Abstract
Subcellular localization of protein synthesis provides a means to regulate the protein composition in far reaches of a cell. This localized protein synthesis gives neuronal processes autonomy to rapidly respond to extracellular stimuli. Locally synthesized axonal proteins enable neurons to respond to guidance cues and can help to initiate regeneration after injury. Most studies of axonal mRNA translation have concentrated on cytoplasmic proteins. While ultrastructural studies suggest that axons do not have rough endoplasmic reticulum or Golgi apparatus, mRNAs for transmembrane and secreted proteins localize to axons. Here, we show that growing axons with protein synthetic activity contain ER and Golgi components needed for classical protein synthesis and secretion. Isolated axons have the capacity to traffic locally synthesized proteins into secretory pathways and inhibition of Golgi function attenuates translation-dependent axonal growth responses. Finally, the capacity for secreting locally synthesized proteins in axons appears to be increased by injury.
Keywords: Axonal protein synthesis, Secretory pathway, Rough endoplasmic reticulum, Golgi apparatus, mRNA localization, Axon guidance
Introduction
Concentrating proteins and other macromolecules in subcellular regions generates the polarity and unique domains of neurons. Targeting membrane and secreted proteins to these regions establishes functional domains in dendrites and axons. Cotranslational secretion of newly synthesized proteins via the rough endoplasmic reticulum (RER) and Golgi apparatus is typically used to traffic membrane and secreted proteins to the correct subcellular locale. Membrane and secreted proteins synthesized in the neuronal cell body progress through RER and cis- and trans-Golgi, ultimately ending up in vesicles that are transported into the dendrites and axons. The endoplasmic reticulum (ER) and Golgi apparatus also provide sites for post-translational modifications, including disulfide bond formation and glycosylation. This soma-centric view for trafficking of nascent proteins into secretory pathways does not take the concept of localized protein synthesis into account (Gerst, 2008). Neuronal proteins that are synthesized locally in dendrites likely make use of the ER- and Golgi-like structures that have been detected at the base of and within dendritic spines (Pierce et al., 2001). These structures are also the likely site of protein glycosylation that has been demonstrated in isolated dendritic processes (Torre and Steward, 1996). These ‘outposts’ of Golgi apparatus in dendrites appear to contribute to polarization of the dendritic compartment in developing neurons, but such has not been seen in the axonal compartment (Horton and Ehlers, 2003b). Axons have been argued to be devoid of such organelles.
In recent years, it has become clear that axonal processes of neurons can also synthesize proteins (Lin and Holt, 2008). Ultra-structurally, only smooth ER-like (SER) structures have been detected in axons and no Golgi or RER has been reported (Tsukita and Ishikawa, 1976, 1979). However, some ER proteins have been detected in axons by immunolocalization (Krijnse-Locker et al., 1995; Weclewicz et al., 1998) and we have shown that ER chaperone proteins can be locally synthesized in axons of cultured rat dorsal root ganglion (DRG) neurons (Willis et al., 2005). We have recently reported that mRNAs encoding membrane and secreted proteins are present in DRG axons in culture (Willis et al., 2007), and others have shown that kappa-opioid receptor mRNA is locally translated in rodent sensory axons (Bi et al., 2006; Tsai et al., 2006). Insertion of locally synthesized proteins into the axoplasmic membrane has been detected in both invertebrate and vertebrate neurons. Spencer et al. (2000) microinjected the mRNA for a mammalian G-protein coupled receptor directly into anucleated Lymnea axons and showed physiologically functional receptor (Spencer et al., 2000). Brittis et al. (2002) transfected isolated axons from developing vertebrate neurons to show that an exogenous alkaline phosphatase mRNA can be locally translated and exported to the cell surface (Brittis et al., 2002). Thus, axonal processes undoubtedly have a secretory mechanism for locally synthesized proteins. However, without any ultrastructurally obvious RER or Golgi in axons the mechanism for secretion is not clear.
Axons could use non-classical mechanisms to target locally synthesized proteins for the axoplasmic membrane and secretion. Alternatively, functional equivalents of RER and Golgi apparatus are present but not recognized ultrastructurally because they lack classical morphological characteristics. To address these two possibilities, we have asked whether axonal processes have the capacity to target locally synthesized proteins to the cell surface, using cultures of DRG and retinal ganglion cells (RGC), two neuronal populations that have been shown to autonomously synthesize proteins in their axons. By immunolocalization studies, distal axons were found to contain components of the co-translational targeting machinery including: the signal recognition particle (SRP); ER proteins needed for protein translocation, folding, and post-translational modifications; and, Golgi apparatus proteins. Dyes that show enriched staining in ER and Golgi membranes also indicate that ER- and Golgi-like structures exist in the DRG and RGC axons. Using anucleated rat sensory axons for metabolic labeling (Zheng et al., 2001), axonally synthesized proteins of varying molecular weights are seen in membrane preparations from the DRG axons. The rat DRG neurons show a transition in growth capacity with injury conditioning that allows for transcription-independent, translation-dependent axonal extension in vitro that is accompanied by robust intra-axonal protein synthesis (Smith and Skene, 1997; Twiss et al., 2000; Zheng et al., 2001). Cultures of these DRG neurons show increased aggregation of axonal RER components after injury conditioning compared to the naive condition suggesting that these neurons alter their capacity for co-translational targeting of axonally synthesized proteins.
Results
mRNAs encoding transmembrane proteins localize to axons
Studies over the past decade have shown that axons are capable of synthesizing a diverse and complex population of different proteins including resident ER proteins (Bassell et al., 1998; Cox et al., 2008; Eng et al., 1999; Hanz et al., 2003; Perlson et al., 2005; Piper et al., 2006; Piper et al., 2008; Willis et al., 2005; Willis et al., 2007; Wu et al., 2005; Yudin et al., 2008). By cDNA array hybridization, we have recently shown that several mRNAs encoding transmembrane and secreted proteins are transported into DRG axons in culture (Willis et al., 2007). These array experiments required amplification of purified axonal RNA and not all of the axonal transcripts identified by array hybridization were confirmed by independent means. To more directly test for existence of mRNAs encoding transmembrane proteins in axons, we used RT-PCR with transcript-specific primers to determine if the mRNAs encoding the transmembrane proteins hyperpolarization-activated cyclic nucleotide-gated (HCN4), α1 subunit of voltage-gated calcium channel (CACNA1), and neural membrane protein 35 (NMP35) localize to DRG axons. For this, 7 d injury-conditioned adult rat DRG neurons were cultured on a porous membrane to allow separation of axonal processes from cell body and non-neuronal cells (Zheng et al., 2001). The axonal RNA used for these amplifications was free of transcripts originating in the cell body and contaminating non-neuronal cells based upon the absence of γ-actin and MAP2 mRNAs, two transcripts that are particularly abundant in the cell body compartment and not transported into axons (Fig. 1A) (Willis et al., 2005). The mRNAs of HCN4, CACNA1, and NMP35 were each amplified from the purified axonal RNA samples in quantities comparable to β-actin mRNA (Fig. 1A). This suggests that sensory axons may have the capacity to locally synthesize these membrane proteins.
Fig. 1.
DRG axons contain mRNAs encoding membrane and channel proteins. A, RNA isolated from DRG axonal and cell-body fractions was used for detection of β-actin, γ-actin and MAP2 mRNA by RT-PCR to assess purity of the axonal preparations (Willis et al., 2005). β-Actin was amplified from both cell body and axonal RNA templates, but γ-actin and MAP2 mRNAs could only be detected in the cell body RNA template. DRG axonal RNA processed for RT-PCR without the addition of reverse transcriptase shows that the β-actin PCR product detected in the axons is specific for amplification of mRNA. NMP35, HCN4, and CACNA1 primers were used for amplification from reverse transcribed cell body and axonal RNA templates. Each of the mRNAs encoding these transmembrane proteins was detected in the DRG axons. B, Immunolocalization was used to determine if proteins encoded by NMP35, HCN4, and CACNA1c (Cav1.2α) are present in the sensory axons. Axonal signals for NMP35 (red) are coarsely granular and concentrated along the periphery of the peripherin (blue) in a single optical XY plane through a segment of distal axon. A reconstructed three-dimensional projection of optical planes of DRG cultures stained in HCN4 (red) is coarsely granular in the axon and is concentrated along the peripheral extents of the neurofilament (blue) signal (18 optical XY planes taken at ~0.21 μm intervals). A single optical XY plane through distal axon stained in CACNA1c (red) is also concentrated along the periphery of the neurofilament (blue) immunoreactivity. [Scale bar=10 μm].
To determine if the transmembrane proteins encoded by axonally localized mRNAs are also present in the DRG axons, we used confocal microscopy to visualize axonal immunoreactivity for HCN4, CACNA1c, and NMP35 in the DRG cultures. Signals generated by anti-HCN4 antibody showed a focal plaque-like distribution along the shaft of the DRG axons in culture (Fig. 1B). Signals for NMP35 appeared more punctate along the axon and single optical plane images through the approximate center of the axon shaft showed that the immunoreactivities for HCN4, CACNA1c, and NMP35 proteins were peripheral to the neurofilament fluorescence, which is suggestive of membrane localization (Fig. 1B).
Axonally synthesized proteins are locally targeted to the membrane
Axonal translation of heterologous mRNA in Lymnea and developing vertebrate spinal cord neurons results in localized insertion of the encoded proteins into the membrane (Brittis et al., 2002; Spencer et al., 2000). Synthesis of resident-ER proteins in the DRG axons (Willis et al., 2005) as well as the axonal localization of mRNAs encoding transmembrane proteins (Fig. 1) suggests that the rat DRG axons may also have the capacity to sort locally synthesized proteins into a secretory pathway. To test this possibility, we performed cell surface biotinylation of metabolically labeled axons. Axons were sheared from cultures of injury-conditioned DRG neurons and subjected to metabolic labeling with [35S]-methionine/cysteine as previously described (Zheng et al., 2001). Cell surface proteins were then biotinylated, precipitated with streptavidin-agarose, and fractionated by SDS/PAGE as outlined in Experimental methods section below. Several biotinylated proteins isolated from the axons were detected by autoradiography; these 35S-labeled bands were clearly enriched compared to the unfractionated lysate and were absent from the non-biotinylated samples (Fig. 2A, lanes 3 vs. 2 and 3 vs. 1, respectively). Although the intensities of these bands are low relative to our previous analyses of axonally synthesized proteins (Zheng et al., 2001), long duration exposure showed at least six bands of axonally synthesized cell surface proteins of varying molecular weights (Fig. 2B). This suggests that at least some locally synthesized proteins are inserted into the axoplasmic membrane. The biotinylated proteins isolated from the sheared axons were distinct from the biotinylated proteins isolated from the metabolically labeled cell body compartment (Figs. 2B vs. 2C,lane 2). The biotinylated proteins isolated from the cell body were also enriched compared to those seen in the unfractionated cell body samples (Figs. 2C, lane 2 vs. 1) arguing that we effectively separated cell surface from cytoplasmic proteins.
