Abstract
Most health system strengthening interventions ignore interconnections between systems components. In particular, complex relationships between medicines and health financing, human resources, health information and service delivery are not given sufficient consideration. As a consequence, populations' access to medicines (ATM) is addressed mainly through fragmented, often vertical approaches usually focusing on supply, unrelated to the wider issue of access to health services and interventions. The objective of this article is to embed ATM in a health system perspective. For this purpose, we perform a structured literature review: we examine existing ATM frameworks, review determinants of ATM and define at which level of the health system they are likely to occur; we analyse to which extent existing ATM frameworks take into account access constraints at different levels of the health system. Our findings suggest that ATM barriers are complex and interconnected as they occur at multiple levels of the health system. Existing ATM frameworks only partially address the full range of ATM barriers. We propose three essential paradigm shifts that take into account complex and dynamic relationships between medicines and other components of the health system. A holistic view of demand-side constraints in tandem with consideration of multiple and dynamic relationships between medicines and other health system resources should be applied; it should be recognized that determinants of ATM are rooted in national, regional and international contexts. These are schematized in a new framework proposing a health system perspective on ATM.
Keywords: Medicines, access barriers, health system, analytical framework
KEY MESSAGES.
Barriers to access to medicines (ATM) are complex and occur at multiple levels of the health system.
Existing frameworks for ATM do not address complexity of barriers and their interconnectedness.
A wider health system perspective may offer an opportunity to embed ATM in the emerging debate around complex adaptive systems and their application to health.
Introduction
In 1975, the World Health Assembly endorsed the concept of essential medicines, triggering the adoption of essential medicines lists and the implementation of national drug policies in most low- and middle-income countries (LMICs). Estimates of the number of people with access to essential medicines almost doubled between 1977 and the late 1990s through a combination of public and private provision (Quick and Hogerzeil 2002). Despite this success, access to medicines (ATM) remains problematic for poor and vulnerable populations.
Peters et al. (2008) define access as ‘the timely use of services according to needs’. Access barriers can stem from the demand side and/or the supply side (Ensor and Cooper 2004): demand-side constraints influence individuals', households' and communities' ability to use services while supply-side constraints are aspects of health services and the health sector that hinder service uptake. Important remaining ATM barriers can be identified along all dimensions of access: geographical and financial accessibility, availability, acceptability and quality (Peters et al. 2008).
In the formal sector of LMICs, average availability of medicines is 35% in public facilities and 66% in the private sector, although prices are often unaffordable in the latter (Cameron et al. 2009). Up to 50% of medicines are inappropriately prescribed or dispensed and up to 50% are used incorrectly by patients (WHO 2004a). This leads to significant wasted resources, the potential to drive the development of drug resistance and to poor health outcomes. Many patients, especially the poor, rely on the informal sector for their health care needs including medicines (Mills et al. 2002), while respective vendors have little or no pharmacy training (Smith 2009). Counterfeit medicines are also prominent in LMICs: 15–50% of all medicines are counterfeit (Cockburn et al. 2005) and tend to be sold at more affordable prices (Burki 2010). Sub-standard medicines should be distinguished from the issue of counterfeits (Newton et al. 2011) and pose a different set of problems: while counterfeit medicines are often limited to the informal market, genuine medicines of poor quality may be dispensed in the formal sector. Sub-standard medicines are a contributing factor of antimicrobial resistance (Newton et al. 2011) and resistant strains for certain communicable diseases such as malaria (Lon et al. 2006). Medicines account for a high proportion of health spending in LMICs, typically between 20 and 60% (Cameron et al. 2009), and 50–90% of this amount is out-of-pocket (WHO 2004b). This inequitable mode of financing can lead to significant financial burden for vulnerable populations.
Policy reforms and interventions aimed at improving ATM have covered issues such as improved efficiency of medicines procurement and supply, quality assurance, formulation and implementation of national essential medicines' lists, standard treatment guidelines, rational prescription (for example, through drug therapeutic committees) or rational use, cost recovery mechanisms for medicines such as revolving drug funds (RDF), and education and training of prescribers and dispensers as well as patients. A bibliometric survey of publications on ATM in LMICs showed 648 publications between 2003 and 2009 (Adam et al. 2012). Many more experiences are not documented, or only in grey literature. There is therefore a body of knowledge on ATM and a variety of approaches adopted in the past decade to address poor ATM in LMICs. So why have these approaches only partially improved ATM for poor people?
Most health system strengthening interventions are designed within single building blocks of the system [health financing, human resources, health information, health service delivery, governance or medicines and health technologies (WHO 2007)] and interconnections between systems components are frequently ignored. In many instances, the pharmaceutical sector itself is seldom considered as a whole: the above bibliometric survey showcases a patchwork of studies on single (yet all important) sub-components of the pharmaceutical sector. For example, only 27 of the 648 aforementioned articles report on broader pharmaceutical policies and reforms in LMICs (Adam et al. 2012). The role of medicines is narrowed down to a system input, a commodity that should be available to allow service delivery. Populations’ ATM is addressed mainly through fragmented, vertical approaches usually focusing on supply. This shortfall has considerable consequences: with such vertical and isolated approach, policies, interventions and actions aimed at improving ATM can only have a limited and short-term effect as many other system constraints do hamper access to care and medicines. This phenomenon may be the source of enduring lack of access to essential medicines for vulnerable populations in LMICs.
The objective of this article is to embed ATM in the wider health system strengthening debate, as a systems approach to improving ATM seeks to ensure that policies are more effective and generate longer-term equitable and sustainable results. For this purpose, we review ATM barriers and define at which level of the health system they occur. We analyse to which extent existing ATM frameworks take into account these demand-and supply-side barriers to treatment and care. Interventions aimed at improving ATM take place at different levels of the health system and by multiple stakeholders, but in a fragmented manner. Our first hypothesis is that ATM frameworks must cover the full range of access barriers at all levels of the health system, to guide research, policy formulation or intervention design. Our second hypothesis is that connections and linkages between system components and relevant stakeholders are as important as the interventions themselves and should be given careful consideration. Based on this analysis, we propose a new conceptual framework, which provides a health system perspective on ATM.
Methods
ATM: identifying frameworks and barriers
A literature search on ATM was conducted as follows:
The WHO Department of Essential Medicines and Health Products repository of publications was searched for the key word ‘access’. Three publications presenting and discussing ATM frameworks were identified through this search: (1) the medicines access framework from a WHO-Management Sciences for Health (MSH) consultative meeting held in Ferney–Voltaire in 2000 (Centre for Pharmaceutical Management 2003); (2) the ‘Equitable access to essential medicines: a framework for collective action’ published in 2004 (WHO 2004c); and (3) a book entitled ‘How do good technologies get to poor people in poor countries?’, which examines the issue of access to pharmaceuticals and medical technologies (Frost and Reich 2010).
Publications identified through the bibliometric survey performed by Adam et al. (2012) were screened to identify papers that would discuss various barriers or determinants of ATM.
We broadened our search to account for key publications on barriers to access to health care in general, acknowledging that ATM and access to health services and interventions are interlinked. For this purpose, we used a recent publication that analyses access barriers to health services for the poor (Jacobs et al. 2012). In this article, the authors performed a Medline search from 1998 onwards to identify access barriers to health services. As this structured literature search was similar to the one applied in the current article, we used their findings to link ATM and access to health services and interventions.
