Abstract
We sought to examine the efficacy and safety of acamprosate augmentation of escitalopram in patients with concurrent major depressive disorder (MDD) and alcohol use disorders. Twenty-three adults (43% female; mean ± SD age, 46 ± 14 years) were enrolled and received 12 weeks of treatment with psychosocial support; escitalopram, 10 to 30 mg/d; and either acamprosate, 2000 mg/d (n = 12), or identical placebo (n = 11). Outcomes included change in clinician ratings of depressive symptoms, MDD response and remission rates, changes in frequency and intensity of alcohol use, retention rates, and adverse events. Twelve subjects (acamprosate, n = 7; placebo, n = 5) completed the study. There was significant mean reduction in ratings of depressive symptoms from baseline in both treatment arms (P < 0.05), with no significant difference between the groups. Those in the acamprosate group had a 50% MDD response rate and a 42% remission rate, whereas those in the placebo arm had a 36% response and remission rate (not significant). Those assigned to acamprosate had significant reduction in number of drinks per week and drinks per month during the trial, whereas those assigned to placebo demonstrated no significant change in any alcohol use parameter, but the between-group difference was not significant. There were no significant associations between change in depressive symptoms and change in alcohol use. Attrition rates did not differ significantly between the 2 arms. Acamprosate added to escitalopram in adults with MDD and alcohol use disorders was associated with reduction in the frequency of alcohol use. The present study was not powered to detect superiority versus placebo. Further study in a larger sample is warranted.
Keywords: acamprosate, alcohol use disorder, AUD, depression, MDD, escitalopram
Among the approximately 17.6 million Americans who develop major depressive disorder (MDD) in a given year, the 12-month prevalence of an alcohol use disorder (AUD) is 16.4%.1 People with comorbid AUD and MDD often have symptoms that are more persistent and severe than people with either disorder alone,2 and this may complicate treatment. Major depressive disorder remission is less likely in patients with concurrent MDD and AUD3; when one disorder increases in severity, the other often increases as well4,5; these individuals also spend more on medical care and are more likely to commit suicide.2 Despite the significant public health burden of comorbid MDD and AUD, there is a notable dearth of research with regard to its optimal management.
Due to concerns over misattribution of symptoms and greater likelihood of adverse events, many physicians are reluctant to provide antidepressant treatment to depressed individuals who are actively using alcohol or other substances.6–9 Nonetheless, the past decade has seen a significant increase in studies that investigate integrated treatment of MDD and AUD.
The approach to the treatment of comorbid MDD and AUD varies greatly, depending on whether the main focus is abstinence, depression alleviation, or both. Whereas results from early studies on the effectiveness of antidepressants in comorbid MDD and AUD were not promising,10– 12 more recent work suggests that antidepressants may in fact be somewhat helpful in this population. A body of at least 9 recent clinical trials of combined antidepressants and psychosocial treatment in patients with a history of comorbid MDD and AUD13–22 consistently revealed a reduction in symptoms of depression, independent of antidepressant type. These recent findings suggest that antidepressant pharmacotherapy, particularly selective serotonin reuptake inhibitors (SSRIs), can be useful in treating depression even if patients continue to drink.9
Attempts to find treatments that reduce alcohol consumption in this comorbid population have been less promising. Among the aforementioned 9 studies, 5 studies showed a beneficial effect of an antidepressant treatment (including desipramine, fluoxetine, nefazodone, sertraline, escitalopram, and the noncompetitive glutamate N-methyl-D-aspartate [NMDA] receptor antagonist memantine) on alcohol consumption.13,15,18,20,22 Memantine in particular showed mood benefits as well as reduction in drinking. 21,22 Three studies reported less encouraging results for imipramine,14 nefazodone,17 and sertraline19 regarding alcohol use reduction. These mixed findings suggest that augmentation of antidepressant treatment may be needed to reduce alcohol consumption while improving mood in those with comorbid MDD.9
Given the relative safety and tolerability of SSRIs compared with other antidepressants such as tricyclic antidepressants, SSRIs may be the preferred choice for the antidepressant component of a combined pharmacotherapy plan for those with comorbid MDD and AUD.23 Regarding antialcohol therapy, acamprosate, which is thought to function by blocking glutamatergic NMDA receptors (analogously to memantine) while also activating gamma-aminobutyric acid (GABA) type A receptors, 24 may be a good candidate for this role. First, it has been shown to help reduce drinking in AUD subjects after detoxification and may also enhance abstinence.25,26 Second, a recent open pilot study in a small sample of patients with anxiety who were partially responding to SSRIs or serotonin-norepinephrine reuptake inhibitors showed that added acamprosate was well tolerated and effective at reducing anxious symptoms.27 This suggests that acamprosate could also be safely combined with SSRIs in depressed patients with AUD and that their combined serotonergic and GABA-ergic mechanisms of action could complement each other to promote abstinence and reduce depressive symptoms.