Fig. 2.
Membrane trafficking of axonally synthesized proteins. Cell surface biotinylation assay was used to determine if axonally synthesized proteins traffic to the membrane. DRG axons were metabolically labeled with [35S]-Met/Cys and axonal cell surface proteins were purified by biotinylation. Panel A shows autoradiogram of matched exposure time (1 week) for these different preparations. Several bands of biotinylated proteins isolated from the axon (lane 3) are enriched by the avidin pull down compared with the unfractionated lysate (lane 2). The non-biotinylated samples did not show any bands in avidin pull downs (lane 1). Panel B shows long duration exposure (10 wk) of the biotinylated, axonally synthesized proteins shown in lane 3 of panel A. Although the signal intensities are low, at least 6 labeled bands of varying molecular weights can be discerned. Panel C shows the samples from the cell body compartments that were metabolically labeled and cell surface biotinylated. The avidin-fractionated proteins isolated from cell body (lane 2) are similarly enriched when compared with unfractionated lysates (lane 1). Comparing the biotinylated axonal proteins (panel A, lane 3) with the cell body proteins (panel C, lane 2) shows that a different population of cell surface proteins appears to be synthesized in axonal vs. cell body compartments. Panel D show blots of the avidin pull-downs vs. lysates from cell body preparations that were probed with antibodies to eIF5 and TrkA, as representative of cytoplasmic and cell surface proteins. Immunoblotting with anti-eIF5 shows no contamination of the avidin pull-downs with cytoplasmic contents and blotting with anti-TrkA confirms enrichment of cell surface proteins in the avidin pull-downs compared with unfractionated lysates.
Immunoblotting for known cell surface and cytoplasmic proteins was used to more rigorously test for any cytoplasmic contents in these biotinylated samples. The translation initiation factor eIF5, whose known activities are restricted to the cytoplasm, was not detected in the streptavidin pull downs but this protein was readily detected in the supernatant from the pull downs from the cell body samples (Fig. 2D). The low protein yields from the biotinylated axonal preparations proved a challenge for immunodetection and we could not detect eIF5 in the axonal samples. However, we have previously shown that eIF protein localizes to PNS axons in vitro and in vivo (Zheng et al., 2001). Furthermore, immunoreactivity for the transmembrane receptor TrkA was enriched in the biotinylated samples compared to the supernatant and here we could detect TrkA in the axonal isolates (Fig. 2D). Together, these data indicate that isolated axons synthesize new proteins and a fraction of these proteins are indeed processed for insertion into the axoplasmic membrane.
ER constituents for classic co-translational secretion pathways are present in growing axons
Although the above experiments provide clear evidence for association of axonally synthesized proteins with the extracellular face of the axoplasmic membrane, these analyses offer no insight into how this might occur. We used immunolabeling for protein constituents of the RER and ER to determine if axons might contain these components needed for classical co-translational secretory mechanisms. Since the DRG neurons are uniquely non-polarized (i.e., they only extend axonal processes in culture (Zheng et al., 2001)), we included RGCs in these analyses as an example of a CNS neuron that has been shown to locally translate proteins in axons (Campbell and Holt, 2001). Because of limitations in antibody reactivity between rodents and Xenopus, we could not perform colocalizations for all components in both the sensory and retinal axons. However, these immunostaining results taken together show that ER components do indeed localize to sensory and retinal axons.
The rat DRG cultures showed clear intra-axonal immunoreactivity for SRP54, a protein component of the signal recognition particle (SRP) that binds to the N-terminal signal peptide of the nascent protein chain (Fig. 3A) (Keenan et al., 2001). Axonal SRP54 signals focally co-localize with immunoreactivity for ribophorin II, an ER protein involved in glycosylation of nascent proteins (Fu et al., 2000). The 7S RNA component of the SRP was also detected by RT-PCR using axonal RNA purified from DRG cultures as a template (data not shown), indicating that the complete SRP ribonucleoprotein complex is likely present in the axons. To determine if axons contain the translocon protein complex that extrudes transmembrane and secreted proteins through the membrane of the ER (Johnson and van Waes, 1999), we asked if the DRG axons showed immunoreactivity for translocon-associated protein α (TRAPα) constituent of the translocon. TRAPα was detectable in the DRG axons and the TRAPα immunoreactivity more frequently overlapped with ribophorin II in the axons than the SRP and ribophorin II colocalization noted above (Fig. 3B). We had previously shown that immunoreactivity for the lumenal ER chaperone proteins, calreticulin, grp78/BiP and ERp29, were vesicular in appearance in DRG axons (Willis et al., 2005). Similarly, immunoreactivity for protein disulfide isomerase (PDI), a lumenal ER chaperone that aids in the formation of disulfide bridges (Wang, 1998), showed vesicular appearing signals and these overlapped focally with TRAPα immunoreactivity (Fig. 3C). PDI signals also showed co-localization with SERCA in the branch points and distal regions of axons (Fig. 3D). Axonal signals for the ER chaperone calnexin also focally overlapped with the immunoreactivity for ribophorin II and PDI (data not shown).
Fig. 3.
Axons contain SRP and ER components for co-translational secretion of locally synthesized proteins. A, SRP54 (red) of the signal recognition particle shows focal co-localization (arrow) with ribophorin II (green). Signal for peripherin is shown in blue. This image displays a three-dimensional average projection through the distal axon of the cultured DRG neurons (22 optical XY planes taken at 0.26 μm intervals in the z-axis). B, Colabeling cultures of DRG neurons for the α subunit of translocon-associated protein (TRAPα; red) and ribophorin II (green) is shown as a merged image DIC. Signals for ribophorin II and TRAPα extend into the distal axon and frequently overlap along the axon shaft and branch points (arrow). C, A three-dimensional average projection of axon from cultures stained for TRAPα, (green), protein disulfide isomerase (PDI; red), and neurofilament (blue) is shown (15 optical XY planes taken at 0.17 μm intervals). TRAPα and PDI focally overlap in the branch point and growth cone (arrows). D, Three-dimensional average projection of distal axon from DRG cultured neurons showing PDI (red), SERCA (green), and neurofilament immunoreactivity (blue). PDI and SERCA focally overlap in the branch point and distal regions of the axon (arrows). Neurofilament is shown in blue (10 optical XY planes taken at ~0.15 μm intervals; inset shows corresponding DIC image). E–F, Xenopus retinal ganglion cell growth cones stained for calreticulin (E) and ribophorin I (F). G–H, Mouse retinal ganglion cell growth cones stained for Sec61α (G) and PDI (H). I, Western blots on protein lysates from Rat DRG cultured neurons (SRP54 and PDI) and J, Western blots on Xenopus st38 brain/eye lysate (ribophorin I and calreticulin) and mouse E18 brain lysates (Sec61-alpha and PDI) demonstrate specificity of antibodies. [Scale bar: A, C=10 μm; B, D=5 μm; E, F=10 μm; G, H=5 μm].
Cultures of Xenopus retina were used to determine if ER constituents also localize to retinal axons and growth cones. Xenopus RGCs have large growth cones where asymmetrical localization of translation factors and newly synthesized proteins has been demonstrated in response to guidance cues (Leung et al., 2006). Axons were stained with a rabbit polyclonal antibody against calreticulin, an ER-associated protein that buffers Ca2+ stores and mediates folding of glycoproteins (Gelebart et al., 2005). Punctate staining for calreticulin was visible all along the axon shaft and in the growth cone (Fig. 3E). The presence of calreticulin in the axon and growth cone agrees with previous results (Weclewicz et al., 1998; Willis et al., 2005) and suggests that ER may be present in these processes. Because a small fraction of total calreticulin is present on the cell surface of many cell types (Johnson et al., 2001), including the neuroblastoma-glioma hybrid cell line NG108-15 (Xiao et al., 1999), we asked whether RGC axons contain ribophorin I, an ER marker with better-defined function and localization than calreticulin, due to its characterized function in glycosylation (Kelleher and Gilmore, 2006). Immunolabeling for ribophorin I showed punctate staining visible all along the axon and in the growth cone similar to calreticulin. The ribophorin I signals were confined to the central domain of the growth cone, with virtually no staining in the lamellipodia or filopodia (Fig. 3F). As a further test for axonal ER in retinal neurons, mouse RGC cultures were used to overcome the relative lack of specific antibodies against Xenopus material. Immunofluorescence using antibodies against Sec61α, a second component of the translocon (Johnson and van Waes, 1999), and PDI showed that both proteins are present in mouse RGC axons and growth cones (Figs. 3G and H).
The above ER marker antibodies gave expected patterns of immunoreactivity on non-neuronal cell bodies. Calreticulin and ribophorin I staining on Xenopus XR1 glial cells and A6 kidney epithelial cells showed perinuclear localization consistent with the distribution of the ER/RER (Supplemental Fig. 1; see also (Guo et al., 2003)). Similar staining patterns were seen on the Schwann cells included in the rat DRG cultures stained with SRP54, ribophorin II, TRAPα and PDI antibodies (Supplemental Fig. 1A). Finally, Western blotting showed that most of the antibodies used for immunolabeling recognized a single band from SDS/PAGE fractionated cell lysates of very near the expected molecular weight (Fig. 3I and J). Taken together, these data argue that at least some axonal processes contain SRP and ER constituents that are focally aggregated into subcellular structures.
Golgi apparatus proteins localize to growing axons
Since both the rodent and Xenopus axons contained ER components needed for nascent protein translocation, folding, and post-translational modifications, we reasoned that Golgi proteins might also localize to these axonal processes. We first asked whether the KDEL receptor, a lumenal protein that binds to the ‘KDEL’ ER-retention signal element and shuttles resident ER proteins from cis-Golgi back to the ER (Cabrera et al., 2003), localized to axons. Immunolabeling DRG cultures with anti-KDEL receptor antibody showed clear intra-axonal signals that partially overlapped with immunoreactivity for the ER chaperone protein grp78/BiP (Fig. 4A). Since the anti-KDEL receptor antibody did not recognize the Xenopus material, we injected Xenopus embryos with a KDEL receptor YFP fusion construct (KDELR-YFP) to visualize localization of KDEL receptor in the RGCs. A6 cells transfected with KDELR-YFP showed perinuclear signals typical of ER and Golgi complex (Fig. 4D). In the Xenopus RGCs, KDELR-YFP extended into the distal axons and showed a punctate appearance similar to what was seen by immunolabeling in the DRG axons (Fig. 4E).
Fig. 4.
