Abstract
The purpose of this manuscript was to study the regulation effects of β-elemene combined with radiotherapy on three different gene expressions in lung adenocarcinoma A549 cell. mTOR gene, HIF-1α gene, Survivin gene were included in the gene group. Cell culture and RT-PCR were applied to finish this research. Hypoxia Control group, Hypoxia β-elemene group, Hypoxia β-elemene combined with irradiation group were set to compare the differences of three different gene expressions. The most active effects were found in the group of Hypoxia irradiation combined with β-elemene. In this group, the mTOR gene, HIF-1α gene, Survivin gene expressions were all down-regulated when compared with the single treatment groups, and there were significantly statistical differences.
Keywords: β-elemene, A549, mTOR, HIF-1α, Survivin, Rhizoma Curcumae
Introduction
β-elemene was an effective antitumour monomer which was isolated from Rhizoma Curcumae roots. And β-elemene was also the type two anticancer new drugs (Wu Z.B. et al., 2011); usually it was used together with radiotherapy in clinical applications. Quite a few papers reported that highly activities were exhibited on many kinds of tumour cells. Some effects were also found on the MDR (multi-drug-resistance) induced by radiotherapy. The emulsion of β-elemene in clinical was used in the treatment of malignant serous cavity effusion, lung, gastrointestinal tumour, brain tumour and other superficial tumours. Many in vitro and in vivo tests demonstrated that β-elemene could significantly increase the sensitivities of lung cancer cells in radiation therapy (Zeng Z.W. et al., 2010). This activity relates to induced cell G2/M phase retardation and cell apoptosis, increased DNA damage, inhibited the damage repair process, and inhibited telomerase activity in the cells (Xu S. et al., 2006).
In vivo lung transplantation tumour experiment of nude mice found that sensitisation doses of β-elemene combined radiation could significantly inhibit HIF-1α and survivin mRNA expression, and could also significantly inhibit mTOR mRNA expression [2]. But if the process was done by mTOR gene to increase the sensitisation of radiation therapy, we can get the answer from this research.
Materials and methods
Cell culture and group (Chen M.W. et al., 2012)
The human lung carcinoma cells of A549 was maintained in monolayer culture at 37□ and 5% CO2 in RPMI 1640 supplemented with 10% heat-inactivated FBS, 10 U/ml of penicillin, 10 µg/ml of streptomycin, and 0.25µg /ml of amphotericin B.
Logarithmic phase cells were used; A549 cell was divided into Normoxia Control Group and Hypoxia Group. Hypoxia Group was divided into four groups: (1) the control group: the cell was dealt with RPMI 1640 medium only; (2) β-elemene group: 20 µ g/mlβ-elemene , 37□ for 24 h; (3) Irradiated group: 6 MV-X-ray single dose 4 GY exposure; (4) β-elemene combined with irradiated group: 20 µ g/mlβ-elemene for 24 h, after 4 GY exposure.
All the groups were put in the 37□ incubator for 24h, and RT-PCR tests were continued to study the effect of β-elemene combined with irradiated action on mTOR/HIF-1α/Survivin mRNA levels.
Target genes in A549 cell was tested by RT-PCR (Meng W. et al., 2007)
As each cell group was treated, 1 ml trizol was added to crack cell, 100 µ l chloroform was also added. each cell was vigorously shaken for 30s, 4□ 14000 RPM centrifugal for 10 min. Supernatant was taken, and same volume isopropyl alcohol was added into it. It stayed on ice for 20 min, 4□, 14000 rpm, centrifuged for 10 min; the supernatant was abandoned, air-dried, 20 µ l DEPC; water was added to make it a solution.
Each sample was centrifuged, and taken 1 µl RNA into PCR pipe. The whole process was according to the RT-PCR kit instructions, kept in 4□ standby. Primer sequence: GAPDH forward primer: 5′-GCA CCG TCA AGG CTG AGA AC-3′; GAPDH reverse primer: 5′-ATGGTGGTGAAGACGCCAGT-3′; annealing temperature changed with target gene temperature. Amplified sequence has 142bp. HIF-1α forward primer: 5′-ATG AAG TGT ACC CTA ACT AGC CG -3′. HIF-1αreverse primer: 5′-GCT TGA GTT TCA ACC CAG ACA TA-3′; annealing temperature was 63□, amplified sequence has 452bp; Survivin forward primer: 5′: AGC CAG ACT TGG CCC AGT GTT TC-3′. Survivin reverse primer: 5′-GCA CTT TCT CCG CAG TTT CCT CA-3′, annealing temperature was 63□, amplified sequence has 243bp; mTOR forward primer: 5′-CGC TGT CAT CCC TTT ATC G-3′ mTOR reverse primer: 5′-ATG CTC AAA CAC CTC CAC C-3′, annealing temperature was 63□, amplified sequence has 193bp.