In view of the foregoing, we investigated the efficacy, safety, and tolerability of a combined regimen of the SSRI escitalopram28,29 plus acamprosate in a population of individuals with MDD and alcohol abuse or dependence (AUD) receiving psychosocial intervention compared to the usual regimen of escitalopram and psychosocial intervention. We hypothesized that the addition of acamprosate to SSRIs in patients with comorbid MDD and AUD would confer a significant benefit for both disorders.
MATERIALS AND METHODS
This was a single-center, 12-week, double-blind, randomized placebo-controlled study comparing the SSRI escitalopram plus psychosocial intervention (the usual treatment for comorbid depression and AUD) against the same regimen with the addition of acamprosate. The study was approved by our institutional review board (IRB), and IRB-approved written informed consent was obtained by licensed physician investigators from all study participants before any study procedures were conducted. The study was carried out in accordance with the Declaration of Helsinki.
Participants
We screened 38 subjects and recruited 23 of both sexes (43%female) of ages 18 to 65 (mean ± SD, 46 ± 14 years) through IRB-approved advertisements and referrals from within our institution and the Boston area. The subjects were required to meet criteria for MDD and an AUD (alcohol abuse or dependence) as diagnosed by the Structured Clinical Interview for DSM-IV Psychiatric Disorders (SCID-P).30
The patients were required to be off any previous antidepressants for at least 2 weeks by the time of the baseline visit (4 weeks in the case of fluoxetine), and off benzodiazepines and other psychotropic medications for at least 1 week by the time of the baseline visit. The decision about whether to taper existing medications was made by prospective study participants in conjunction with their primary physicians.
Exclusion criteria included the following: (1) suicidal ideation where study participation was deemed unsafe by the study clinician; (2) women who were pregnant or breastfeeding, or women of childbearing potential not using a medically accepted means of contraception; (3) serious or unstable medical illness; (4) clinical or laboratory evidence of untreated hypothyroidism; (5) lifetime history of organic mental disorder, schizophrenia spectrum illness, bipolar disorder, MDD with psychotic features, substance use disorder other than alcohol or nicotine active in the prior 