Axons contain Golgi components for secretion of locally synthesized proteins. A, Three-dimensional average projection image from DRG cultures stained with KDEL-receptor (red), BiP/grp78 (green), and neurofilament (blue). KDEL receptor and BiP/grp78 focally overlap in the axon shaft (16 optical XY planes taken at ~0.18 μm intervals). B, A single optical XY plane through the axon of the cultured DRG neuron stained for GM130 (red) and SERCA (green) is illustrated. Note that the axonal signals for GM130 are punctuate in appearance (arrow) and do not show any overlap with the vesicular signals for SERCA (arrowhead). C, A single optical XY plane through the axon of the cultured DRG neuron stained for TGN38 (red), SERCA (green), and neurofilament (blue) is displayed. Similar to panel B, TGN38 shows focal vesicular-appearing signals in the distal axons (arrow) that does not co-localize with the axonal signals for SERCA (arrowhead). D, A6 cell transfected with KDELR-YFP shows expected localization of KDEL receptor to the Golgi complex with some signal in the ER. E, Xenopus retinal ganglion cell expressing KDELR-YFP shows KDEL receptor localized in the central domain of the growth cone. F, Xenopus retinal ganglion cell growth cone stained for GM130. G, Isolated retinal cell on same cover slip as growth cone in (F) stained for GM130 shows expected Golgi localization. H, I, Western blot rat DRG (H) and Xenopus st40 eye (I) lysates demonstrate the specificity of the GM130 antibodies. [Scale bars: A, F=5 μm; B, C, E=10 μm; D, G=15 μm].
Immunostaining for the cis- and trans-Golgi proteins showed that the DRG axons displayed immunoreactivity for both Golgi matrix protein 130 (GM130) and trans-Golgi network protein 38 (TGN38) (Figs. 4B and C). GM130 is a cytoplasmic protein tightly bound to the Golgi complex; in other cellular systems, GM130 has been shown to partially overlap with medial- and trans-Golgi (Nakamura et al., 1995). However, we saw no definite overlap between GM130 and TGN38 immunoreactivity (data not shown), suggesting that separate cis- and trans-Golgi exist within the axonal compartment of the DRG neurons. Moreover, the signals for GM130 and TGN38 showed no overlap with axonal sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) immunoreactivity, suggesting the existence of distinct cis-Golgi and ER compartments (Figs. 4B and C). The anti-GM130 antibody signal was not as robust in the Xenopus materials, but the fluorescent signal in the axons and growth cone was clearly higher than background fluorescence (Fig. 4F). In A6 cells anti-GM130 produced a characteristic Golgi pattern of staining (Fig. 4G). GM130 and TGN38 immunoreactivity in Schwann cells and neuronal cell bodies showed the typical appearance of Golgi apparatus (Fig. 4G and Supplemental Fig. S1). Immunoblots for GM130 using lysates from rat DRG cultures detected a single band migrating at approx. 130 kDa (Fig. 4H). The predicted molecular weight of GM130 from the amino acid sequence is 111 kDa, but the protein has been characterized in rats as having a molecular weight of 130 kDa, but with a possible proteolytic product at 116 kDa (Nakamura et al., 1995). Immunoblotting lysates from Xenopus stage 40 eye gave a strong band at ~110 kDa and a weaker band at ~130 kDa (Fig. 4I). Taken together, these localization studies suggest that both DRG and RGC axons contain some components of the Golgi apparatus.
ER and Golgi membrane compartments in growing axons
The immunolabeling and transfection studies above clearly show that ER and Golgi apparatus proteins can localize into the axonal compartment of both PNS sensory neurons and CNS retinal ganglion cells. To gain a better estimate of ER and Golgi content in axonal processes of living neurons, cell permeant stains were used to visualize ER and Golgi apparatus. ER-Tracker Red is a BODIPY (boron dipyromethene difluoride) - Texas Red conjugate of Glibenclamide (glyburide), which binds to the sulphonylurea receptors of ATP-sensitive K+ channels that are prominent along the ER membrane (Hambrock et al., 2002). BODIPY FL C5-ceramide is a BODIPY FL conjugate of a sphingolipid analogue. It accumulates at high concentrations in the Golgi apparatus, most likely due to lipid components specific to the Golgi. BODIPY FL C5-ceramide fluoresces green at low concentrations, but at high concentrations it also gains a red emission due to intermolecular excimer formation, so red fluorescence can be used as a marker for the Golgi complex (Pagano et al., 1991).
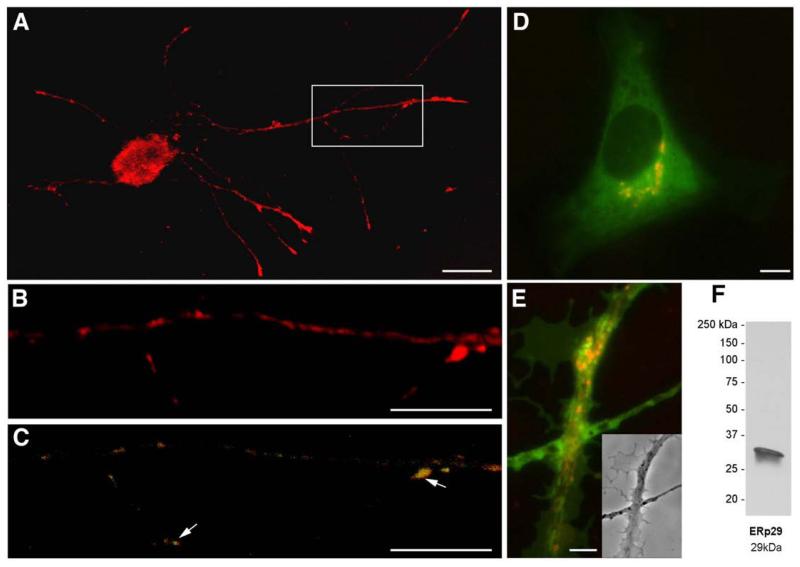
The DRG cultures treated with the ER-Tracker dye showed clear signal along the axonal processes (Fig. 5A and B). To confirm that this fluorescence was from axons and not from closely apposed Schwann cells, the DRG cultures were fixed with cold methanol, immunostained with antibodies to neurofilament and ERp29 and loaded with ER-Tracker. Clear axonal signals for ER-tracker were seen that appeared as focal puncta suggestive of vesicular compartments and showed focal overlap with immunoreactivity for ERp29 (Fig. 5C), an ER chaperone protein that we have previously shown is synthesized in adult rat DRG axons (Willis et al., 2005). Note that some ER-Tracker dye reactive foci were separate from the ERp29 immunoreactivity, suggesting that not all of the axonal ER compartment is reactive for this ER chaperone protein (Fig. 5C). In the cell body, the RER and SER form a single continuous lumen. This raises the possibility that the ER-like structures seen by immunostaining are membrane compartments of ER that are contiguous with that in the cell body. Imaging live DRG neurons loaded with the ER-Tracker dye showed identical discontinuous puncta along the axons (data not shown), arguing that the vesicular pattern noted above was not an artifact of fixing the cells for immunolabeling. To determine if we were missing contiguous spans of ER in these images, 3D maximal projections were generated from optical planes taken through the entire thickness of the axon shaft running from proximal to distal axonal processes (Fig. 5B). Even though the ER-Tracker dye showed that we were likely missing some ER compartments with the immunolabeling, there was no visibly discernable connection between ER-Tracker positive punctae along the axon shaft. This suggests that the ER-like structures defined by the immunolocalization and staining methods above represent discontinuous compartments along the axon shaft rather than constituting an extension of the cell body ER.
Fig. 5.
The axonal ER compartment is not contiguous with the cell body organelle network. Cultures of injury-conditioned DRG neurons were fixed and stained with the ER-Tracker red dye (red) and by indirect immunofluorescence for ERp29 (green) and neurofilament (blue). A–C, Representative confocal image sequences from an XYZ stack of a DRG neuron are shown where cell body, proximal axon, and distal axon can easily be traced in a single field. Panel A shows a three-dimensional average projection of confocal image of ER-Tracker red signal in cell body and axonal process. The boxed regions of the mid axon shaft in panel A are shown at higher magnification in panels B and C. Panel B shows a high magnification of the ER-Tracker signal in the mid-axon shaft from panel A. This three-dimensional maximal projection was generated from 10 XY optical planes taken over 0.15 μm in the z-axis. Note that the ER-Tracker signals show focal concentrations moving from proximal to distal (left to right) that show no clear continuity despite the projecting maximal signals. Panel C shows the same ER-Tracker signals (red) from panel B merged with immunosignals for ERp29 (green). Note that the areas of highest ER-Tracker signals from panel B show co-localization with ERp29 (arrows). D, A6 cell stained with BODIPY FL shows green staining of all cell membranes, with selective red staining of the Golgi complex. E, Axons stained with BODIPY FL show green staining throughout, with occasional areas of brighter red fluorescence, suggesting the presence of structures with Golgi-like lipid composition. F, Western blots on protein lysates from rat DRG cultured neurons to exhibit the specificity of the ERp29 antibody. [Scale bars: A=25 μm; B, C=15 μm; D, E=5 μm].
Staining A6 cells with BODIPY FL C5-ceramide revealed a characteristic Golgi pattern, where all cellular membranes are stained green, but only the Golgi complex is stained red (Fig. 5D). In the RGC axons, there are areas that stain red more prominently than green, suggesting that they might have Golgi-like lipid composition (Fig. 5E). The DRG neurons showed a similar staining pattern with red emitting punctae for BODIPY FL C5-ceramide along the axons (data not shown).
Pharmacological disruption of the Golgi apparatus prevents growth cone turning
We next asked whether the axonal protein secretory pathway is functionally important for axonal development. To test this, we used brefeldin A (BFA), a fungal metabolite that disrupts intracellular membrane trafficking, especially ER-Golgi traffic, by inhibiting ADP-ribosylation factor (ARF) guanine nucleotide exchange factors (GEFs) (Anders and Jurgens, 2008). BFA acutely and reversibly causes the redistribution of the Golgi into the ER within 10 min (Doms et al., 1989), which we confirmed using BODIPY-labeled A6 cells (Fig. 6A–C). We asked whether this pharmacological disruption of the Golgi apparatus would affect growth cone turning. Engrailed-2 (En-2) is a homeodomain transcription factor that also acts as a secreted guidance cue that directly repels temporal axons in vitro through a protein synthesis-dependent mechanism (Brunet et al., 2005). A gradient of En-2 caused repulsion of temporal axons in a growth cone turning assay (mean turning angle −11°±4.2°, n=14), which was blocked by pre-incubation of growth cones with 10 μg/ml BFA for 10 min (+3.0°±3.7°, n=18) (Fig. 6D–K). Importantly, growth cone turning was eliminated without significantly changing the growth rate (p=0.9394, Mann-Whitney test) (Fig. 6L). These observations suggest that the translation-dependent growth cone turning response of developing axons also requires localized synthesis and Golgi-associated processing of membrane proteins. Interestingly, Hess et al. (1999) showed that the growth cones of the injury conditioned rat DRG neurons rapidly retract upon exposure to BFA (Hess et al., 1999). Thus, regenerating axons may similarly utilize locally synthesized proteins.