Reverse transcription reaction conditions: 30□ preliminary degeneration, 10min; 42□ degeneration, 30 min; 99□ annealing, 5 min; 5□ extensions, 5 min. PCR amplification conditions: 94□ preliminary degeneration, 2 min; 94□, 30 sec; 63□ for 30 sec; 72□, 1 min; 72□ extension, 7min. 35 cycle in total.
PCR product electrophoresis and gel analysis
As polymerase chain reaction (PCR) was ended, 10 µ l samples were blended with 2 µl 6 × Loading Buffer to continue 2% of the agarose gel electrophoresis and EB dyeing, scanned by µVP gel imaging system. The quantity one electrophoresis image analysis software (BIO-RAD company) was used in the result analysis with purpose gene amplification banding and NaCan GAPDH banding relative gray ratio to represent mRNA expression relative quantity.
Results
Expression of mTOR mRNA in A549
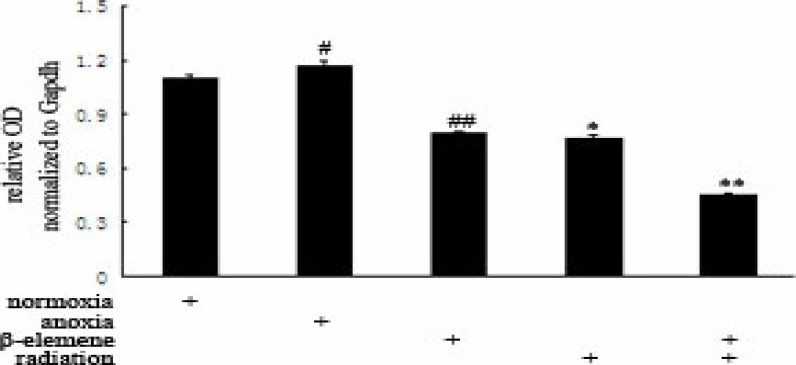
We found that mTOR mRNA expression in Hypoxia Control Group (1.1699±0.0248) was increased, and has statistical significance when compared with Normoxia Control Group (1.0964±0.0181). The mTOR mRNA expression in Hypoxia Drug Group (0.7933±0.0045) and Hypoxia Irradiation Group were all lower than Hypoxia Control Group (p<0.01). The mTOR mRNA expression in Hypoxia Combitional Group (0.4476±0.0129) was decreased, and has statistical significance when compared with all the other groups (p<0.01). See Table 1 and Table 2.
Hypoxia compared with Normoxia group, #p<0.01; Drug, irradiation, Hypoxia Group compared, ##p, *p<0.01; Combination Group compared with other groups, **p<0.01
Expression of HIF-1α mRNA in A549
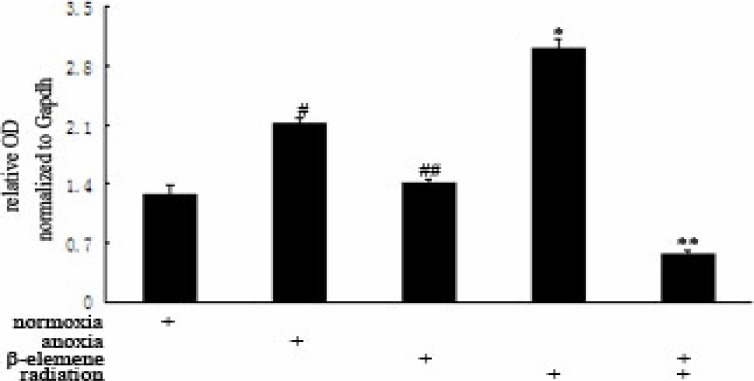
We found that HIF-1α mRNA expression in Hypoxia Control Group (2.1086±0.0918) was higher than Normoxia Control Group (1.2666±0.1069), and has statistical significance. The HIF-1α mRNA expression in Hypoxia Drug Group (1.4025±0.0408) was all lower than Hypoxia Control Group (p<0.01). The HIF-1α mRNA expression in Hypoxia Irradiation Group (2.9963±0.1319) was increased (p<0.01). The HIF-1α mRNA expression in Hypoxia Combitional Group (0.5550±0.0609) was obviously decreased when compared with all the other groups (p<0.01). See Table 3 and Table 4.
Hypoxia compared with Normoxia group, #p<0.01; Drug, irradiation, Hypoxia Group compared, ##p, *p<0.01; Combination Group compared with other groups, **p<0.01.