Analysing barriers and existing ATM frameworks
Hanson et al. (2003) analysed access constraints to scaling-up priority health interventions for the poor, whereby they used a five-level framework to capture the range and intensity of these constraints. We have slightly adapted these constraint levels to capture specific issues related to ATM:
Individuals, households and community;
Health service delivery;
Health sector level;
Public policies cutting across sectors; and
International and regional level
Level 1 is now labelled to encompass individuals in addition to their household and community, hereby acknowledging that health-seeking behaviour and attitude towards health and health services depends on individual preferences, in the context of cultural and social constraints of households and communities. Other authors categorize this level as the demand-side (Ensor and Cooper 2004; Peters et al. 2008; Jacobs et al. 2012).
Levels 2–5 are related to the supply side. We adapted Levels 4 and 5 by:
– Merging ‘environmental and contextual characteristics’ with ‘public policies cutting across sectors’. Level 4 now represents the national level and includes sectors other than health, which influence health and medicine policies. A specific focus on this level was required to capture influences of trade, industry or legal sectors.
– Adding ‘international and regional level’ constraints as the fifth level because elements such as international markets and regulations often play an important role in ATM.
We used this five-level framework to categorize ATM barriers and to analyse the multiple links between access constraints in complex health systems.
Results
The results of the structured literature review are presented in three sections: first, an overview of three existing ATM frameworks is presented, followed by a review and categorization of identified ATM barriers and a brief examination of the frameworks’ consideration of the full range of ATM constraints and their linkages. Finally, a new framework that presents a system-wide perspective on ATM is proposed.
Existing ATM frameworks
A WHO-MSH consultative meeting in Ferney–Voltaire in 2000 set the ground for the first ATM framework (Centre for Pharmaceutical Management 2003). This framework (hereinafter named ‘WHO-MSH 2000'), developed from the work of Penchansky and Thomas (1981) is based on the 4As of ‘Availability’, ‘Accessibility’, ‘Acceptability’ and ‘Affordability’, with ‘Quality’ of products and services as a cross-cutting determinant (Table 1). Each of the As has two components pertaining to demand and supply.
Table 1.
Domains and determinants covered in existing frameworks for ATM
| ATM framework | Domains | Specific determinants | Cross-cutting determinant |
|---|---|---|---|
| 1. WHO-MSH 2000 (Centre for Pharmaceutical Management 2003) | Availability |
|
Quality of products and services |
| Affordability |
|
||
| Acceptability |
|
||
| Accessibility |
|
||
| 2. WHO (2004c) | Rational use |
|
Quality of medicines |
| Affordable prices |
|
||
| Sustainable financing |
|
||
| Reliable health and supply systems |
|
||
| 3. Frost and Reich (2010) | Availability |
|
Architecture: organization relationships at national and international levels |
| Affordability |
|
||
| Adoption |
|
Source: authors.
The WHO 2004 ‘Equitable access to essential medicines framework (hereinafter named ‘WHO 2004')’ presents four dimensions of ATM, (WHO 2004c) summarized in Table 1. ‘Rational selection’ proposes to rationalize therapeutic choices. Improved use of medicines by consumers is also part of this dimension of access. ‘Affordable prices’ deals with supply-side aspect of affordability. ‘Sustainable financing’ addresses resource mobilization and pooling as well as reduction of out-of-pocket and catastrophic expenditures. ‘Reliable health and supply system’ is meant to include all aspects of health system strengthening that are not covered by the other three dimensions: procurement and supply of medicines, regulation and human resources. Quality assurance and management systems are assumed to underpin all access components.
Frost and Reich (2010) examine how poor populations access health technologies, including medicines, in poor countries. The authors also adopt a 4A framework for ATM (hereinafter named ‘Frost and Reich 2010'), though different from the WHO-MSH 4A model (Table 1): ‘Architecture’, ‘Availability’, ‘Affordability’ and ‘Adoption’ are the determinants of access. ‘Availability’ represents supply and includes manufacturing, forecasting, procurement, distribution and delivery functions. ‘Adoption’ represents demand at all levels. ‘Affordability’ integrates costs, at government, non-government and end-user levels. All three are co-ordinated by organizational relationships at national and international levels, represented in the pharmaceutical ‘Architecture’ function.
These three models intend to present comprehensive frameworks for ATM. However, although they overlap on several aspects (‘affordability’ and ‘availability’), they also diverge on other components such as ‘architecture’ or ‘reliable health systems’. The strengths and weaknesses of these ATM frameworks are summarized in Table 2. All three ATM frameworks adopt a supply-side approach to address demand-side constraints and focus on products rather than services (except for WHO-MSH 2000). Governance is limited to the pharmaceutical sector and usually remains within public sector boundaries. Linkage with national policies beyond the health sector is limited, if any, as well as consideration of the international context. Each of these frameworks has been formulated at a certain point in time, corresponding to the evolution of the debate on health systems strengthening, whereby they respond to the needs of respective relevant stakeholders. The WHO-MSH 2000 framework is clearly centred on health service delivery through quality, availability, accessibility and acceptability of medicines. Four years later, the WHO 2004 access framework had evolved to capture the pharmaceutical sector and intends to guide pharmaceutical policy formulation. The most recent framework by Frost and Reich pays more attention to the international aspects of partnerships for ATM. However, none of these frameworks reflects the most recent debates on health systems, their complexity and system dynamics (de Savigny and Adam 2009; Paina and Peters 2011; Sheikh et al. 2011; van Olmen et al. 2012). To address these shortcomings, we categorize ATM constraints by health system level in the following section.
Table 2.
Strengths and weaknesses of existing ATM frameworks vis-à-vis ATM constraints at different levels of the health system
| Level of the health system | ATM constraints | Strength and weaknesses of ATM frameworks (WHO-MSH 2000; WHO 2004c; Frost and Reich 2010) |
|---|---|---|
| I. Individual, household and community | Perceived quality of medicines and health services | All three ATM frameworks address demand-side barriers, although through a classical supply-oriented approach. |
| Cost of medicines and services | The most comprehensive one is WHO-MSH 2000. | |
| Irrational health-seeking behaviour, demand for and use of medicines | All fail to picture the full range of social and cultural constraints affecting access. | |
| Social and cultural barriers (stigma related to poverty, ethnicity and gender) | ||
| II. Health Service Delivery | Irregular availability | WHO-MSH 2000 is the most comprehensive at this level and links products and services. |
| High medicine prices | Other frameworks are focused on products rather than services. | |
| Irrational prescription and dispensing | All three fail to acknowledge the pluralism of health service delivery in LMICs. | |
| Low quality/sub-standard and counterfeit medicines | ||
| Low quality of health services | ||
| Competition between public and private health service delivery | ||
| III. Health Sector | Pharmaceutical sector governance | WHO-MSH 2000 does not provide a view on determinants of ATM at health sector level and beyond. |
| Medicines price control | WHO 2004 and Frost and Reich (2010) generally limit governance to the pharmaceutical sector. | |
| Weak health sector governance affecting all health system building blocks | All frameworks seem limited to the public sector | |
| Health sector pluralism and stewardship over private sector | ||
| IV. Public policies cutting across sectors | Low public accountability and transparency | All three ATM frameworks generally neglect the issue of national policies beyond the health sector. |
| Low priority attached to social sectors | The WHO-MSH 2000 and the WHO 2004 frameworks have a limited international perspective on governance, mainly centered on donor funding for medicines | |
| High burden of government bureaucracy | ||
| Conflict between trade and economic goals for pharmaceutical markets and public health goals | Frost and Reich (2010) extend their analysis to partnerships and collaborations at international level under the Architecture function of their framework. | |
| V. International and regional level | Unethical use of patents and intellectual property rights | |
| International donors’ agenda | ||
| Distorted research and development, not targeting disease burden in LMICs |
Source: authors.