Fig. 6.
Disruption of Golgi apparatus with brefeldin A (BFA) prevents growth cone turning away from Engrailed-2 gradient. A–C, Prominent perinuclear Golgi labeling in BODIPY FL-stained A6 cells (A) is abolished by 10 min treatment with 10 μg/ml BFA (B). Degree of Golgi disruption is quantified in (C). D-I, Xenopus stage 32 temporal retinal axons turn away from a source of En-2 after 60 min (D–F), but BFA (G-I) blocks this turning response. Trajectory plots of axons for En-2 (F) and En-2+BFA (I). J, Mean turning angles to En-2, with and without BFA (*p<0.05, Mann–Whitney test). K, Cumulative distribution plot of turning angles to En-2, with (red squares) and without (blue diamonds) BFA. L, BFA does not affect the mean axon extension rate during the turning assay. Numbers inside bars indicate number of growth cones analyzed for each condition. [Scale bars: A, B=10 μm; D, G=15 μm].
Axotomy alters the distribution of targeting machinery in regenerating axons
Most analyses of axonal protein synthesis have focused on growing axons. Our own observations indicate that axonal protein synthesis is particularly robust in the rapidly growing axons of injury-conditioned DRG (Willis et al., 2005). An increase in localized protein synthesis could be accompanied by a relative increase in the capacity to target locally synthesized proteins for secretory pathways. To address this possibility, we compared the immunoreactivity for SRP54, ER and Golgi apparatus proteins in cultures of injury conditioned vs. naive DRG neurons. The DRGs were cultured overnight in the presence of the RNA polymerase II inhibitor, DRB. DRB inhibits ~95% of new RNA synthesis in rat DRG cultures over 24 h but does not affect neurite growth (Smith and Skene, 1997; Twiss et al., 2000; Willis et al., 2007). Thus, the results implicate post-transcriptional events in the two preparations rather than alterations in gene expression that might occur in vitro. There were no apparent changes in immunoreactivity for SRP54, ER, or Golgi proteins in the cell bodies or Schwann cells (data not shown). Using matched exposures, axonal signals for SRP54, PDI, SERCA and KDEL receptor appeared more intense in the axons of injury-conditioned neurons compared with those of naive neurons (Figs. 7A vs. E, D vs. H, I vs. M, and J vs. N). On the other hand, immunoreactivity for ribophorin II, TRAPα, GM130, and TGN38 showed no apparent difference in intensity of immunofluorescent signals (Figs. 7B vs. F, C vs. G, K vs. O, and L vs. P). Interestingly, the aggregation of SRP54 and the ER proteins shifted with injury. SRP54 shifted from small puncta to larger plaque-like distribution in the axons of injury-conditioned neurons (Figs. 7A vs. E). The ER proteins, PDI and SERCA, also appeared to aggregate in the axons of injury conditioned neurons (Figs. 7D vs. H and I vs. M). Although the relative immunoreactivity for ribophorin II and TRAPα did not appreciably change with injury, these proteins did shift their aggregation in axons of the injury conditioned neurons. Ribophorin II showed more defined vesicular appearing loci in the axons of injury-conditioned neurons compared to more diffuse immunoreactivity in axons of naive neurons (Figs. 7B vs. F). TRAPα was concentrated at foci along the axons of the injury-conditioned DRG neurons (Fig. 7G). The cis- and trans- Golgi proteins showed no change in levels or localization with injury conditioning.
Fig. 7.
Components for co-translational targeting machinery are enriched in axons of injury-conditioned DRG neurons. Cultures of 7 day injury-conditioned (E–H and M–P) and naive DRG neurons (A-D and I-L) were immunostained with antibodies to NF and SRP54 (A, E), ribophorin II (B, F), TRAPα (C, G), PDI (D, H), SERCA (I, M), KDEL receptor (J, N), GM130 (K, O), or TGN38 (L, P). Each panel displays a representative merged epifluorescent image of mid-axon shaft of 20 h DRG cultures with NF signals in blue and SRP, RER, ER and Golgi proteins in red. The immunoreactivity for SRP54, PDI, SERCA and KDEL receptor are relatively increased in the axons of injury-conditioned neurons compared with those of naive neurons. Immunoreactivity for ribophorin II, TRAPα, GM130 and TGN38 appeared relatively equivalent between the injury-conditioned and naive DRG neurons. [Scale bar=10 μm].
Discussion
Most studies of axonal protein synthesis have focused on synthesis of cytoplasmic and cytoskeletal proteins. A number of different cytoplasmic proteins have been shown to be synthesized in developing and regenerating axons (Bassell et al., 1998; Cox et al., 2008; Eng et al., 1999; Hanz et al., 2003; Willis et al., 2005; Wu et al., 2005; Yudin et al., 2008; Zheng et al., 2001). However, sensory axons from injury conditioned adult DRG neurons contain several mRNAs encoding membrane and secreted proteins (Willis et al., 2007) and mRNAs encoding transmembrane proteins have been detected in developing axons (Brittis et al., 2002; Piper et al., 2008). Both invertebrate and vertebrate axons have been shown to target exogenous epitope-tagged receptor proteins encoded by axonal mRNAs to the axoplasmic membrane (Brittis et al., 2002; Spencer et al., 2000), suggesting that axonally synthesized proteins can be appropriately trafficked to the membrane. Many of the mRNAs encoding membrane and secreted proteins that we have identified in DRG axons encode a signal peptide (e.g., HCN4, NMP35, CACNA1); thus, these locally synthesized proteins would presumably need access to classical secretory mechanisms. EphA2, which is translated in developing chick commissural axons (Brittis et al., 2002), similarly contains a classic signal peptide. The cell surface biotinylation analyses of isolated axons shown in Fig. 2 supports the notion that at least some axons can insert locally synthesized proteins into the membrane. With the low sensitivity of the metabolic labeling and inability to detect secreted proteins, we surely have underestimated the complexity of axonally synthesized membrane and secreted proteins with these analyses; nonetheless, this is the first biochemical evidence for membrane localization of axonally synthesized proteins.
Without ultrastructural evidence for RER or Golgi apparatus, the mechanism for getting axonally synthesized proteins to the cell surface has not been known. Previous studies have localized some ER proteins to axons (Krijnse-Locker et al., 1995; Weclewicz et al., 1998) and the resident-ER chaperone proteins, calreticulin, grp78/BiP, and ERp29 are synthesized in DRG axons (Willis et al., 2005). However, these ER chaperone proteins could have additional functions in the axonal compartment. For example, there have been frequent reports that calreticulin has extra-ER functions (Johnson et al., 2001). Despite the possibility of non-ER functions for these proteins, the breadth of RER proteins that we have detected in the DRG and RGC axons, as well as the colocalization of these axonal proteins with well-characterized ER markers, make a strong case for some RER equivalent structure existing in axons. Ribophorin proteins are part of the complex that transfers oligosaccharides to nascent proteins in the initial step of N-linked glycosylation (Fu et al., 1997) and dendritic localization of ribophorins was previously used to support evidence that these processes can glycosylate newly synthesized proteins (Torre and Steward, 1996). Ribophorin is retained in the ER (Fu and Kreibich, 2000) and associates with the Sec61 of the translocon complex (Shibatani et al., 2005). Thus, detection of TRAPα and Sec61α in axons provides evidence that the full ER-membrane translocon complex is likely transported into axons. Extension of SRP54 and 7S RNA into axons indicates that these processes also contain the machinery needed for targeting ribosomes that are generating proteins with signal peptides to the ER, with the nascent polypeptides gaining access to the ER lumen through the translocon.
Although the ensemble of ER components that we have detected in axons argues that classical co-translational targeting mechanisms for protein secretion exist in axons, ribosomes docking with the ER should form ultrastructurally recognized RER. While there are several reports of ribosomes localizing into vertebrate axons, including polysomal profiles (Bassell et al., 1998; Koenig and Martin, 1996; Koenig et al., 2000; Tennyson, 1970; Zelena, 1970; Zelena, 1972; Zheng et al., 2001), none have shown that ribosomes are bound to membranes, the defining ultrastructural feature of RER. One possible explanation for these negative findings is that ribosomes may be only rarely attached to endomembranes, if local translation of membrane proteins is used only under certain conditions, such as rapid responses to extracellular stimuli, as might be necessary in growing or regenerating axons that are actively pathfinding. For example, Xenopus retinal axons grown in serum-free medium show significant amino acid incorporation only after acute stimulation with guidance cues (Campbell and Holt, 2001). In contrast, previous electron microscopy studies of axons have used in vivo axons (Tennyson, 1970; Zelena, 1970; Zelena, 1972) or axons grown under constant low-level stimulation by growth factors (Bunge, 1973; Yamada and Wessells, 1971). Though tonic stimulation may accurately reflect in vivo exposure to guidance cues, it may not reveal the axon’s full secretory capacity. Moreover, minimal quantities of Golgi are enough to restore secretory capacity in cytoplasts lacking Golgi (Pelletier et al., 2000), suggesting that axons may only need tiny fragments of Golgi and RER, which may be difficult to detect ultrastructurally.
In the cell body, the RER and SER form a single contiguous membrane bound organelle. This raises the possibility that the ER-like structures that we have defined by immunolocalization could be contiguous with the cell body RER and ER. Dailey and Bridgman (1991) demonstrated ER-like tubulovesicular arrays that paralleled micro-tubules in axonal growth cones (Dailey and Bridgman, 1991), but this study was limited to growth cones so continuity with cell body organelles was not considered. The immunoreactivity for RER and ER proteins that we detected in the axons and growth cones is more consistent with separate vesicular compartments distributed along the axon. Even the ER Tracker dye, which concentrates in ER but could effectively stain non-ER components, showed obvious discontinuity between the ER-like vesicular compartments in the axons. This would be similar to the ‘Golgi outposts’ that have been demonstrated in developing Drosophila (Herpers and Rabouille, 2004) and the ‘satellite secretory’ machinery that has been demonstrated in dendritic spines (Pierce et al., 2001). It is conceivable that the classical morphology of ER (and Golgi) is only seen in the perikaryon for polarized cells like neurons, with the morphology of these organelles being different in distal cytoplasmic processes. In budding yeast, an actin-based transport mechanism gives a means for cortical ER inheritance by anchoring cytoplasmic ER tubules at the bud tip (Du et al., 2004). Compartmentalization of the ER at the neck of the budding yeast is due in part to the activities of septin proteins (Luedeke et al., 2005). Septin 7 has also been shown to localize to the base of axonal processes of cultured hippocampal neurons (Tada et al., 2007). Thus, one could envision a similar budding mechanism for delivering ER into axons, and myosin-based transport of SER vesicles on microfilaments has been documented in the squid giant axon (Langford,1999; Tabb et al.,1998). Moreover, the primitive eukaryote Giardia lamblia during the nonencysting phase appears not to have morphologically recognizable Golgi apparatus, yet can still secrete proteins using a brefeldin A-sensitive mechanism (Lujan et al., 1995). It is unclear what role the stacked morphology of the Golgi apparatus plays, as the two budding yeasts Pichia pastoris and Saccaromyces cerevisiae have similar secretion kinetics, but Golgi cisternae are stacked in P. pastoris but not in S. cerevisiae (Losev et al., 2006; Mogelsvang et al., 2003; Wooding and Pelham, 1998). These findings suggest that an unconventional secretory apparatus in axons may be an evolutionarily ancient secretory mechanism.