Expression of Survivin mRNA in A549
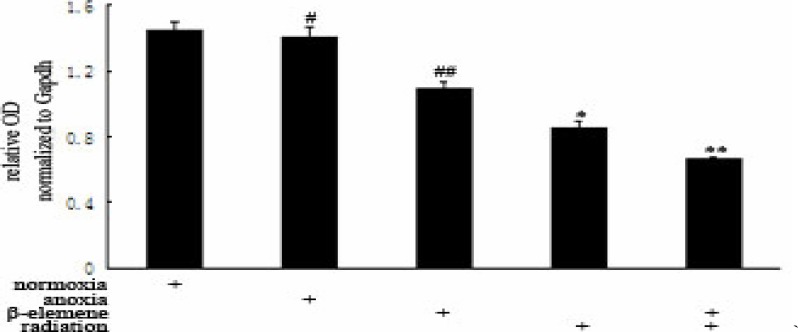
We found that Survivin mRNA expression in Hypoxia Control Group (1.4045±0.0613) was lower than Normoxia Control Group (1.4519±0.0433), and has no statistical significance (p>0.05). The Survivin mRNA expression in Hypoxia Drug Group (1.0855±0.0468) and Hypoxia Irradiation Group (0.8507±0.0444) was all lower than Hypoxia Control Group (p<0.01). The Survivin mRNA expression in Hypoxia Combitional Group (0.6635±0.0150) was obviously decreased when compared with all the other groups (p<0.01). See Table 5 and Table 6.
Normoxia group compared with Hypoxia, #p>0.01; Drug, irradiation, Hypoxia Group compared, ##p, *p<0.01; Combination Group compared with other groups, **p<0.01.
Discussion
The inhibition of β-elemene induced cancer cell proliferation is mainly due to the apoptotic cell death and cell cycle arrest (Lu J.J. et al, 2012). β-elemene could also lead to phosphorylation of p38 MAPK, cell-cycle arrest in G0/G1 phase, anti-proliferation of glioblastoma cells (Yao Y.Q. et al., 2008). β-elemene induced inhibition growth in H22 tumour cells through down-regulation of c-Met. (Qin Y. et al, 2012). Combinations of β-elemene with docetaxel could induce big inhibition on p53 mutant H23 cells and p53 null H358 cells, as well as H460 and A549 cells (Zhao J. et al., 2007). In addition to this inhibition on lung cancer cells, Atg5-Atg12 conjugated protein was up-regulated by β-elemene treatment; PI3K/Akt/mTOR/p70S6K1 activity was inhibited by β-elemene; it protected autophagy and prevented human gastric cancer cells from undergoing apoptosis (Liu J et al., 2011).
In this research, we set up Normoxia Control Group and Hypoxia Group. Hypoxia Group was divided into four groups: the control group, β-elemene group, Irradiated group, and Combination group. The results found that β-elemene combined with irradiation could obviously down-regulate the expression of mTOR mRNA, HIF-1α mRNA, and Survivin mRNA. And the effect was better than the single method group.
β-elemene ranging from 28.68µg/mL to 35.43µg/ml are highly cytotoxic to A549 cell. (Wu Z.B. et al, 2012). In clinical, β-elemene injection could inhibit the proliferation of HepG-2 cell by inhibiting the tubulin polymerisation of HepG-2 cell (Mao Y.Q. et al, 2012). β-elemene, in addition to inhibiting kidney cancer, has a stronger effect on proliferation inhibition and could induce apoptosis of gastric cancer cells. (Zhang Y. et al, 2012). β-elemene could crack PARP, and has anti-tumour action on Kidney cancer 786-0 cell since it can down-regulate the expression of Bcl-2 and Survivin (Zhan H.Y. et al, 2012). Numerous studies have shown that salvia miltiorrhiza, turmeric, etc. have obvious tumour cell apoptosis effect. β elemi olefine is a kind of volatile oil isolated from Chinese traditional medicine Rhizoma Curcumae roots, which belongs to the second grade national cancer drug. β-elemene can also reduce the expression of CTGF and FN proteins in Glomerular mesangial cells induced by fibrosis (Zeng H.B. et al., 2012).
Figure 1.
Expression of mTOR mRNA in A549
Figure 2.
Expression quantities of mTOR mRNA in A549
Figure 3.
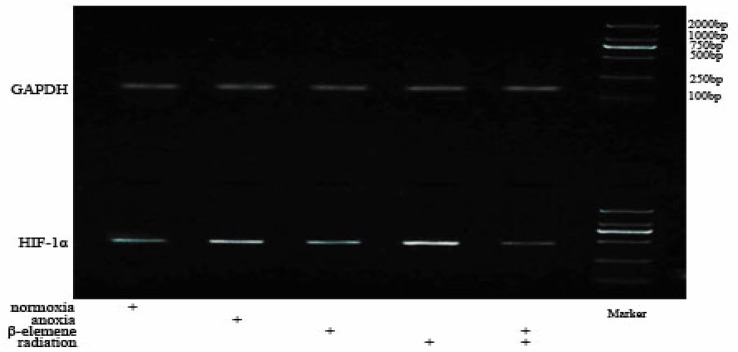
Expression of HIF-1αmRNA in A549
Figure 4.