12 months; (6) current use of other psychotropic drugs other than antihistamines; (7) failure to respond during the course of the current depressive episode to 2 or more adequate antidepressant trials (defined as ≥6 weeks of escitalopram ≥20 mg/d or its equivalent); (8) participation in depression-focused or addiction-focused psychotherapy (participation in Alcoholics Anonymous was allowed); (9) investigational psychotropic drug use in the prior year; (10) need for medical alcohol detoxification in the opinion of the screening physician in accordance with methods used in the multicenter STAR-D study.31
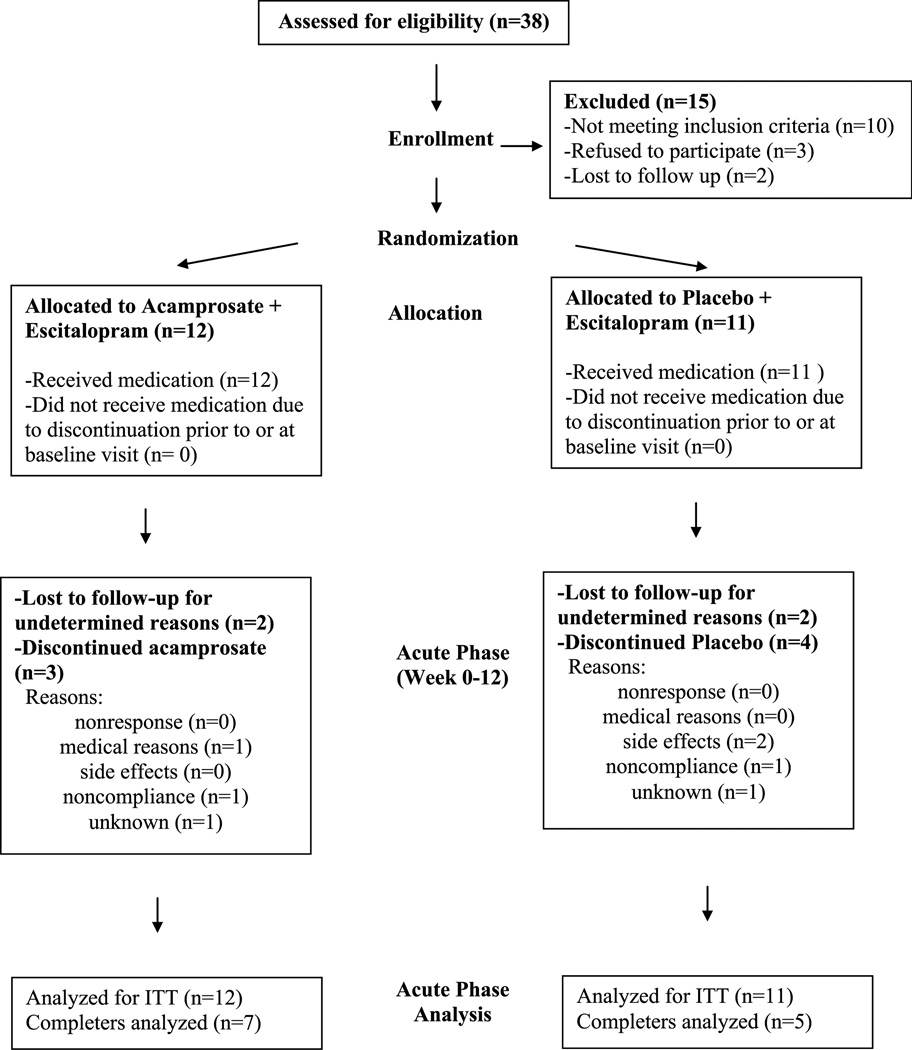
Recruitment and patient flow are diagrammed in Figure 1.
FIGURE 1.
Patient flow diagram.
Assessments
Screening assessments included medical and psychiatric history and physical examination. Rating scales administered at screen included the SCID-P,25 17-item Hamilton Depression Rating Scale (HAM-D-17),32–34 Consumptive Habits Questionnaire, 35 Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR),36 Quality of Life Enjoyment and Satisfaction questionnaire (Q-LES-Q),37 Clinical Global Impression—Severity of Illness (CGI-S),38 CGI—Improvement (CGI-I),38 36-item Short-Form Health Survey (SF-36),39 Obsessive Compulsive Drinking Scale (OCDS)40 and Alcohol Timeline Followback.41
Because study the subjects were actively drinking at the time of admission, no formal instruments for withdrawal were administered. However, alcohol withdrawal symptoms were monitored by clinical interview at each visit. All entering subjects received a list of withdrawal symptoms to watch for, and the pager number of the principal investigator, who was available 24/7 to answer questions and triage a subject to the appropriate level of care. An alcohol withdrawal management protocol was put in place, with primary emphasis on ensuring the subject’s medical safety. For mild symptoms (loss of appetite, irritability, and tremulousness), a chlordiazepoxide taper for up to 4 days would be provided. A study physician would contact the subject daily during the taper to determine whether the subject required a more intensive level of care. In case of more serious withdrawal symptoms (including tremulousness, heart rate persistently more than 100 beats per minute, severe insomnia, hypervigilance, hallucinations, mental status changes, seizures, or delirium), the patient would be immediately transferred to the nearest emergency department.