Studies in developing neurons suggest that the distinct morphology of axons and dendrites is derived, at least in part, by differential reliance on secretory mechanisms. Disrupting ER to Golgi transport seems to preferentially deplete membrane supply of dendrites but not axons in developing Drosophila and rodent neurons (Ye et al., 2007). Horton and Ehlers (2003a) have detected two distinct modes for ER to Golgi transport for dendrites: one that transports membranes and lipids from the cell body and a second localized or ‘outpost’ version that is used for locally synthesized proteins (Horton and Ehlers, 2003a). This differential membrane trafficking contributes to neuronal polarity in hippocampal and cortical neurons (Horton and Ehlers, 2003b), and even can define asymmetric growth within dendritic arbors (Horton et al., 2005). These studies on polarizing CNS neurons seem to exclude any role in axonal outgrowth for secretion of locally synthesized axonal proteins. However, studies of axonal protein synthesis emerging over the last decade indicate that axonally and dendritically synthesized proteins provide very different functions for these processes (Wang et al., 2007). With the effect of inhibiting Golgi function on axonal turning shown in Fig. 6, it is quite likely that locally synthesized proteins, including membrane and/or secreted proteins, serve quite different functions in developing axons than in the dendrites.
Now that we know that growing axons can also use an outpost-like mechanism to secrete locally synthesized proteins, the actual function of the axonally synthesized membrane proteins needs to be defined. Though slow axonal transport is too slow to account for the maintenance of cytosolic proteins in long axons (Alvarez et al., 2000), in theory fast axonal transport could, at 50–200 mm/day, maintain the baseline level of membrane and secreted proteins. Thus, the function of local translation of membrane and secreted proteins is less likely to be axonal maintenance and more likely to be rapid responses to local conditions. Indeed, our results show that repulsive turning by temporal retinal axons in response to the guidance cue En-2 requires intracellular membrane trafficking, because it is inhibited by brefeldin A. An axonal site of action by brefeldin A is supported by the finding that axons severed from their cell bodies can also be repelled by En-2 (Brunet et al., 2005), indicating that the intracellular membrane trafficking required for turning must occur in the axon. It is true that brefeldin A disrupts other intracellular membrane trafficking pathways besides ER-Golgi trafficking, such as endosome-lysosome trafficking (Anders and Jurgens, 2008), although it apparently does not disrupt trafficking between endosomes and the plasma membrane (Schonhorn and Wessling-Resnick, 1994), possibly because the GEF that activates ARF1 for endocytosis is unaffected by brefeldin A (Kumari and Mayor, 2008). As with any pharmacological approach, we cannot entirely rule out the possibility that the blockade of turning is due to off-target effects of brefeldin A. Still, together with the redistribution of ER markers after injury conditioning (Fig. 7) and the attenuation of rapid axonal outgrowth from injury conditioned DRG neurons previously published (Hess et al., 1999), these results suggest that the axonal ER and Golgi we have observed in this study is functionally important for axon guidance and regeneration. This is consistent with circumstantial evidence pointing to a role for axonal synthesis of the EphA2 guidance receptor in spinal commissural axons after crossing the midline, to allow the growth cone to be repelled from the midline (Brittis et al., 2002).
Why might local protein secretion be important for axon guidance and regeneration? One possibility is local synthesis of transmembrane cell adhesion molecules. For example, the mRNA of the protocadherin NFPC has been detected in RGC axons (Piper et al., 2008). Another possibility is that local synthesis of receptors may be involved in growth cone adaptation to changing levels of guidance cues. Growth cones lose sensitivity to guidance cues after exposure to a low dosage, but recover their responsiveness after a period of resensitization. The latter process requires protein synthesis (Ming et al., 2002; Piper et al., 2005), perhaps of guidance cue receptors. Although our En-2 experiments did not involve adaptation, growth cone attractive turning has been reported to involve ‘zig-zag’ patterns of outgrowth, suggesting that the growth cone goes through alternating period of desensitization and resensitization (Ming et al., 2002); such adaptive responses may be important for turning away from En-2. It remains unclear why the transmembrane proteins whose mRNAs we have found in DRG axons should be locally translated and exported. Some of them (HCN4, CACNA1c) are ion channels, perhaps allowing local regulation of the axon’s primary function, transmission of action potentials. NMP35 has been localized to synapses in the CNS (Schweitzer et al., 2002), but otherwise its function is unknown. Local translation of ER chaperone proteins (Willis et al., 2005) may also serve to rapidly increase the translational and secretory capacity of the axon.
Experimental methods
Cell culture preparations
For DRG cultures, adult male Sprague–Dawley rats (140–160 g) were injury-conditioned by sciatic nerve crush at mid-thigh level 7 d prior to culture (Smith and Skene, 1997). L4-6 DRGs were dissociated using collagenase and trypsin and cultured on poly-l-lysine/laminin-coated surfaces at 37 °C with 5% CO2 for 16–24 h as previously described (Twiss et al., 2000). Cultures were then processed for immunofluorescence or fractionation of axonal and cell body compartments for RNA and protein analyses as described below. For the immunofluorescence studies, dissociated DRGs were cultured at low density on glass coverslips. For isolation of axons, the dissociated DRGs were plated onto a porous polyethyline-tetrathalate [PET] membrane (8 μm pores; BD Falcon) (Zheng et al., 2001).
For preparation of retinal cultures, Xenopus embryos were obtained by in vitro fertilization and raised in 0.1x Modified Barth’s Solution (MBS) at 14–20 °C. Embryos were anesthetized and dissected in a 1:1 mixture of 60% L-15 (Gibco) and 0.4 mg/ml in MS222 solution at pH 7.7 (1xMBS) with 100 units/ml penicillin, 100 μg/ml streptomycin and 250 ng/ml fungizone (PSF; Gibco). The eyes were dissected out and cut into 3–4 pieces. For En-2 turning assays, only the extreme temporal portions of stage 32 eyes were used. Eye pieces were gently washed in culture medium and plated ganglion cell side down on cover slips pre-coated with 10 μg/ml poly-l-lysine (Sigma) and 10 μg/ml laminin (Sigma) or 1 μg/ml fibronectin (Sigma). These cultures were incubated at 20 °C overnight to allow axon outgrowth. To generate mouse retinal cultures, retinas were dissected from E15 mouse embryos (strain C57/BL6, gift of A. Ferguson-Smith) in a 1:1 mixture of D-MEM and F-12 medium with PSF, and the lens, vitreous humor, and pigment epithelium were removed. Eight pieces were cut from the peripheral retina and plated onto cover slips pre-coated with 25 μg/ml poly-l-lysine and 12.5 μg/ml laminin. Explants were cultured overnight in DF/PS supplemented with N1, 1% BSA, and 0.4% methylcellulose (Sigma), pH 7.4, at 37 °C.
XR1 glial cells (Sakaguchi et al., 1989) and A6 kidney epithelial cells were maintained in 60% L15 medium with PSF and 5% FBS (for A6 cells) or 10% FBS (for XR1 cells), at 20°C.
Fractionation of axons
Axons were physically separated from the cell body using dissociated rat DRG cultures prepared as above on porous PET membranes that had been coated with poly-l-lysine/laminin. To isolate the axonal compartment, the cellular contents from the upper membrane surface were repeatedly scraped away using with a cotton-tipped applicator as previously described (Zheng et al., 2001). To isolate the cell body compartment, the axonal processes were scraped away from the lower membrane surface leaving cell bodies, axonal segments that had not traversed the membrane, and non-neuronal cells. These fractionated axonal and cell body preparations were then used for metabolic labeling or RNA isolation as outlined below. Amplification of β-actin, γ-actin, and MAP2 mRNAs was used to assess purity of the axonal preparations (Willis et al., 2005).
Metabolic labeling and cell surface biotinylation
For metabolic labeling, the axonal and cell body compartments were isolated as above at 16 h in culture. These were transferred to a second tissue culture dish containing 2 mCi/ml [35S]-methionine/cysteine (ICN Biochemicals) in DMEM/F12,1% N1 supplement, and 10% horse serum for 3.5 h at 37 °C, 5% CO2. Cell surface proteins were biotinylated as previously described with minor modifications (Chang et al., 2003). Briefly, the metabolically labeled preparations were rinsed with phosphate buffered saline (PBS) and then incubated with 0.5 mg/ml Sulfo-NHS-LC-Biotin (Pierce) in PBS for 2 h at 4 °C. Controls consisted of axons and cell body preparations that were identically processed but without the addition of Sulfo-NHS-LC-Biotin. Biotinylation solutions were removed and replaced with ice-cold DMEM with glutamine for 10 min at 4 °C. Samples were then rinsed gently with ice-cold PBS and lysed in radio-immunoprecipitation assay (RIPA) buffer. The lysates were cleared by centrifugation at 15,000 ×g and biotinylated proteins were precipitated overnight at 4 °C with streptavidin-agarose beads (Calbiochem). The beads were gently pelleted by centrifugation (3000 ×g), washed 5 times with 1 ml of ice-cold PBS, and resuspended in 2× Laemmli sample buffer. After denaturation by boiling for 5 min, proteins were resolved by denaturing polyacrylamide electrophoresis (SDS/PAGE). For visualizing labeled proteins, gels were fixed in isopropanol:acetic acid: water (25:10:65) for 30 min and transferred to Amplify fluorography reagent (Amersham Biosciences) for 30 min, dried, and exposed to pre-flashed Kodak Hyper film (Amersham) with enhancing screen at −80 °C. To test whether we had enriched for cell surface proteins and if any cytoplasmic contents contaminated the biotinylated isolates, a portion of each isolated protein samples was fractionated by SDS/PAGE and used for immunoblotting (see below).
Axonal RNA analyses
RNAqueous Micro kit (Ambion) was used to extract RNA from DRG cultures per the manufacturer’s protocol. RNA isolated from adult rat brain using 4 M guanidium isothiocyanate/phenol-chloroform extraction (Chromozinski and Sacchi,1987) was used as a positive control for polymerase chain reactions (PCR). RNA concentrations were determined by fluorometry using RiboGreen reagent (Molecular Probes) (Willis et al., 2005).