Expression quantities of HIF-1αmRNA in A549
Figure 5.
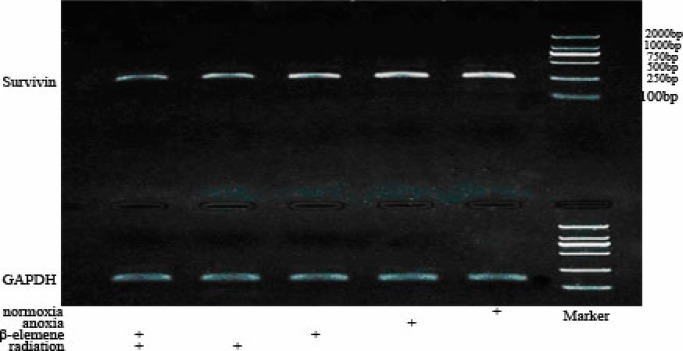
Expression of survivin mRNA in A549
Figure 6.
Expression quantities of survivin mRNA in A549
References
- 1.Chen MW, Zhong ZF, Wang SP, et al. Research progress in anticancer activity and novel delivery system of β-elemene. Chinese Journal of New Drugs. 2012;21(12):1358–1361. 1388. [Google Scholar]
- 2.Liu J, Zhang Y, Qu J, et al. β-Elemene-induced autophagy protects human gastric cancer cells from undergoing apoptosis. BMC Cancer. 2011;11:183. doi: 10.1186/1471-2407-11-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu JJ, Dang YY, Huang M, et al. Anti-cancer properties of terpenoids isolated from Rhizoma Curcumae. Journal of Ethnopharmacology. 2012;143(2):406–411. doi: 10.1016/j.jep.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Mao YQ, Liang XM, Fu HY, et al. Effects of β-elemene on α-tubulin of Human Hepatocarcinoma HepG-2 Cells. China Cancer. 2012;21(02):145–149. [Google Scholar]
- 5.Meng W, Wang YF, Xiong S, et al. Chinese Journal of Health Laboratory Technology. Target genes were tested by RT-PCR. 2007;17(1):51–52. [Google Scholar]
- 6.Qin Y, Guo YJ, et al. Anti-tumor effect of β-elemene in murine hepatocellular carcinoma cell line H22 depends on the level of c-Met downregulation. Biomedicine & Preventive Nutrition. 2012;2(2):91–98. [Google Scholar]
- 7.Wu ZB, Ma SL, Chen XQ, et al. Experiment Research on Synergistic Effect of Elemene Combined with Hyperthermia on Cell line A549 of Lung Adenocarcinoma. Chinese archives of traditional chinese medicine. 2012;30(04):742–744. [Google Scholar]
- 8.Yao YQ, Ding X, Jia YC, et al. Anti-tumor effect of β-elemene in glioblastoma cells depends on p38 MAPK activation. Cancer Letters. 2008;264(1):127–134. doi: 10.1016/j.canlet.2008.01.049. 8. [DOI] [PubMed] [Google Scholar]
- 9.Xu S, Xu CF. Mechanism of multidrug resistance in tumor and reversal effect of traditional Chinese medicine: An advancement. 2006;13(06):404–411. [Google Scholar]
- 10.Zeng HB, Liu JG, Du XG, Gao JC. Suppression effect of β-elemene on the mesangial cell fibrosis. China Journal of Modern Medicine. 2012;22(24):27–30. [Google Scholar]
- 11.Zeng ZW, Zhou GL, Zhan XR, et al. Separation of Free Drug of Elemene Emulsion and Determination of Entrapment Efficiency by UPLC. Traditional Chinese Drug Research and Clinical Pharmacology. 2010;21(03):304–307. [Google Scholar]
- 12.Zhan HY, Wang KF, Liu J, et al. The anti-tumor effect of β-elemene on renal cell carcinoma cells and the mechanisms involved. Journal of Modern Oncology. 2012;20(01):1–3. [Google Scholar]
- 13.Zhao J, Li QQ, Zou B, et al. In vitro combination characterization of the new anticancer plant drug beta-elemene with taxanes against human lung carcinoma. Int J Oncol. 2007;31(2):241–252. [PubMed] [Google Scholar]
- 14.Zhang Y, Zhao MF, Liu YP, et al. The effects of β-elemene on the activation of Akt and the expressions of apoptosis-related proteins in gastric cancer. Journal of Modern Oncology. 2012;20(03):451–454. [Google Scholar]