytotoxic effects of extracts from plants used in South African ethnovertinary medicine. Vert J. 2007;173:366–372. doi: 10.1016/j.tvjl.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Melzer D. New drug treatment for Alzheimer's disease: lessons for healthcare policy. Br Med J. 1998;316:762–764. doi: 10.1136/bmj.316.7133.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mossmann T. Rapid colorimetric assay for cellular growth and survival. Application to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 23.Park JH, Joo HS, Yoo K-Y, Shin BN, Kim IH, Lee CH, Choi JH, Byun K, Lee B, Lim SS, Kim MJ, Won M-H. Extract from Terminalia chebula seeds protect against experimental ischemic neuronal damage via maintaining SODs and BDNF levels. Neurochem Res. 2011;36:2043–2050. doi: 10.1007/s11064-011-0528-9. [DOI] [PubMed] [Google Scholar]
- 24.Pawlowska AM, Camangi F, Bader A, Braca A. Flavonoids of Zizyphus jujuba (L) and Zizyphus spina-christi (L.) Willd (Rhamnaceae) fruits. Food Chem. 2009;112:858–862. [Google Scholar]
- 25.Pereira C, Agostinho P, Moreira PI, Cardoso SM, Oliveira CR. Alzheimer's disease-associated neurotoxic mechanisms and neuroprotective strategies. Curr Drug Targets: CNS and Neurol Disord. 2005;4:383–403. doi: 10.2174/1568007054546117. [DOI] [PubMed] [Google Scholar]
- 26.Puttfarcken PS, Manelli AM, Neilly J, Frail DE. Inhibition of age-induced beta-amyloid neurotoxicity in rat hippocampal cells. Exp Neurol. 1996;138:73–81. doi: 10.1006/exnr.1996.0048. [DOI] [PubMed] [Google Scholar]
- 27.Qian XZ. The common Chinese herbal medicine Vol 2 [in Chinese] 1st ed. Beijing: Ren Min Wei Sheng Chu Ban She; 1996. The colour pictorial handbook of the Chinese herbal. [Google Scholar]
- 28.Schulz V. Gingko extract or cholinesterase inhibitors in patients with dementia: what clinical trial and guidelines fail to consider. Phytomedicine. 2003;10:74–79. doi: 10.1078/1433-187x-00302. [DOI] [PubMed] [Google Scholar]
- 29.Silva BA, Dias ACP, Ferreres F, Malva JO, Oliveira CR. Neuroprotective effect of H. perforatum extracts on β-amyloid induced neurotoxicity. Neurotox Res. 2004;6:119–130. doi: 10.1007/BF03033214. [DOI] [PubMed] [Google Scholar]
- 30.Taati M, Alirezaei M, Moshkatalsadat MH, Rasoulian B, Moghadasi M, Sheikhzadeh F, Sokhtezari A. Protective effects of Ziziphus jujuba fruit extract against ethanol-induced hippocampal oxidative stress and spatial memory impairment in rats. J Med Plants Res. 2011;5:915–921. [Google Scholar]
- 31.Wing YK. Herbal treatment of insomnia. Hong Kong Med J. 2001;7:392–402. [PubMed] [Google Scholar]
- 32.Yu M-S, Leung SK-Y, Lai S-W, Che C-M, Zee S-Y, So K-F, Yuen W-H, Chang RC-C. Neuroprotective effects of anti-aging oriental medicine Lycium barbarum against β-amyloid peptide neurotoxicity. Exp Gerontol. 2005;40:716–727. doi: 10.1016/j.exger.2005.06.010. [DOI] [PubMed] [Google Scholar]