Reverse transcription coupled PCR (RT-PCR) was used to detect axonal mRNAs. For this, 200 ng of RNA from cell body or axonal compartment was used as a template for RT using the iScript cDNA synthesis kit per the manufacturer’s protocol (Biorad). Reverse transcription reactions were diluted 5-fold for PCR with HotstarTaq Mastermix (Qiagen). The primers for rat β-actin, γ-actin, and MAP2 mRNAs have been published (Willis et al., 2005). Other primers used were as follows: NMP35 (accession number AF044201) — forward, 5′-ACCTGACTCTGGCTTGCTGT and reverse, 5′-CGAGGAGGAGTCCACTGAAG; HCN4 (accession number AF247453) — forward, 5′-CAGCCTCCTGGAGAGTTGTC-3′ and reverse, 5′-GAGCTTCAGGTCCTGTGTG-3′; CACNA1 (accession number NM012517) — forward, 5′-TTCCCTCAGGCTGTGCTACT-3′ and reverse, 5′-ATGGGGACCAAGGATAGACC-3′; and, 7S RNA (generated from conserved regions of human and Xenopus 7S RNA sequences, accession numbers X01037 and X01056, respectively) — forward, 5′-GCATCAATATGGTGACCTCCC-3′ and reverse, 5′-TTCCCACTACTGATCAGCACG-3′. After 15 min at 95 °C for hotstart, 35 cycles of PCR was performed consisting of 45 s at 95 °C, 45 s at 58 °C, and 3 min at 72 °C. Negative control consisted of RT-PCR performed without the addition of reverse transcriptase. PCR products were resolved on ethidium bromide containing agarose gels and visualized under UV light.
Immunofluorescence
All steps below were performed at room temperature unless specified otherwise. The conditions for fixation, permeabilization and blocking were slightly different for the DRG and retinal cultures. For DRG cultures, coverslips were fixed in 4% paraformaldehyde (PFA) for 20 min, rinsed in PBS, and then permeabilized in PBS +0.02% Triton X-100 for 15 min. The DRG cultures were rinsed in PBS and then blocked in 5% donkey serum in PBS for 1 h. The retinal cultures and cell lines were fixed in 2% PFA for 30 min, rinsed in PBS, and then permeabilized by 0.1% Triton X-100 for 3–5 min. These cultures were rinsed in PBS, and then blocked in 5% heat-inactivated goat serum for 30–60 min. Both the DRG and retinal culture samples were incubated in primary antibodies diluted in blocking buffer for 1–3 h at room temperature or overnight at 4 °C. Samples were incubated in secondary antibody diluted in blocking buffer for 45–60 min, followed by three PBS washes and mounting. The following primary antibodies were used: mouse anti-neurofilament (1:400; Sigma), chicken anti-neurofilament (1:1000; Millipore/Chemicon), rabbit anti-peripherin (1:200; Millipore/Chemicon), mouse anti-SRP54 (1:200; BD-Transduction Lab.), mouse anti-ribophorin I (Serotec, 1:50), goat anti-ribophorin II (1:100; Santa Cruz Biotech.), rabbit anti-TRAPα (1:300; Christopher Nicchitta, Duke Univ., Durtham, NC), rabbit anti-Sec61α (1:250; Affinity Bioreagents), mouse anti-calreticulin (1:200; BD-Transduction Lab.), rabbit anti-calreticulin (1:100; Novus), rabbit anti-SERCA (1:200; Jonathan Lytton, Univ of Calgary, Canada), rabbit anti-calnexin (1:100; Millipore/Chemicon), rabbit anti-ERp29 (1:400; Affinity Bioreagents); mouse and rabbit anti-PDI (1:200 and 1:500, resp.; Stressgen); mouse anti-KDEL-receptor (1:800; Stressgen), mouse anti-GM130 (1:200; Abcam or 1:50; BD Transduction), mouse anti-TGN38 (1:200; Affinity Bioreagents), rabbit anti-CACNA1c (Cav1.2, 1:100; Alamone Labs), and rabbit anti-HCN4 (1:100; Millipore/Chemicon). Antibody to rat NMP35 was generated in chicken using a synthetic NMP35 peptide (SYEEATSGEGLKAGAF; Swisprot accession # AAC32463) as previously described (Schweitzer et al., 1998) by Gene-Tel Labs (Madison, WI); the chick anti-NMP35 was used at 1:200. Secondary antibodies were as follows: Cy3 conjugated goat anti-rabbit IgG (1:700; Calbiochem); Cy3 conjugated goat anti-mouse (1:700; Calbiochem); FITC, Texas red, or Cy5 conjugated donkey anti-goat, -chicken, -rabbit or -mouse antibodies (1:300; Jackson Immunoresearch).
For confocal imaging, fluorescence was analyzed using a Leica TCS/SP2 confocal system on an inverted Leica DMIRE2 microscope equipped with galvanometer stage for fine Z sectioning. All confocal images were acquired and processed using Leica confocal Software package; processing for individual images is described in the results section. For RGC growth cones and cell lines, fluorescent images were captured on a Nikon TE2000-U microscope at 100×, using a Nikon C-SHG1 mercury lamp and DAPI, FITC, and G-2A filters (Nikon). For epifluorescent imaging of DRG neurons, images were captured at 63X using Orca ER CCD camera (Hamamatsu) mounted on a Leica DMRXA2 fluorescent microscope. Epifluorescent images were processed using Openlab software package (Improvision).
Dye-based detection of ER and Golgi apparatus
ER-Tracker Red dye (Molecular Probes) was resuspended in anhydrous dimethylsulfoxide (DMSO) and was diluted to 1 μM in PBS immediately prior to use. ER-Tracker Red was added to cultures in a final concentration was 1 nM per the manufacturer’s instruction. In some instance, the cultures were methanol (cold) fixed for 10 min and colabeled with anti-NF as above. The BODIPY FL C5-ceramide (Molecular Probes) was resuspended in sterile deionized water and diluted to 5 μM in Hank’s buffered salt solution (HBSS) immediately prior to use. This working dilution was added directly to cultures at a final concentration of 5 μM and incubated for 30 min at 4 °C followed by several cold washes and 30 min room temperature incubation prior to imaging. Brefeldin A (BFA) induced disruption of the Golgi apparatus was measured by blinded scoring of BODIPY labeled A6 cells treated with 0, 5, and 10 μg/ml BFA.
Immunoblotting
For western blotting, protein lysates were prepared from cultures or tissues by lysis in RIPA buffer and normalized for content by Bradford assay. Proteins from these extracts as well as from the cell surface biotinylation assays were resolved by SDS/PAGE and immobilized on polyvinylidene difluoride (for DRG neurons) or nitrocellulose membranes (for retinal cultures and tissues). Membranes were blocked with 5% non-fat milk diluted in TRIS-buffered saline with 0.05%-0.2% Tween 20 (TBST) for 1 h. Blots were then incubated in primary antibody diluted in blocking buffer for 1 h at room temperature or overnight at 4 °C. Blots were then rinsed several times in TBST and incubated in secondary antibody conjugated to HRP for 1 h at room temperature. After a second series of rinses, immunoreactivity was detected by chemiluminescence (ECLplus or ECLadvance; Amersham, Piscataway, NJ). Primary antibodies were as follows: rabbit anti-TrkA-ex (1:5,000) (Molliver et al., 1995), rabbit anti-eIF5 (1:1000; Santa Cruz), mouse anti-ribophorin I (1:1000, Serotec), rabbit anti-calreticulin (1:2000; Novus), rabbit anti-Sec61α (1:5000; Affinity Bioreagents), rabbit anti-PDI (1:4000; Stressgen), and mouse anti-GM130 (1:250; BD Transduction). The following horse radish peroxidase (HRP) conjugated secondary antibodies were used: goat anti-mouse (1:5000; Abcam), goat anti-rabbit (1:20,000; Zymed), and donkey anti-rabbit (1:4000; Cell Signaling).
Expression of fluorescent fusion proteins
The KDEL receptor-EYFP fusion construct (gift from H.-D. Soeling, M.P.I. Goettingen) consisted of the human KDEL receptor (also known as ERD2) cloned into pEYFP-N1 (Clontech) (Majoul et al., 2001). 4-, 8-, and 16-cell stage Xenopus embryos were de-jellied with 2% cysteine (1X MBS, pH 8) and injected with 250–500 pg of DNA (5 nl of solution). Embryos expressing the fusion construct in the eye were selected under epifluorescence for dissection.
A6 cells were transfected with KDELR-YFP using LipofectaminePlus (Invitrogen). For this, 0.4 μg plasmid was incubated with 1 μl Lipofectamine and 4 μl Plus reagents in 60% L-15 for 15 min, and cells were incubated with the DNA mixture in serum- and PSF-free medium. After 3 h, serum was replenished by adding an equal volume of 60% L-15 with 10% serum. Transfected cells were microscopically analyzed 1 day after transfection.
Protein production
En-2 protein (a gift from A. Prochiantz) was produced in isopropyl-β-D-thiogalactoside-inducible bacteria as described previously (Montesinos et al., 2001). Purity of the protein was analyzed using SDS-polyacrylamide gel electrophoresis, and its concentration determined by the Nanodrop ND-1000 Spectrophotometer (version 3.0.1, Nano-Drop Technologies). It was then stored at −20°C in culture medium supplemented with 10% glycerol. Prior to experiments, dialysis was performed against PBS to remove glycerol.
Xenopus growth cone turning assays
Growth cone turning assays were performed as previously described (Lohof et al., 1992). Briefly, a stable gradient of En-2 was generated by pulsatile ejection (3 psi, 20 ms, 2 Hz) from a micropipette (inner diameter 1–2 μm). The micropipette was placed 100 μm from the center of a growth cone, at a 45° angle to the direction of growth. Micropipettes were pulled using a Flaming/Brown micropipette puller Model P-97 (Sutton), and pulsation was generated by a picospritzer (General Valve). Growth cones were exposed to the gradient for 1 h. BFA (BioChemika) was bath-applied for 10 min prior to the application of the En-2 gradient to disrupt the Golgi apparatus. The turning angle for each growth cone was defined as the angle between the direction of growth at the onset of the experiment and the final position of the centre of the growth cone (Lohof et al., 1992). Only growth cones that showed a net extension of >10 μm were used for analysis. The final patterns of axon extension were traced from digital images to generate trajectory plots.
Acknowledgments
This work was supported by funds from Paralyzed Veterans America (PVA-2442 to TTM), NINDS (R01-NS049041 to JLT), the Nemours Foundation (JLT), the Wellcome Trust (Programme Grant to CEH), NSF, and Gates Trust (CEH). Drs. Jan van Minnen and Mike Fainzilber provided critical input along the course of these studies. We thank the following for providing antibodies: Christopher Nicchitta, Duke University for TRAPα; Jonathan Lytton, UCHSC for SERCA N1; Jeremy Skepper, Cambridge University for anti-calreticulin; and Stuart Feinstein, UCSB for anti-TrkA. We thank Anne Ferguson-Smith for providing mouse embryos, Alain Prochiantz for the En2 protein, and H.-D. Soeling for the KDELR-YFP plasmid.