ATM constraints by health system levels
Barriers to accessing medicines, identified through the structured literature review and categorized by health system level are summarized in Table 2.
Level 1: individuals, households and community
This level is usually considered as the demand side (Ensor and Cooper 2004; Jacobs et al. 2012). Demand-side ATM barriers include perceived quality, health workers' attitude, as well as affordability of medicines and services (Kiwanuka et al. 2008; Chuma et al. 2010; Patel et al. 2010). Such barriers tend to be used in reference to the interaction between patients and service providers at the time of illness. Irrational health seeking behaviour, medicine demand and use are also considered as contributing to reduced access. This first level of the health system is not limited to individual patients but extends to households and communities. As mentioned, demand-side barriers are present beyond the individual as they also relate to social and cultural characteristics, including stigma, determined by the household and community affiliations (Ensor and Cooper 2004; Ruxin et al. 2005).
Level 2: health service delivery
Constraints to ATM at health service delivery concern the supply side. First, they relate to irregular availability and high prices (Saleh and Mohamed 2005; Babar et al. 2007; Cameron et al. 2009; Carasso et al. 2009; Kotwani 2009); second, to irrational prescription and dispensing (Laing et al. 2001; Shankar 2009; Holloway and van Dijk 2011); and finally, to medicines quality, including sub-standard and counterfeit medicines (Cockburn et al. 2005; Burki 2010; Newton et al. 2011). ATM and access to health services are closely interlinked: more general constraints in access to health services, either public or private, affect ATM and vice versa. Medicines availability is cited as a key determinant in several studies of access to and utilization of health services (Chukwuani et al. 2006; Kiwanuka et al. 2008; Pariyo et al. 2009). From a management perspective, availability of essential medicines has been used as a measure of quality of care (Ameli and Newbrander 2008; Jacobs et al. 2010). Essential medicines are referred to as playing a major role in primary health care performance (Rohde et al. 2008; Walley et al. 2008). Interaction between medicines and service delivery is essential in interventions targeted to mothers, newborn and children (Mavalankar and Rosenfield 2005; Pariyo et al. 2005; de Brouwere et al. 2010) or in disease-specific areas where researchers insist on the importance of adopting a broader vision of access to treatment and care rather than ATM only (Reilley et al. 2002; Beran and Yudkin 2006; Beran et al. 2008; Chuma et al. 2010). To these constraints that apply to both public and private health service delivery structures, we should add the interaction between public and private supply of services in LMICs. The private health market, ranging from drug shops to clinics and hospitals, for-profit or not, formal or informal, is widely used and preferred by patients (Mills et al. 2002; Maïga et al. 2003; Chalker et al. 2005). In weakly regulated systems, boundaries between private and public services are blurred and both providers and patients constantly shift from one to another (Van Damme et al. 2008; Meessen et al. 2011). This situation is exacerbated in the pharmaceutical sector, as health professionals of diverse training may own and operate medicines outlets (Mills et al. 2002).
Level 3: health sector
Governance of the pharmaceutical sector is related to eight functions (Kohler and Baghdadi-Sabeti 2011): registration, selection, procurement, distribution, licensing of pharmaceutical establishment, inspection, control of promotion and control of clinical trials. Medicines' prices are an element that will affect several of these functions, especially procurement. WHO Health Systems Strengthening framework highlights that ‘multiple, dynamic relationships [ … ] between building blocks [of the health system are] essential for achieving better outcomes’ (WHO 2007). Thus, weak governance will negatively impact all building blocks of the health system. Finally, the issue of health sector pluralism, previously highlighted under Level 2 (health service delivery), is also a determinant of access at health sector level.
Levels 4 and 5: above the health sector: national and international contexts
Low public accountability, low priority attached to the social sector, corruption or government bureaucracy, are constraints that affect the health sector. In addition, economic, trade and industry objectives on one hand and public health goals on the other, may be at best disconnected or worse, conflicting (Cohen-Kohler 2007; Zakus et al. 2010). Although this has been often reported at the international level with the use of patents and intellectual property rights, it may as well occur at national level, in countries aiming at a strong local pharmaceutical production (e.g. Vietnam or Iran). Tensions can also occur with international donors’ agendas related to medicines, for example in global health partnerships, which have highly contributed to relieving the burden of certain diseases such as malaria, HIV or tuberculosis, but have also diverted donors' attention from the burden of non-communicable diseases and to a certain extent from maternal and child health issues. Chirac and Torreele (2006) also document the disconnect between global pharmaceutical research and development and the burden of diseases affecting lower income countries. Equitable access to quality health care, including medicines, depends on global and national forces operating beyond the health sector, as illustrated in a socio-ecological framework by Tomson (2010). This model warns against the potential erosion of public health system stewardship in favour of national or international market dynamics.
As this review demonstrates, barriers to ATM are multiple and complex. Table 2 also summarizes strengths and weaknesses of existing ATM frameworks in addressing these access constraints at each level of the health system. The WHO-MSH 2000 framework is strongest at service delivery level (Level 2) and also captures adequately the interaction between services and individual patients or ‘users’. The WHO 2004 access framework is focused on Level 3 and adopts a pharmaceutical policy perspective, which fits the normative and policy advice mandate of the organization. Frost and Reich propose a broader access framework that attempts to capture most constraint levels from users (Level 1) to global level governance (Level 5) but still lacks adequate perspective on national constraints above the health sector (Level 4) and more complex demand-side issues beyond the individual user. More importantly all three frameworks limit their scope to the pharmaceutical sector in relative isolation from other health system building blocks.
A health system perspective on ATM
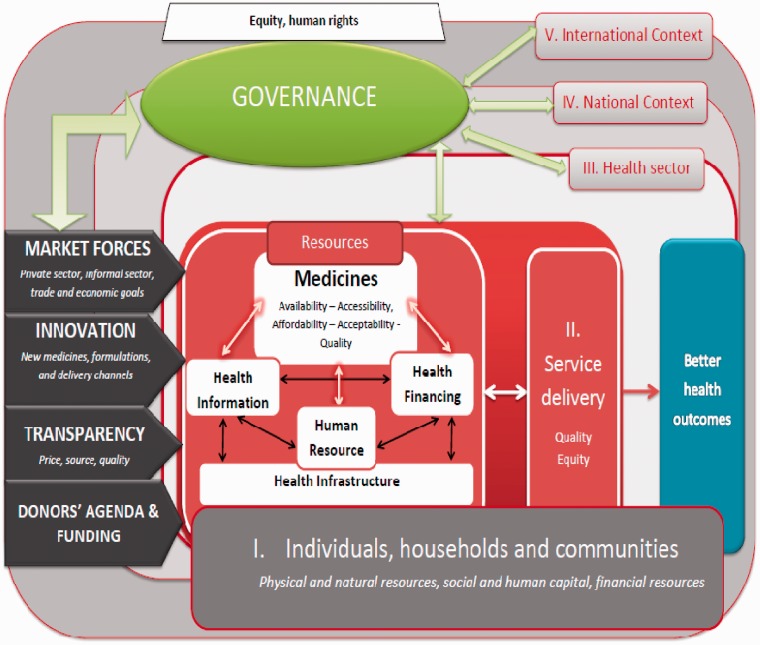
The above analysis reveals important ATM barriers that have not been given full consideration in the pharmaceutical sector thus far. To address this shortfall, we need a conceptual framework adopting a system-wide perspective on ATM (Figure 1).