Interventions
Potential participants who were deemed eligible returned 1 week after enrollment (or as noted earlier in cases of antidepressant taper) for a baseline visit. At this visit, all participants were assigned to escitalopram, 10 to 30 mg/d, a behavioral intervention for AUD as described in the Medical Management Treatment Manual from the National Institute of Alcohol Abuse and Alcoholism’s Combining Medications and Behavioral Interventions Monograph Series,42 and randomized double-blind augmentation with acamprosate, 2000 mg/d, or identical placebo for 12 weeks.
The initial medical management treatment session at the baseline visit (40–60 minutes) involved discussion of the alcohol abuse/dependence diagnosis and negative consequences from drinking, a recommendation to abstain from consuming alcohol, medication information, strategies to enhance medication adherence, and referral to support groups such as Alcoholics Anonymous. A phone call was made by the doctor 3 days after the baseline visit to check on progress and adverse events. The 6 subsequent study visits included medical management sessions (15- to 25-minute visits) in which the physician assessed the participant’s drinking, overall functioning, medication adherence, and any adverse events.
Initial dosing was acamprosate, 333 mg, capsules or identical placebo, 2 capsules 3 times a day, plus escitalopram, 10 mg/d. The escitalopram was dosed flexibly per the discretion of the study physician, starting at 10 mg/d and modified based on apparent efficacy and tolerability. Increases were allowed to 20 mg/d at week 2 or later, and to 30 mg/d at week 4 or later. Whereas the highest recommended escitalopram dose is 20 mg/d, clinical practice has shown that higher doses are often safe and effective; and for this reason, we allowed a maximum dose of 30 mg in cases where patients may have had limited improvement on the lower doses.
Adverse events were carefully documented at each visit. Study visits were conducted at weeks 1, 2, 4, 6, 9, and 12.
Analysis
Power analysis was originally based on intent-to-treat (ITT) analysis with 40 subjects and assumed a 30% difference in alcohol consumption (50% reduction vs 20% reduction in the acamprosate and placebo groups, respectively). With α = 0.05, we estimated 74% power to detect a difference between the 2 groups. Results would be used to estimate effect size to set the stage for an adequately powered larger study in the future.
Demographic information was documented using descriptive statistics. Intent-to-treat analysis was carried out, using last observation carried forward (LOCF).
Changes in HAM-D-17 scores, alcohol use parameters, and other continuous outcome measures from baseline to end point for each treatment arm were assessed by paired sample t tests. Because of the small sample size, the results were also analyzed by the nonparametric Wilcoxon signed ranks test. Comparisons between treatment arms (acamprosate vs placebo) were made by the independent samples t test and the nonparametric Mann-Whitney U test.
Antidepressant response was defined as a 50% or greater improvement in the HAM-D-17 score from baseline to end point. Remission was defined as a final HAM-D-17 score of less than 8. Response and remission rates were compared between the 2 treatment arms using the χ2 and Fisher exact tests.
Changes in alcohol use parameters were also compared between responders and nonresponders within each intervention arm using the independent samples t test and the Mann-Whitney U test. Linear regression was used to examine any association between the change in HAM-D-17 score and the drinking parameters. Logistic regression was used to examine any association between response rates and the drinking parameters.