Footnotes
Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.mcn.2008.09.008.
References
- Alvarez J, Giuditta A, Koenig E. Protein synthesis in axons and terminals: significance for maintenance, plasticity and regulation of phenotype. With a critique of slow transport theory. Prog. in Neurobiol. 2000;62:1–62. doi: 10.1016/s0301-0082(99)00062-3. [DOI] [PubMed] [Google Scholar]
- Anders N, Jurgens G. Large ARF guanine nucleotide exchange factors in membrane trafficking. Cell. Mol. Life Sci. 2008 doi: 10.1007/s00018-008-8227-7. [epub ahead of print; DOI: 10.1007/s00018-008-8227-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassell GJ, Zhang H, Byrd AL, Femino AM, Singer RH, Taneja KL, Lifshitz LM, Herman IM, Kosik KS. Sorting of beta-actin mRNA and protein to neurites and growth cones in culture. J. Neurosci. 1998;18:251–265. doi: 10.1523/JNEUROSCI.18-01-00251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi J, Tsai NP, Lin YP, Loh HH, Wei LN. Axonal mRNA transport and localized translational regulation of kappa-opioid receptor in primary neurons of dorsal root ganglia. Proc. Natl. Acad. Sci. U. S. A. 2006;103:19919–19924. doi: 10.1073/pnas.0607394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittis PA, Lu Q, Flanagan JG. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell. 2002;110:223–235. doi: 10.1016/s0092-8674(02)00813-9. [DOI] [PubMed] [Google Scholar]
- Brunet I, Weinl C, Piper M, Trembleau A, Volovitch M, Harris W, Prochiantz A, Holt C. The transcription factor Engrailed-2 guides retinal axons. Nature. 2005;438:94–98. doi: 10.1038/nature04110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge MB. Fine structure of nerve fibers and growth cones of isolated sympathetic neurons in culture. J. Cell Biol. 1973;56:713–735. doi: 10.1083/jcb.56.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera M, Muniz M, Hidalgo J, Vega L, Martin ME, Velasco A. The retrieval function of the KDEL receptor requires PKA phosphorylation of its C-terminus. Mol. Biol. Cell. 2003;14:4114–4125. doi: 10.1091/mbc.E03-04-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D, Holt C. Chemotropic responses of reginal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1016. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- Chang JH, Mellon E, Schanen NC, Twiss JL. Persistent TrkA activity is necessary to maintain transcription in neuronally differentiated PC12 cells. J. Biol. Chem. 2003;278:42877–42885. doi: 10.1074/jbc.M308155200. [DOI] [PubMed] [Google Scholar]
- Chromozinski P, Sacchi N. Single-step method of RNA isolation by guanidine isothiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cox LJ, Hengst U, Gurskaya NG, Lukyanov KA, Jaffrey SR. Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival. Nat. Cell Biol. 2008;10:149–159. doi: 10.1038/ncb1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey ME, Bridgman PC. Structure and organization of membrane organelles along distal microtubule segments in growth cones. J. Neurosci. Res. 1991;30:242–258. doi: 10.1002/jnr.490300125. [DOI] [PubMed] [Google Scholar]
- Doms RW, Russ G, Yewdell JW. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J. Cell Biol. 1989;109:61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Ferro-Novick S, Novick P. Dynamics and inheritance of the endoplasmic reticulum. J. Cell. Sci. 2004;117:2871–2878. doi: 10.1242/jcs.01286. [DOI] [PubMed] [Google Scholar]
- Eng H, Lund K, Campenot RB. Synthesis of beta-tubulin, actin, and other proteins in axons of sympathetic neurons in compartmented cultures. J. Neurosci. 1999;19:1–9. doi: 10.1523/JNEUROSCI.19-01-00001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Kreibich G. Retention of subunits of the oligosaccharyltransferase complex in the endoplasmic reticulum. J. Biol. Chem. 2000;275:3984–3990. doi: 10.1074/jbc.275.6.3984. [DOI] [PubMed] [Google Scholar]
- Fu J, Pirozzi G, Sanjay A, Levy R, Chen Y, De Lemos-Chiarandini C, Sabatini D, Kreibich G. Localization of ribophorin II to the endoplasmic reticulum involves both its transmembrane and cytoplasmic domains. Eur. J. Cell. Biol. 2000;79:219–228. doi: 10.1078/S0171-9335(04)70025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Ren M, Kreibich G. Interactions among subunits of the oligosaccharyltransferase complex. J. Biol. Chem. 1997;272:29687–29692. doi: 10.1074/jbc.272.47.29687. [DOI] [PubMed] [Google Scholar]
- Gelebart P, Opas M, Michalak M. Calreticulin, a Ca2+-binding chaperone of the endoplasmic reticulum. Int. J. Biochem. Cell Biol. 2005;37:260–266. doi: 10.1016/j.biocel.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Gerst JE. Message on the web: mRNA and ER co-trafficking. Trends Cell Biol. 2008;18:68–76. doi: 10.1016/j.tcb.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Guo L, Groenendyk J, Papp S, Dabrowska M, Knoblach B, Kay C, Parker JM, Opas M, Michalak M. Identification of an N-domain histidine essential for chaperone function in calreticulin. J. Biol. Chem. 2003;278:50645–50653. doi: 10.1074/jbc.M309497200. [DOI] [PubMed] [Google Scholar]
- Hambrock A, Loffler-Walz C, Quast U. Glibenclamide binding to sulphonylurea receptor subtypes: dependence on adenine nucleotides. Br. J. Pharmacol. 2002;136:995–1004. doi: 10.1038/sj.bjp.0704801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, Fainzilber M. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- Herpers B, Rabouille C. mRNA localization and protein sorting mechanisms dictates the usage of tER-Golgi units involved in Gurken transport in Drosophila oocytes. Mol. Biol. Cell. 2004;15:5306–5317. doi: 10.1091/mbc.E04-05-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DT, Smith DS, Patterson SI, Kahn RA, Skene JH, Norden JJ. Rapid arrest of axon elongation by brefeldin A: a role for the small GTP-binding protein ARF in neuronal growth cones. J. Neurobiol. 1999;38:105–115. doi: 10.1002/(sici)1097-4695(199901)38:1<105::aid-neu8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Horton AC, Ehlers MD. Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J. Neurosci. 2003a;23:6188–6199. doi: 10.1523/JNEUROSCI.23-15-06188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton AC, Ehlers MD. Neuronal polarity and trafficking. Neuron. 2003b;40:277–295. doi: 10.1016/s0896-6273(03)00629-9. [DOI] [PubMed] [Google Scholar]
- Horton AC, Racz B, Monson EE, Lin AL, Weinberg RJ, Ehlers MD. Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron. 2005;48:757–771. doi: 10.1016/j.neuron.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Johnson AE, van Waes MA. The translocon: a dynamic gateway at the ER membrane. Annu. Rev. Cell Dev. Biol. 1999;15:799–842. doi: 10.1146/annurev.cellbio.15.1.799. [DOI] [PubMed] [Google Scholar]
- Johnson S, Michalak M, Opas M, Eggleton P. The ins and outs of calreticulin: from the ER lumen to the extracellular space. Trends Cell Biol. 2001;11:122–129. doi: 10.1016/s0962-8924(01)01926-2. [DOI] [PubMed] [Google Scholar]
- Keenan R, Freymann D, Stroud R, Walter P. The signal recognition particle. Ann. Rev. Biochem. 2001;70:755–775. doi: 10.1146/annurev.biochem.70.1.755. [DOI] [PubMed] [Google Scholar]
- Kelleher DJ, Gilmore R. An evolving view of the eukaryotic oligosaccharyltransferase. Glycobiology. 2006;16:47R–62R. doi: 10.1093/glycob/cwj066. [DOI] [PubMed] [Google Scholar]
- Koenig E, Martin R. Cortical plaque-like structures identify ribosome-containing domains in the Mauthner cell axon. J. Neurosci. 1996;16:1400–1411. doi: 10.1523/JNEUROSCI.16-04-01400.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig E, Martin R, Titmus M, Sotelo-Silveira JR. Cryptic peripheral ribosomal domains distributed intermittently along mammalian myelinated axons. J. Neurosci. 2000;20:8390–8400. doi: 10.1523/JNEUROSCI.20-22-08390.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krijnse-Locker J, Parton RG, Fuller SD, Griffiths G, Dotti CG. The organization of the endoplasmic reticulum and the intermediate compartment in cultured rat hippocampal neurons. Mol. Biol. Cell. 1995;6:1315–1332. doi: 10.1091/mbc.6.10.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S, Mayor S. ARF1 is directly involved in dynamin-independent endocytosis. Nat. Cell Biol. 2008;10:30–41. doi: 10.1038/ncb1666. [DOI] [PubMed] [Google Scholar]
- Langford GM. ER transport on actin filaments in squid giant axon: implications for signal transduction at synapse. FASEB J. 1999;13(Suppl. 2):S248–250. doi: 10.1096/fasebj.13.9002.s248. [DOI] [PubMed] [Google Scholar]
- Leung KM, van Horck FP, Lin AC, Allison R, Standart N, Holt CE. Asymmetrical beta-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nat. Neurosci. 2006;9:1247–1256. doi: 10.1038/nn1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AC, Holt CE. Function and regulation of local axonal translation. Curr. Opin. Neurobiol. 2008;18:60–68. doi: 10.1016/j.conb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohof AM, Quillan M, Dan Y, Poo MM. Asymmetric modulation of cytosolic cAMP activity induces growth cone turning. J. Neurosci. 1992;12:1253–1261. doi: 10.1523/JNEUROSCI.12-04-01253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losev E, Reinke CA, Jellen J, Strongin DE, Bevis BJ, Glick BS. Golgi maturation visualized in living yeast. Nature. 2006;441:1002–1006. doi: 10.1038/nature04717. [DOI] [PubMed] [Google Scholar]
- Luedeke C, Frei SB, Sbalzarini I, Schwarz H, Spang A, Barral Y. Septin-dependent compartmentalization of the endoplasmic reticulum during yeast polarized growth. J. Cell Biol. 2005;169:897–908. doi: 10.1083/jcb.200412143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan HD, Marotta A, Mowatt MR, Sciaky N, Lippincott-Schwartz J, Nash TE. Developmental induction of Golgi structure and function in the primitive eukaryote Giardia lamblia. J. Biol. Chem. 1995;270:4612–4618. doi: 10.1074/jbc.270.9.4612. [DOI] [PubMed] [Google Scholar]
- Majoul I, Straub M, Hell SW, Duden R, Soling HD. KDEL-cargo regulates interactions between proteins involved in COPI vesicle traffic: measurements in living cells using FRET. Dev. Cell. 2001;1:139–153. doi: 10.1016/s1534-5807(01)00004-1. [DOI] [PubMed] [Google Scholar]