Figure 1.
ATM from a health system perspective: a conceptual framework (Source: authors).
The design of this framework is inspired by the emerging interest in systems thinking for health systems strengthening (de Savigny and Adam 2009) and complex adaptive systems (CAS) (Paina and Peters 2011; Sheikh et al. 2011; van Olmen et al. 2012). Systems thinking moves away from a linear input–output-outcome chain and adopts a circular dynamic thinking (de Savigny and Adam 2009) that better reflects the complexity of health systems. Paina and Peters (2011) propose to adopt the lens of CAS to improve pathways to scaling up interventions. Sheikh et al. (2011) distinguish between ‘system hardware’, in other terms the usual health system building blocks that are concrete and tangible expressions of health systems; and ‘system software’ that create interconnectedness and system complexity: ideas and interests, relationships and power, values and norms. In traditional approaches, policy decisions are meant to influence the ‘system hardware’, whereas they actually also influence and are influenced by ‘system software’. Furthermore, health systems are embedded in a social and political context and the complex social construct of health systems must consider international, national, sub-national and local arena and their intersections. Using a similar lens, van Olmen et al. (2012) propose a generic framework for analysing health system dynamics. Our framework applies these novel approaches to ATM and reorganizes existing building blocks of the health system to highlight their dynamic interactions with medicines. For this purpose three important paradigm shifts are considered necessary: (1) adopting a holistic view on demand-side constraints, (2) considering the multiple and dynamic relationships between all building blocks of the health system, especially between resources to enable health service delivery (medicines, human resources, financial resources, health information and health infrastructure), and (3) considering leadership and governance of the health sector in their local, national and international contexts, including analysis of innovations, market forces and international agendas influencing the health and pharmaceutical sectors. These are examined in detail in the following section and their schematic representation in Figure 1 is explained as we proceed.
A holistic view of demand-side constraints
Our framework places the population at the centre and addresses demand-side barriers to access in as much detail as possible. This first paradigm shift acknowledges the fact that the population is an integral part of a health system (Level 1): this is illustrated in Figure 1 by the block labelled ‘I. Individuals, households and communities’. Health seeking behaviour depends on the ‘vulnerability context’ of an individual or household in a community which is determined by five livelihood assets: natural, physical, human, social and financial capital (Obrist et al. 2007). Interventions such as health equity funds (Bigdeli and Annear 2009), maternal voucher schemes (Ahmed and Khan 2011) or conditional cash transfers (Lagarde et al. 2007) improve access to a number of livelihood assets and impact access to health services. The ACCESS programme in Tanzania has successfully adopted this approach (Obrist et al. 2007) to design a range of interventions aimed at improving malaria treatment. To achieve better access to treatment and care, it is important to mobilize the full human capital available at community level and to remove the strict distinction between providers and patients (Haines et al. 2007; Van Damme et al. 2008). Moving away from passive users, patients, communities and community members become ‘expert patients’ and are valuable and available resources in supporting other patients or building collective networks and actions. Although the mentioned authors apply this concept to child survival interventions or scaling-up antiretroviral treatment (ART); it is also applicable for access to many other essential treatments, especially for chronic and lifelong conditions (van Olmen et al. 2011).
Multiple and dynamic relationships between health sector resources
The WHO Health System Strengthening Strategy has been instrumental in defining the essential functions of a health system. An unforeseen misinterpretation of the strategy has however been, in practice, a verticalization of each building block, although, as highlighted earlier, the document itself states that ‘multiple, dynamic relationships [ … ] between building blocks [are] essential for achieving better outcomes’ (WHO 2007). Following van Olmen et al. (2012), we have separated the health service delivery building block on one hand and we have grouped together the resources that a health system requires to deliver health services to the population on the other hand: these are represented by the medicines, human resources, health financing and health infrastructure blocks (Figure 1). To represent interconnections between building blocks and reflect the existence of ‘system software’, we have linked these resources one to another, as illustrated by the double-sided arrows between all components of health system resources. The basic idea that funding is required for medicines in a health system is already sketched in the WHO 2004 framework, under the component ‘Sustainable financing’. Inversely, in many LMICs where the domestic health budget is insufficient, medicines may become a substantial source of funding under the Bamako Initiative (Audibert and Mathonnat 2000). Revenues generated through medicine fees are often used to provide staff incentives and salaries or operational budget for facilities. Beyond financial incentives, medicines are also an obvious determinant of health workers' ability to perform their task; they contribute to recognition of their role, importance and even power, allowing them to exercise their profession in a credible manner and hence influencing their motivation. This will in turn influence patients’ and communities’ trust in health workers and health services. Any intervention aimed at one of the ‘resources’ building blocks will not only influence service delivery but also the other building blocks.
A better understanding of national and international contexts
Our framework highlights four important determinants of ATM at national (above the health sector) and international levels. These four determinants are represented by the left arrows in Figure 1: market forces, innovation, transparency and donors’ agenda and funding. At these levels, determinants of ATM become more complex and intricate. They have also been less documented in published literature. Therefore, more research is probably needed to identify an exhaustive list of ATM constraints at these levels.
Market forces
Although inevitable and maybe even necessary, pluralism of service delivery is a challenge to the governance of the health sector. The classical approach is to issue regulations, which are difficult to implement by weak public administrations. Lack of enforcement and the evidence that informal drug vendors or traditional healers routinely infringe legal restrictions has consequences on the credibility of the steward. However, in remote, underserved areas, these providers have a local legitimacy, de facto recognized by official inspection and enforcement officers (Goodman et al. 2007). For scaling up ART treatment in LMICs, Van Damme et al. (2008) propose to de-medicalize treatment provision, and make better use of unskilled human resources in health available at community level. Goodman et al. (2007) propose to bring the top down de jure policies and regulations of private and informal drug outlets more in line with a bottom-up de facto policy making, grounded in local legitimacy. Patouillard et al. (2007) showed that experiences of working with the private-for-profit sector may be successful in poor communities although adequate regulation and control are needed. These approaches frame innovative ways of exercising stewardship over the entire health sector, tapping into the resources of private and informal health sectors to achieve public health goals. At national level, barriers to ATM include low importance given to social sectors and its impact on the level of public funding for health. Less obvious is the potential distorted effect of local pharmaceutical production, which has been promoted since the Alma Ata declaration on Primary Health Care (PHC). It is wrong to believe that local pharmaceutical production will necessarily contribute to public health objectives. Rather, it may be easily diverted to serve trade and industry objectives. Also, local production is not necessarily cheaper if economies of scale are not achieved; this may lead to actual higher prices of locally produced medicines, and sometimes lower quality. When health authorities set national targets for consumption of locally produced medicines, they may actually indirectly trigger irrational prescription and/or decreased financial access. However, local pharmaceutical production may also help access cheap generic medicines if adequately harnessed to contribute to public health goals. The paradigm shift we propose to overcome these shortfalls consists in considering health as a human right and extending this concept to medicines, as proposed by Hogerzeil et al. (2006) or Perehudoff et al. (2010). The right to access essential medicines should drive national policies such as public administration reform, decentralization, finance or trade, rather than the opposite (i.e. national policies adversely impacting ATM). Tomson (2010) forwards the need for strong leadership ‘to ensure that values imposed upon national health systems can remain firmly grounded in the public sector’ rather than steered by privatization of health care and market dynamics.