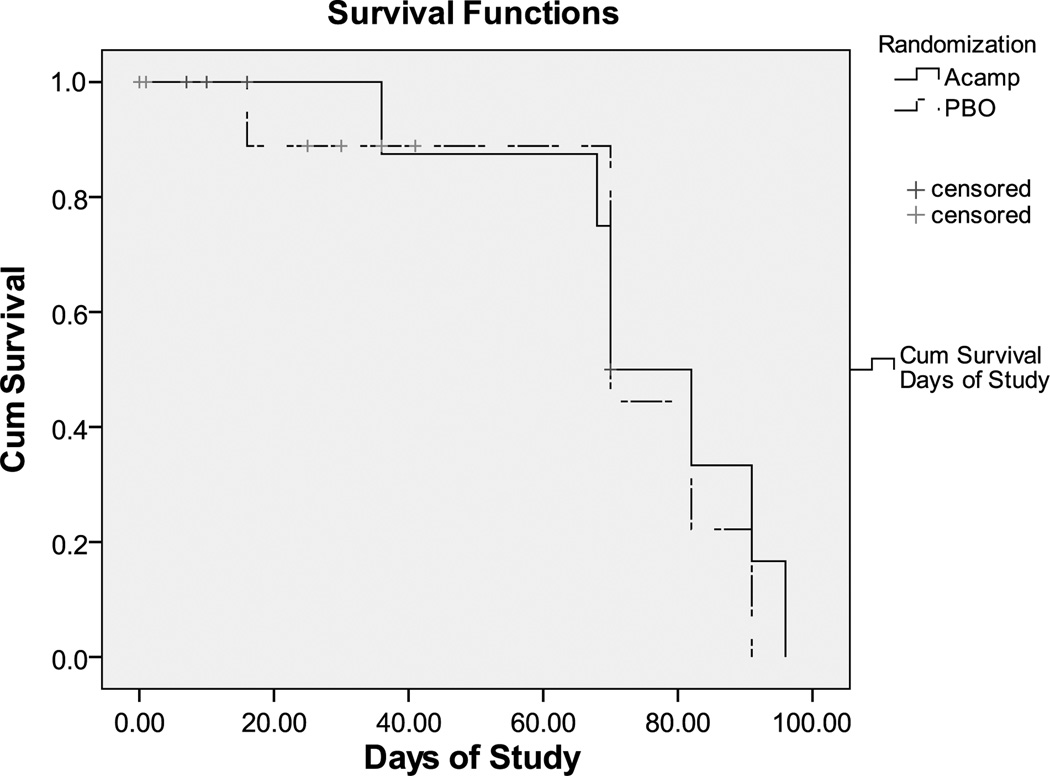
Kaplan-Meier survival analysis was used to examine the difference in attrition rates between the acamprosate and placebo groups.
For all analyses, 2-tailed statistical significance was set at P < 0.05. All calculations were performed with SPSS version 17.0 (Chicago, Ill).
RESULTS
Sample demographics are summarized on Table 1. The acamprosate and placebo groups were similar with regard to age, sex, ethnicity, history of lifetime drug abuse, presence of current alcohol abuse or dependence, and presence of recurrent MDD. Of note, there was a significant difference in baseline HAM-D-17 and CGI-S scores between the acamprosate group and the placebo group (Table 2). Twelve patients (7 patients in the acamprosate group and 5 patients in the placebo group) completed the study, and the difference between the arms was not significant (Table 2). Kaplan-Meier survival analysis showed no significant difference in attrition rates between the 2 treatment arms (Fig. 2).
TABLE 1.
Sample Demographics
| Acamprosate (n = 12) | Placebo (n = 11) | Significance | |
|---|---|---|---|
| Age, mean ± SD, y | 49 ± 13 | 43 ±14 | t = 0.997, df = 21, P = 0.330 |
| Female | 4 | 6 | χ2 = 1.05, P = 0.414 |
| White | 12 | 9 | χ2 = 1.26, P = 0.455 |
| Drug abuse: lifetime | 4 | 1 | χ2 = 1.98, P = 0.317 |
| Alcohol abuse: current | 1 | 0 | χ2 = 0.96, P = 0.33 |
| Alcohol dependence: current | 11 | 11 | χ2 = 0.96, P = 0.33 |
| MDD: recurrent | 7 | 3 | χ2 = 2.25, P = 0.214 |
TABLE 2.