- Ming GL, Wong ST, Henley J, Yuan XB, Song HJ, Spitzer NC, Poo MM. Adaptation in the chemotactic guidance of nerve growth cones. Nature. 2002;417:411–418. doi: 10.1038/nature745. [DOI] [PubMed] [Google Scholar]
- Mogelsvang S, Gomez-Ospina N, Soderholm J, Glick BS, Staehelin LA. Tomographic evidence for continuous turnover of Golgi cisternae in Pichia pastoris. Mol. Biol. Cell. 2003;14:2277–2291. doi: 10.1091/mbc.E02-10-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver DC, Radeke MJ, Feinstein SC, Snider WD. Presence or absence of TrkA protein distinguishes subsets of small sensory neurons with unique cytochemical characteristics and dorsal horn projections. J. Comp. Neurol. 1995;361:404–416. doi: 10.1002/cne.903610305. [DOI] [PubMed] [Google Scholar]
- Montesinos ML, Foucher I, Conradt M, Mainguy G, Robel L, Prochiantz A, Volovitch M. The neuronal microtubule-associated protein 1B is under homeoprotein transcriptional control. J. Neurosci. 2001;21:3350–3359. doi: 10.1523/JNEUROSCI.21-10-03350.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Rabouille C, Watson R, Nilsson T, Hui N, Slusarewicz P, Kreis TE, Warren G. Characterization of a cis-Golgi matrix protein, GM130. J. Cell Biol. 1995;131:1715–1726. doi: 10.1083/jcb.131.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano RE, Martin OC, Kang HC, Haugland RP. A novel fluorescent ceramide analogue for studying membrane traffic in animal cells: accumulation at the Golgi apparatus results in altered spectral properties of the sphingolipid precursor. J. Cell Biol. 1991;113:1267–1279. doi: 10.1083/jcb.113.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier L, Jokitalo E, Warren G. The effect of Golgi depletion on exocytic transport. Nat. Cell Biol. 2000;2:840–846. doi: 10.1038/35041089. [DOI] [PubMed] [Google Scholar]
- Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Segar R, Fainzilber M. Vimentin-dependent spatial translocation of an activated MAP kinse in injured nerve. Neuron. 2005;45:715–726. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Mayer T, McCarthy JB. Evidence for a satellite secretory pathway in neuronal dendritic spines. Curr. Biol. 2001;11:351–355. doi: 10.1016/s0960-9822(01)00077-x. [DOI] [PubMed] [Google Scholar]
- Piper M, Anderson R, Dwivedy A, Weinl C, van Horck F, Leung KM, Cogill E, Holt C. Signaling mechanisms underlying Slit2-induced collapse of Xenopus retinal growth cones. Neuron. 2006;49:215–228. doi: 10.1016/j.neuron.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M, Dwivedy A, Leung L, Bradley RS, Holt CE. NF-protocadherin and TAF1 regulate retinal axon initiation and elongation in vivo. J. Neurosci. 2008;28:100–105. doi: 10.1523/JNEUROSCI.4490-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M, Salih S, Weinl C, Holt CE, Harris WA. Endocytosis dependent desensitization and protein synthesis-dependent resensitization in retional growth cone adaptation. Nat. Neurosci. 2005;8:179–186. doi: 10.1038/nn1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi DS, Moeller JF, Coffman CR, Gallenson N, Harris WA. Growth cone interactions with a glial cell line from embryonic Xenopus retina. Dev. Biol. 1989;134:158–174. doi: 10.1016/0012-1606(89)90086-9. [DOI] [PubMed] [Google Scholar]
- Schonhorn JE, Wessling-Resnick M. Brefeldin A down-regulates the transferrin receptor in K562 cells. Mol. Cell. Biochem. 1994;135:159–169. doi: 10.1007/BF00926519. [DOI] [PubMed] [Google Scholar]
- Schweitzer B, Suter U, Taylor V. Neural membrane protein 35/Lifeguard is localized at postsynaptic sites and in dendrites. Brain Res. Mol. Brain Res. 2002;107:47–56. doi: 10.1016/s0169-328x(02)00445-x. [DOI] [PubMed] [Google Scholar]
- Schweitzer B, Taylor V, Welcher AA, McClelland M, Suter U. Neural membrane protein 35 (NMP35): a novel member of a gene family which is highly expressed in the adult nervous system. Mol. Cell. Neurosci. 1998;11:260–273. doi: 10.1006/mcne.1998.0697. [DOI] [PubMed] [Google Scholar]
- Shibatani T, David LL, McCormack AL, Frueh K, Skach WR. Proteomic analysis of mammalian oligosaccharyltransferase reveals multiple subcomplexes that contain Sec61, TRAP, and two potential new subunits. Biochemistry. 2005;44:5982–5992. doi: 10.1021/bi047328f. [DOI] [PubMed] [Google Scholar]
- Smith DS, Skene P. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J. Neurosci. 1997;17:646–658. doi: 10.1523/JNEUROSCI.17-02-00646.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer GE, Syed NI, van Kesteren E, Lukowiak K, Geraerts WP, van Minnen J. Synthesis and functional integration of a neurotransmitter receptor in isolated invertebrate axons. J. Neurobiol. 2000;44:72–81. doi: 10.1002/1097-4695(200007)44:1<72::aid-neu7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Tabb JS, Molyneaux BJ, Cohen DL, Kuznetsov SA, Langford GM. Transport of ER vesicles on actin filaments in neurons by myosin V. J. Cell. Sci. 1998;111(Pt. 21):3221–3234. doi: 10.1242/jcs.111.21.3221. [DOI] [PubMed] [Google Scholar]
- Tada T, Simonetta A, Batterton M, Kinoshita M, Edbauer D, Sheng M. Role of Septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Curr. Biol. 2007;17:1752–1758. doi: 10.1016/j.cub.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennyson VM. The fine structure of the axon and growth cone of the dorsal root neuroblast of the rabbit embryo. J. Cell Biol. 1970;44:62–79. doi: 10.1083/jcb.44.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre ER, Steward O. Protein synthesis within dendrites: glycosylation of newly synthesized proteins in dendrites of hippocampal neurons in culture. J. Neurosci. 1996;16:5967–5978. doi: 10.1523/JNEUROSCI.16-19-05967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai NP, Bi J, Loh HH, Wei LN. Netrin-1 signaling regulates de novo protein synthesis of kappa opioid receptor by facilitating polysomal partition of its mRNA. J. Neurosci. 2006;26:9743–9749. doi: 10.1523/JNEUROSCI.3014-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Ishikawa H. Three-dimensional distribution of smooth endoplasmic reticulum in myelinated axons. J. Electron. Microsc. (Tokyo) 1976;25:141–149. [PubMed] [Google Scholar]
- Tsukita S, Ishikawa H. Morphological evidence for the involvement of the smooth endoplasmic reticulum in axonal transport. Brain Res. 1979;174:315–318. doi: 10.1016/0006-8993(79)90853-9. [DOI] [PubMed] [Google Scholar]
- Twiss J, Smith D, Chang B, Shooter E. Translational control of ribosomal protein L4 is required for rapid neurite extension. Neurobiol. Dis. 2000;7:416–428. doi: 10.1006/nbdi.2000.0293. [DOI] [PubMed] [Google Scholar]
- Wang CC. Isomerase and chaperone activities of protein disulfide isomerase are both required for its function as a foldase. Biochemistry (Mosc) 1998;63:407–412. [PubMed] [Google Scholar]
- Wang W, van Niekerk E, Willis DE, Twiss JL. RNA transport and localized protein synthesis in neurological disorders and neural repair. Develop. Neurobiol. 2007;67:1166–1182. doi: 10.1002/dneu.20511. [DOI] [PubMed] [Google Scholar]
- Weclewicz K, Svensson L, Kristensson K. Targeting of endoplasmic reticulum-associated proteins to axons and dendrites in rotavirus-infected neurons. Brain Res. Bull. 1998;46:353–360. doi: 10.1016/S0361-9230(98)00013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis DE, Li KW, Zheng J-Q, Smit AB, Kelly TK, Merianda TT, Sylvester J, van Minnen J, Twiss JL. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J. Neurosci. 2005;25:778–791. doi: 10.1523/JNEUROSCI.4235-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis DE, van Niekerk EA, Sasaki Y, Mesngon M, Merianda TT, Williams GG, Kendall M, Smith DS, Bassell GJ, Twiss JL. Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. J. Cell Biol. 2007;178:965–980. doi: 10.1083/jcb.200703209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooding S, Pelham HR. The dynamics of golgi protein traffic visualized in living yeast cells. Mol. Biol. Cell. 1998;9:2667–2680. doi: 10.1091/mbc.9.9.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KY, Hengst U, Cox LJ, Macosko EZ, Jeromin A, Urquhart ER, Jaffrey SR. Local translation of RhoA regulates growth cone collapse. Nature. 2005;436:1020–1024. doi: 10.1038/nature03885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Chung TF, Fine RE, Johnson RJ. Calreticulin is transported to the surface of NG108–15 cells where it forms surface patches and is partially degraded in an acidic compartment. J. Neurosci. Res. 1999;58:652–662. doi: 10.1002/(sici)1097-4547(19991201)58:5<652::aid-jnr6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Wessells NK. Axon elongation. Effect of nerve growth factor on microtubule protein. Exp. Cell Res. 1971;66:346–352. doi: 10.1016/0014-4827(71)90687-2. [DOI] [PubMed] [Google Scholar]
- Ye B, Zhang Y, Song W, Younger SH, Jan LY, Jan YN. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell. 2007;130:717–729. doi: 10.1016/j.cell.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin D, Hanz S, Yoo S, Iavnilovitch E, Willis D, Segal-Ruder Y, Vuppalanchi D, Ben-Yaakov K, Hieda M, Yoneda Y, Twiss J, Fainzilber M. Localized regulation of axonal RanGTPase controls retrograde injury signaling in peripheral nerve. Neuron. 2008;59:241–252. doi: 10.1016/j.neuron.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelena J. Ribosome-like particles in myelinated axons of the rat. Brain Res. 1970;24:359–363. doi: 10.1016/0006-8993(70)90120-4. [DOI] [PubMed] [Google Scholar]
- Zelena J. Ribosomes in myelinated axons of dorsal root ganglia. Zeitschrift fur Zellforschung und Mikroskopische Anatomie. 1972;124:217–229. doi: 10.1007/BF00335680. [DOI] [PubMed] [Google Scholar]
- Zheng J-Q, Kelly T, Chang B, Ryazantsev S, Rajasekaran A, Martin K, Twiss J. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J. Neurosci. 2001;21:9291–9303. doi: 10.1523/JNEUROSCI.21-23-09291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]