Innovation
In the early part of 20th century and until the 1970s, the role of the pharmaceutical industry was crucial in the fight against endemic tropical diseases, for example, manufacturing and marketing of chloroquine and major anthelminthics (Pecoud et al. 1999). This situation has drastically changed since 1970s: today only 1% of new chemical entities commercialized are relevant for tropical diseases and few of these are the direct result of pharmaceutical industry’s research and development (Chirac and Torreele 2006). The role of innovation in tackling disease burden of LMICs is important and there is a need to maintain this input through adequate funding, untied to commercial interests, as proposed by the report of the Consultative Expert Working Group on R&D (2012). Innovation is limited not only to new medicines but also to new formulations and new delivery channels. Effective adherence interventions, for example, include new solutions such as simplification of dosage and packaging (Holloway and van Dijk 2011), including combination therapies. These innovative solutions may influence price, supply and stock management and therefore impact ATM.
Transparency
Lack of information on price, source and quality of medicines procured, distributed and used in health sectors of LMICs is a constraint to access. Enforcing transparency on these issues is very often beyond the scope of the health sector alone, as it does require broader interventions cutting across sectors at national level, touching upon economic sectors (growth of local production, fiscal policies), trade, customs, law enforcement agencies and other aspects. Partnerships with civil society and consumer organizations as well as international collaborations are essential in collecting and sharing transparent information that impact ATM. Several initiatives such as the Medicines Transparency Alliance have emerged recently (MeTA 2010). Other initiatives such as the Health Action International—WHO medicines pricing surveys, offer a web-based access to data collected in a number of countries on medicines prices, availability and affordability (http://www.haiweb.org/medicineprices/).
Donors’ agenda and funding
The last important determinant of ATM at international level is donors' agendas and commitments that influence development aid as well as national policies and plans. Despite the Paris Declaration on Aid Effectiveness, harmonization and alignment of donor funding has not been optimal, and donor assistance for financing consumables such as medicines are highly debated (WHO 2004c). Adopting the same human rights perspective, Cometto et al. (2009) propose a ‘Global Health Fund’ for Millenium Development Goals (MDGs) that would set aside criteria of financial sustainability imposed on recipient countries, adopt a new model of globally shared financial contributions to health and clarify financial commitments to tackle system bottlenecks. This concept derives from the work of Ooms (2008), who proposed a new ‘Global Health Aid Paradigm’, similar to the support provided for combating AIDS. Such endeavour aided in designing interventions and policies that triggered funding to curb the HIV/AIDS epidemic in the past 20 years. This new global health aid paradigm should promote access to essential medicines, not only access to antiretroviral therapy.
The Governance function is usually understood from the perspective of health sector governance, sometimes even limited to pharmaceutical sector governance. In our framework, governance is represented as a function cutting across Levels 3, 4 and 5. It encompasses the traditional stewardship of the public health sector but covers also private health markets, both domestic and international. Governance must also be exercised over non-health sectors to maintain focus on public health goals in a given economic or legal context.
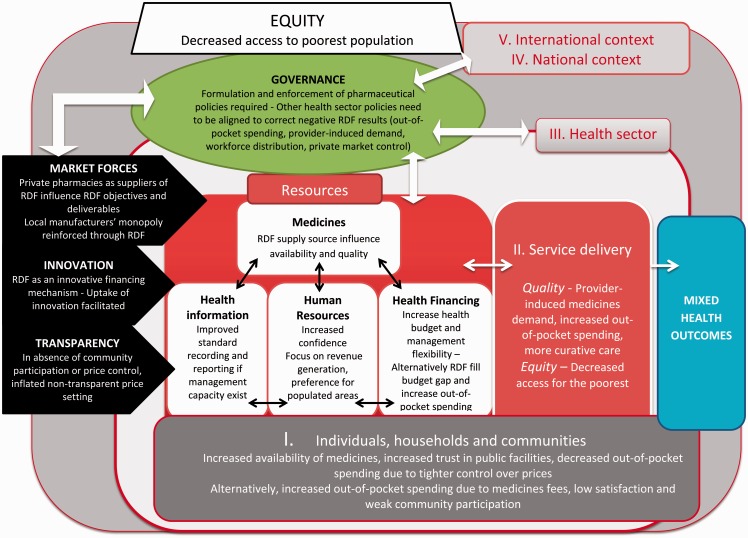
To illustrate a practical application of our framework, we have developed a case study presented in Box 1 and Figure 2. This case study is an application of our framework for a system-wide evaluation of RDF. Several linear assessments of RDF identified during our literature review have been combined to produce this more complex view of both intended and unintended effects of RDF at system level in lower income countries.
Box 1 A case study of RDF in dynamic health systems.
RDF enable either centralized or decentralized procurement of medicines that are in turn sold to patients. Revenues from medicine sales are used to replenish the fund and in some instances to finance other expenses such as health facilities operational costs or incentives to health staff. Although RDF have been established with the primary objective of increasing medicines availability (i.e. one component of ATM), their potential effect on health systems are more complex.
Level 1—Individuals, households and communities. Compared with a baseline situation of medicines shortage, RDF increase availability of medicines (Ali 2009) and affordability, through a closer control over price (Carasso et al. 2009). Communities’ satisfaction and trust in health services may increase (Ali 2009) and out-of-pocket expenses (OOPE) may decrease. However, fees charged for medicines at facilities may deter poor patients from seeking care. Dissatisfaction with medicine fees and communities’ lack of ownership over RDF have been documented (Uzochukwu and Onwujekwe 2005; Murakami et al. 2001).
- Level 2—Health service delivery
- Health system resources
- Medical supplies: Both centralized and decentralized medicines procurement may result in timely supply but may inversely create shortages, depending on capacity of RDF managers. Absence of reliable procurement sources impacts on medicine quality (Ali 2009) and prices, thus affecting their affordability.
- Health financing: Establishment of RDF may increase available domestic funding for other health priorities (Audibert and Mathonnat 2000), improve cash availability and management flexibility at facility level although RDF revenues may simply fill a budget gap without solving funding shortage; OOPE is increased.
- Human resources: Health staff may feel confident to perform their work as medicines are available. There is a risk, however, that they focus on revenue generation activities at the expense of delivering preventive services (Uzochukwu and Onwujekwe 2005). Health staff may prefer posting in populated areas with potential high utilization rates and consequent superior RDF sales volumes at the expense of remote areas.
- Health information: Standard recording and reporting is needed to improve supply forecasting and management; RDF may therefore result in improved and integrated health information systems. However, such required managerial capacity is usually missing in LMICs (Murakami et al. 2001).
- Service delivery
- Quality: RDF as a source of revenue for the health facility may induce provider-induced demand for medicines, with potential negative outcomes resulting from poly-medication and antimicrobial resistance as well as increased OOPE. Curative care may be favoured at the expense of health promotion and education (Uzochukwu and Onwujekwe 2005).
- Equity: Costs of medicine may deter the poorest segments of population from accessing quality care.
Level 3—Health sector level To support RDF, pharmaceutical policies (national essential medicines list, standard treatment guidelines, price control policies such as tax and duty exemption or mark-up regulation) need to be formulated and enforced while other essential health policies for service delivery, health financing and human resources need to be aligned. Although RDF establishment requires a ‘business-oriented management’ (Ali 2009) or ‘market-oriented thinking’ (Murakami 2001), a strong governance, regular supervision, monitoring and evaluation need to be in place to keep the focus on public health goals.
Levels 4 and 5—Cross-sectoral policies and international context When procurement is decentralized to facility level, private pharmacies are both competitors of RDF as well as their suppliers (Murakami et al. 2001) whereby any change in the private market (price or quality variation, introduction of new medicines, etc.) will directly affect the operations of RDF. When governance is weak, private pharmacies—driven by profits—will therefore affect RDF and alter their public health objectives. In centralized procurement systems, RDF may be an avenue for promoting locally produced medicines. If domestic economic considerations dominate over public health goals, RDF may become the main and secured clients of local manufacturers, with potential negative consequences on medicines prices and quality. In the absence of community participation or price control mechanisms, RDF may result in non-transparent inflated price setting. On the positive side, RDF may be a financing mechanism to support innovative initiatives such as the peer educator network for diabetic patients in Cambodia (www.mopotsyo.org); competition with the private market may also trigger earlier adoption of innovative medicines and formulations.
Figure 2.
A case study of RDF in dynamic health system (Source: authors).
Discussion and conclusions
Existing ATM frameworks have been designed with specific purposes but insufficiently encompass the complex role of medicines in dynamic health systems. Our review suggests that ATM barriers are interrelated, that they occur simultaneously at different levels of the health system, where multiple stakeholders operate. Therefore, we propose to adopt a health system view on ATM that will support implementing effective reforms at different levels of the health system to achieve desired results. In support of this view, it is argued that paradigm shifts are necessary at each system level. A more holistic view of demand-side constraints in tandem with consideration of multiple and dynamic relationships between medicines and other health system resources should be applied. It should be recognized that determinants of ATM are rooted in local, national and international contexts. Our framework offers a comprehensive view of this complexity and points to the variety and diversity of ATM barriers, enablers and their interactions. It builds on the emergence of complex systems thinking in health systems strengthening and stimulates a deeper understanding of ATM issues.
Paina and Peters (2011) argue that, despite large investments in global health initiatives, efforts to scale up health services in LMICs will not meet the expectations of MDGs. They attribute this shortfall to traditional linear approaches and propose to adopt the lens of CAS for planning, implementing and evaluating interventions. Our framework adopts a CAS lens and could be used as a guide to scale up existing small-scale or fragmented, otherwise successful, ATM interventions. At planning stage, our framework identifies linkages and relevant stakeholders for enabling ATM and therefore allows exploration of context for scaling up. At implementation and evaluation stages, our framework explores both the intended and unintended effects of ATM interventions, as illustrated by the RDF case study in Box 1. Our example of RDF illustrates how multiple linear assessments of an ATM intervention can be combined to provide a more complex and comprehensive view. Establishment of RDF may increase medicines availability, whereby improving health service quality; it may also increase the domestic health budget available for other priorities. But medicine fees charged in RDF may also increase out-of-pocket spending, medicines-related household debt and foregone treatment. A negative feedback loop (Paina and Peters 2011) is created if medicine fees generate revenues for staff and trigger irrational prescription and provider-induced demand. This negative feedback loop may be reinforced by the influence of private markets, through competition between RDF and private pharmacies, and if private pharmacies become suppliers of RDF. Our example shows that evaluations of ATM interventions should not be linear but should adopt a dynamic system view and should guide an iterative process whereby system responses to ATM interventions can be adequately considered in re-design and positive feedback loops.
By integrating values such as equity or human rights, our framework does capture the ‘system software’ forwarded by Sheikh et al. (2011) and which was missing in previous ATM frameworks. Other ‘system software’ such as relationships and power can be better visualized through the linkages represented in our framework. Software elements are also highlighted at individual and community level through human and social capital. Our RDF illustration points to several ‘system software’ elements influenced by this specific financing arrangement: confidence of health staff may improve because they have medicines to work with; this may in turn influence their trust and power relationships with patients. RDF also affect patients’ trust in health services, either positively (if medicines are available and affordable) or negatively (in case of medicine shortage and un-transparent and unaffordable prices). This influences their health-seeking behaviour and may trigger a ‘neighbourhood effect’ (Paina and Peters 2011), affecting communities’ health seeking preferences and trust. These elements are important not only for formulating ATM policies but also for health policy and system research questions (Sheikh et al. 2011). International, national, sub-national and local levels necessary for a complex social construct of health systems (Sheikh et al. 2011) are adequately schematized in the five levels of our framework.
Experiences in using CAS theories in health system strengthening are limited (Paina and Peters 2011) and indeed it is difficult to find documented examples of using complex systems thinking, especially in ATM. Van Olmen et al. (2012) present two case studies of the application of their health system dynamic framework: one related to creation of medical schools in DR Congo and the other on delivery of chronic care in a local health system in India. Another case study has been presented in de Savigny and Adam (2009) using results-based financing. Apart from these examples, concrete applications of systems thinking in health decision making and policy design appear limited, although similar approaches have been successful in other sectors. It is therefore likely that practical applications of our framework will similarly present many challenges, especially related to capacity of stakeholders and limitations of conventional health plans and programs. However, de Savigny and Adam (2009) propose several options for overcoming these challenges and moving the systems thinking agenda forward, including systematically exploring issues from a health system perspective, fostering more system-wide planning, evaluation and research, and building a community of practice. We believe that our framework contributes to this effort although more implementation research is needed to guide its practical applications.
Acknowledgements
The authors are grateful to Kent Ranson, Taghreed Adam, Anita Wagner, Hans Hogerzeil and Dean Shuey for their extensive and very useful comments on earlier versions of this manuscript.
Funding
This work was completed as part of the ATM program at the Alliance for Health Policy and System Research. This program is funded through a research grant from the UK Department for International Cooperation (DfID).
REFERENCES
- Adam T, Ahmad S, Bigdeli M, et al. Trends in health policy and systems research over the past decade: still too little capacity in low-income countries. PLoS One. 2012;6:1–10. doi: 10.1371/journal.pone.0027263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Khan MM. A maternal health voucher scheme: what have we learned from the demand-side financing scheme in Bangladesh? Health Policy and Planning. 2011;26:25–32. doi: 10.1093/heapol/czq015. [DOI] [PubMed] [Google Scholar]
- Ali GKM. How to establish a successful revolving drug fund: the experience of Khartoum state in the Sudan. Bulletin of the World Health Organization. 2009;87:139–42. doi: 10.2471/BLT.07.048561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameli O, Newbrander W. Contracting for health services: effects of utilization and quality on the costs of the Basic Package of Health Services in Afghanistan. Bulletin of the World Health Organization. 2008;86:920–9. doi: 10.2471/BLT.08.053108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audibert M, Mathonnat J. Cost recovery in Mauritania: initial lessons. Health policy and planning. 2000;15:66–75. doi: 10.1093/heapol/15.1.66. [DOI] [PubMed] [Google Scholar]
- Babar ZUD, Ibrahim MIM, Singh H, et al. Evaluating drug prices, availability, affordability, and price components: implications for access to drugs in Malaysia. PLoS Medicine. 2007;4:466–75. doi: 10.1371/journal.pmed.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran D, McCabe A, Yudkin JS. Access to medicines versus access to treatment: the case of type 1 diabetes. Bulletin of the World Health Organization. 2008;86:648–9. doi: 10.2471/BLT.07.048710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran D, Yudkin J. Diabetes care in sub-Saharan Africa. Lancet. 2006;368:1689–95. doi: 10.1016/S0140-6736(06)69704-3. [DOI] [PubMed] [Google Scholar]
- Bigdeli M, Annear PL. Barriers to access and the purchasing function of health equity funds: lessons from Cambodia. Bulletin of the World Health Organization. 2009;87:560–4. doi: 10.2471/BLT.08.053058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burki T. The real cost of counterfeit medicines. Lancet Infectious Diseases. 2010;10:585–6. doi: 10.1016/s1473-3099(10)70173-0. [DOI] [PubMed] [Google Scholar]
- Cameron A, Ewen M, Ross-Degnan D, et al. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet. 2009;373:240–9. doi: 10.1016/S0140-6736(08)61762-6. [DOI] [PubMed] [Google Scholar]
- Carasso BS, Lagarde M, Tesfaye A, Palmer N. Availability of essential medicines in Ethiopia: an efficiency–equity trade-off? Tropical Medicine and International Health. 2009;14:1394–400. doi: 10.1111/j.1365-3156.2009.02383.x. [DOI] [PubMed] [Google Scholar]
- Centre for Pharmaceutical Management. Defining and Measuring Access to Essential Drugs, Vaccines, and Health Commodities: Report of the WHO-MSH consultative meeting. 2003. Ferney-Voltaire, France, December 11–13, 2000. [Google Scholar]
- Chalker J, Ratawajitrasin S, Chuc NTK, et al. Effectiveness of a multi-component intervention on dispensing practices at private pharmacies in Vietnam and Thailand—a randomized controlled trial. Social Science and Medicine. 2005;60:131–41. doi: 10.1016/j.socscimed.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Chirac P, Torreele E. Global framework on essential health R & D. Lancet. 2006;367:1560–1. doi: 10.1016/S0140-6736(06)68672-8. [DOI] [PubMed] [Google Scholar]
- Chukwuani CM, Olugboji A, Ugbene E. Improving access to essential drugs for rural communities in Nigeria: the Bamako initiative re-visited. Pharmacy World and Science. 2006;28:91–5. doi: 10.1007/s11096-006-9010-1. [DOI] [PubMed] [Google Scholar]
- Chuma J, Okungu V, Molyneux C. Barriers to prompt and effective malaria treatment among the poorest population in Kenya. Malaria Journal. 2010;9:144. doi: 10.1186/1475-2875-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn R, Newton P, Agyarko E, et al. The global threat of counterfeit drugs: why industry and governments must communicate the dangers. PLoS Medicine. 2005;2:302–8. doi: 10.1371/journal.pmed.0020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Kohler JC. The morally uncomfortable global drug gap. Clinical Pharmacology and Therapeutics. 2007;82:610–4. doi: 10.1038/sj.clpt.6100359. [DOI] [PubMed] [Google Scholar]
- Cometto G, Ooms G, Starrs A, et al. A global fund for the health MDGs? Lancet. 2009;373:1500–2. doi: 10.1016/S0140-6736(09)60835-7. [DOI] [PubMed] [Google Scholar]
- Consultative Expert Working Group on Research and Development. Research and Development to Meet Health Needs in Developing Countries: Strengthening Global Financing and Coordination. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- de Brouwere V, Richard F, Witter S. Access to maternal and perinatal health services: lessons from successful and less successful examples of improving access to safe delivery and care of the newborn. Tropical Medicine & International Health. 2010;15:901–9. doi: 10.1111/j.1365-3156.2010.02558.x. [DOI] [PubMed] [Google Scholar]
- de Savigny D, Adam T. Alliance for Health Policy and Systems Research. Geneva, Switzerland: World Health Organization; 2009. Systems thinking for health systems strengthening. [Google Scholar]
- Ensor T, Cooper S. Overcoming barriers to health service access: influencing the demand side. Health Policy and Planning. 2004;19:69–79. doi: 10.1093/heapol/czh009. [DOI] [PubMed] [Google Scholar]
- Frost LJ, Reich MR. Boston, MA: Harvard Center for Population and Development Studies; 2010. How do good health technologies get to poor people in poor countries. [Google Scholar]
- Goodman C, Kachur SP, Abdulla S, et al. Drug shop regulation and malaria treatment in Tanzania—why do shops break the rules, and does it matter? Health Policy and Planning. 2007;22:393–403. doi: 10.1093/heapol/czm033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K, Ranson MK, Oliveira-Cruz V, Mills A. Expanding access to priority health interventions: a framework for understanding the constraints to scaling-up. Journal of International Development. 2003;15:1–14. [Google Scholar]
- Haines A, Sanders D, Lehmann U, et al. Achieving child survival goals: potential contribution of community health workers. Lancet. 2007;369:2121–31. doi: 10.1016/S0140-6736(07)60325-0. [DOI] [PubMed] [Google Scholar]
- Hogerzeil HV, Samons M, Casano JV, et al. Is access to essential medicines as part of the fulfilment of the right to health enforceable through the courts? Lancet. 2006;368:305–11. doi: 10.1016/S0140-6736(06)69076-4. [DOI] [PubMed] [Google Scholar]
- Holloway K, van Dijk L. Rational Use of Medicines. The World Medicines Situation 2011. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- Jacobs B, Ir P, Bigdeli M, Annear PL, Van Damme W. Addressing access barriers to health services for the poor: an analytical framework for selecting appropriate interventions in low income countries. Health Policy and Planning. 2012;27:288–300. doi: 10.1093/heapol/czr038. [DOI] [PubMed] [Google Scholar]
- Jacobs B, Thomé JM, Overtoom R, et al. From public to private and back again: sustaining a high service-delivery level during transition of management authority: a Cambodia case study. Health Policy and Planning. 2010;25:197–208. doi: 10.1093/heapol/czp049. [DOI] [PubMed] [Google Scholar]
- Kiwanuka SN, Ekipara EK, Peterson S, et al. Access to and utilisation of health services for the poor in Uganda: a systematic review of available evidence. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2008;102:1067–74. doi: 10.1016/j.trstmh.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Kohler JC, Baghdadi-Sabeti G. Good Governance for the Pharmaceutical Sector. The World Medicines Situation 2011. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- Kotwani A. Availability, price and affordability of asthma medicines in five Indian states. The International Journal of Tuberculosis and Lung Disease. 2009;13:574–9. [PubMed] [Google Scholar]
- Lagarde M, Haines A, Palmer N. Conditional cash transfers for improving uptake of health interventions in low- and middle-income countries: a systematic review. Journal of the American Medical Association. 2007;298:1900–10. doi: 10.1001/jama.298.16.1900. [DOI] [PubMed] [Google Scholar]
- Laing R, Hogerzeil H, Ross-Degnan D. Ten recommendations to improve use of medicines in developing countries. Health Policy and Planning. 2001;16:13–20. doi: 10.1093/heapol/16.1.13. [DOI] [PubMed] [Google Scholar]
- Lon C, Tsuyuoka R, Phanouvong S, et al. Counterfeit and substandard antimalarial drugs in Cambodia. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2006;100:1019–24. doi: 10.1016/j.trstmh.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Maïga FI, Haddad S, Fournier P, et al. Public and private sector responses to essential drugs policies: a multilevel analysis of drug prescription and selling practices in Mali. Social Science & Medicine. 2003;57:937–48. doi: 10.1016/s0277-9536(02)00462-8. [DOI] [PubMed] [Google Scholar]
- Mavalankar DV, Rosenfield A. Maternal mortality in resource-poor settings: policy barriers to care. American Journal of Public Health. 2005;95:200–3. doi: 10.2105/AJPH.2003.036715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MeTA. Medicines Transparency Alliance—A Review of the Pilots. 2010. Medicines Transparency Alliance. http://www.medicinestranparency.org (Accessed 5 December 2011) [Google Scholar]
- Meessen B, Bigdeli M, Kannarath C, et al. Composition of pluralistic health systems. How much can we learn from household surveys? An exploration in Cambodia. Health Policy and Planning. 2011;26:i30–44. doi: 10.1093/heapol/czr026. [DOI] [PubMed] [Google Scholar]
- Mills A, Brugha P, Hanson K, et al. Public health reviews. What can be done about the private health sector in low-income countries? Bulletin of the World Health Organization. 2002;80:325–30. [PMC free article] [PubMed] [Google Scholar]
- Murakami H, Phommasak B, Oula R, et al. Revolving drug funds at front-line health facilities in Vientiane, Lao PDR. Health Policy and Planning. 2001;16:98–106. doi: 10.1093/heapol/16.1.98. [DOI] [PubMed] [Google Scholar]
- Newton PN, Amin AA, Bird C, et al. The primacy of public health considerations in defining poor quality medicines. PLoS Medicine. 2011;8:e1001139. doi: 10.1371/journal.pmed.1001139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrist B, Iteba N, Lengeler C, et al. Access to health care in contexts of livelihood insecurity: a framework for analysis and action. PLoS Medicine. 2007;4:1584–8. doi: 10.1371/journal.pmed.0040308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms G. The Right to Health and the Sustainability of Healthcare: Why a New Global Health Aid Paradigm Is Needed. Belgium: University of Ghent; 2008. [Google Scholar]
- Paina L, Peters DH. Understanding pathways for scaling up health services through the lens of complex adaptive systems. Health Policy and Planning. 2011;26:1–9. doi: 10.1093/heapol/czr054. [DOI] [PubMed] [Google Scholar]
- Pariyo GW, Ekipara-Kiracho E, Okui O, et al. Changes in utilization of health services among poor and rural residents in Uganda: are reforms benefitting the poor? International Journal for Equity in Health. 2009;8:39. doi: 10.1186/1475-9276-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariyo GW, Gouws E, Bryce J, et al. Improving facility-based care for sick children in Uganda: training is not enough. Health Policy and Planning. 2005;20(Suppl 1):58–68. doi: 10.1093/heapol/czi051. [DOI] [PubMed] [Google Scholar]
- Patel A, Gauld R, Norris P, et al. “This body does not want free medicines”: South African consumer perceptions of drug quality. Health Policy and Planning. 2010;25:61–9. doi: 10.1093/heapol/czp039. [DOI] [PubMed] [Google Scholar]
- Patouillard E, Goodman C, Hanson K, et al. Can working with the private for-profit sector improve utilization of quality health services by the poor? A systematic review of the literature. International Journal for Equity in Health. 2007;6:17. doi: 10.1186/1475-9276-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoud B, Chirac C, Trouiller P, et al. Access to essential drugs in poor countries. Journal of the American Medical Association. 1999;282:631. [Google Scholar]
- Penchansky R, Thomas JW. The concept of access: definition and relationship to consumer satisfaction. Medical Care. 1981;19:127–40. doi: 10.1097/00005650-198102000-00001. [DOI] [PubMed] [Google Scholar]
- Perehudoff S, Laing RO, Hogerzeil HV. Access to essential medicines in national constitutions. Bulletin of the World Health Organization. 2010;88:800. doi: 10.2471/BLT.10.078733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters DH, Garg A, Bloom G, et al. Poverty and access to health care in developing countries. Annals of the New York Academy of Sciences. 2008;1136:161–71. doi: 10.1196/annals.1425.011. [DOI] [PubMed] [Google Scholar]
- Quick JD, Hogerzeil HV. Perspectives: twenty-five years of essential medicines. Bulletin of the World Health Organization. 2002;80:913–4. [PMC free article] [PubMed] [Google Scholar]
- Reilley B, Abeyasinghe R, Pakianathar MV. Barriers to prompt and effective treatment of malaria in northern Sri Lanka. Tropical Medicine & International Health. 2002;7:744–9. doi: 10.1046/j.1365-3156.2002.00919.x. [DOI] [PubMed] [Google Scholar]
- Rohde J, Cousens S, Chopra M, et al. Alma-Ata: Rebirth and Revision 4 30 years after Alma-Ata: has primary health care worked in countries? Lancet. 2008;372:950–61. doi: 10.1016/S0140-6736(08)61405-1. [DOI] [PubMed] [Google Scholar]
- Ruxin J, Paluzzi JE, Wilson PA, et al. Emerging consensus in HIV/AIDS, malaria, tuberculosis, and access to essential medicines. Lancet. 2005;365:618–21. doi: 10.1016/S0140-6736(05)17914-8. [DOI] [PubMed] [Google Scholar]
- Saleh K, Ibrahim MI. Are essential medicines in Malaysia accessible, affordable and available? Pharmacy World & Science. 2005;27:442–6. doi: 10.1007/s11096-005-1318-8. [DOI] [PubMed] [Google Scholar]
- Shankar PR. Medicines Use in Primary Care in Developing and Transitional Countries: Fact Book Summarizing Results from Studies Reported between 1990 and 2006. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- Sheikh K, Gilson L, Agyepong IA, et al. Building the field of health policy and systems research : framing the questions. PLoS Medicine. 2011;8:1–6. doi: 10.1371/journal.pmed.1001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith F. The quality of private pharmacy services in low and middle-income countries: a systematic review. Pharmacy World and Science. 2009;31:351–61. doi: 10.1007/s11096-009-9294-z. [DOI] [PubMed] [Google Scholar]
- Tomson G. The impact of global processes on health systems in Europe. Global Health Europe. 2010;2:1–47. [Google Scholar]
- Uzochukwu B, Onwujekwe O. Healthcare reform involving the introduction of user fees and drug revolving funds: influence on health workers’ behavior in southeast Nigeria. Health Policy. 2005;75:1–8. doi: 10.1016/j.healthpol.2005.01.019. [DOI] [PubMed] [Google Scholar]
- van Damme W, Kober K, Kegels G. Scaling-up antiretroviral treatment in Southern African countries with human resource shortage: How will health systems adapt? Social Science & Medicine. 2008;66:2108–21. doi: 10.1016/j.socscimed.2008.01.043. [DOI] [PubMed] [Google Scholar]
- van Olmen J, Ku GM, Bermejo R, et al. The growing caseload of chronic life-long conditions calls for a move towards full self-management in low-income countries. Globalization and health. 2011;7:1–10. doi: 10.1186/1744-8603-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Olmen J, Criel B, Bhojani U, Marchal B, et al. The health system dynamics framework: the introduction of an analytical model for health system analysis and its application to two case-studies. Health, Culture and Society. 2012;2:10–21. [Google Scholar]
- Walley J, Lawn J, Tinker A, et al. Alma-Ata: making Alma-Ata a reality. Lancet. 2008;372:1001–7. doi: 10.1016/S0140-6736(08)61409-9. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Medicines Startegy: Countries at the Core. 2004-2007. Geneva, Switzerland: WHO; 2004a. [Google Scholar]
- World Health Organization. The World Medicines Situation 2004. Geneva, Switzerland: WHO; 2004b. [Google Scholar]
- World Health Organization. Equitable access to essential medicines: a framework for collective action. Policy Perspectives on Medicines. 2004c;8:1–6. [Google Scholar]
- World Health Organization. Everybody's Business—Strengthening Health Systems to Improve Health Outcomes. Geneva, Switzerland: WHO; 2007. [Google Scholar]
- Zakus D, Kohler J, Zakriova V, et al. Achieving a dream: meeting policy goals related to improving drug access. The Open AIDS Journal. 2010;4:25–7. doi: 10.2174/1874613601004020025. [DOI] [PMC free article] [PubMed] [Google Scholar]