Depression Outcomes (ITT Sample Based on Last Observation Carried Forward [LOCF]
| Acamprosate (n = 12) | Placebo (n = 11) | Significance (Acamprosate vs Placebo) | |
|---|---|---|---|
| HAM-D-17 at baseline, mean ± SD | 15.6 ± 5.2 | 20.7 ± 5.8 | P = 0.036* Mann-Whitney: U = 33.00, Z = −2.03, P = 0.042* |
| HAM-D-17 at end point, mean ± SD | 10.0 ± 7.2 | 12.9 ± 9.8 | t = −0.814, df = 21, P = 0.425 |
| Change in HAM-D-17 score, mean ± SD | −5.6 ± 8.5 | −7.8 ± 9.9 | t = 0.579, df = 21, P = 0.566 |
| Significance for change in HAM-D-17 score | t = 2.28, P = 0.043* Wilcoxon: Z = −2.05, P = 0.041*) | t = 2.62, P = 0.026* Wilcoxon: Z = − 2.14, P = 0.033* | NA |
| QIDS-SR at baseline, mean ± SD | 10.7 ± 3.5 | 13.2 ± 3.7 | t = −1.667, df = 21, P = 0.110 |
| QIDS-SR at end point, mean ± SD | 7.5 ± 5.1 | 9.4 ± 6.2 | t = −0.788, df = 21, P = 0.439 |
| Change in QIDS-SR, mean ± SD | −3.2 ± 5.0 | −3.8 ± 5.2 | t = 0.308, df = 21, P = 0.761 |
| Significance for change in QIDS-SR score | t = 2.22, P = 0.049* Wilcoxon: Z = −2.05, P = 0.040* | t = 2.44, P = 0.035* Wilcoxon: Z = −2.11, P = 0.035* | NA |
| CGI-S at baseline, mean ± SD | 3.3 ± 0.8 | 4.4 ± 0.7 | t = −3.378, df = 21, P = 0.003* Mann Whitney: U = 23.00, Z = −2.83, P = 0.005* |
| CGI-S at end point, mean ± SD | 2.6 ± 1.2 | 3.0 ± 1.6 | t = −0.705, df = 18, P = 0.490 |
| Change in CGI-S, mean ± SD | −0.75 ± 1.4 | −1.4 ± 1.7 | t = 0.945, df = 21, P = 0.355 |
| Significance for change in CGI-S score | t = 1.92, P = 0.082 Wilcoxon: Z = −1.64, P = 0.101 | t = 2.59, P = 0.027* Wilcoxon: Z = −2.04, P = 0.041* | NA |
| CGI-I at end point, mean ± SD | 2.8 ± 1.2 | 2.9 ± 1.5 | t = −0.279, df = 21, P = 0.482 |
| Response rate | 50% | 36% | χ2 = 0.43, P = 0.68 |
| Remission rate | 42% | 36% | χ2 = 0.07, P = 1.00 |
| Completers | 7 | 5 | χ2 = 0.38, P = 0.68 |
Statistically significant (P < 0.05).
FIGURE 2.
Attrition in acamprosate versus placebo group.
Dosing Schedules
In the escitalopram/acamprosate group (n = 12), 8 subjects received a maximum dose of 10 mg of escitalopram per day, one subject received 20 mg/d, and 3 subjects received 30 mg/d (mean ± SD, 15.8 ± 9.0). In the escitalopram/placebo group (n = 11), 4 subjects received a maximum dose of 10 mg of escitalopram per day, 7 subjects received 20 mg/d, and none received 30 mg/d (mean ± SD, 16.4 ± 5.0). Mean escitalopram doses did not differ significantly between the treatment groups (P > 0.05).
Adherence
Medication adherence was assessed in part with the medication adherence question of the QIDS-SR at each study visit. The patients were asked how often they missed their medication, with answers ranging from 0 (not applicable), 1 (never), 2 (rarely), 3 (sometimes), 4 (less than half of the time), 5 (approximately half of the time), 6 (more than half of the time), 7 (very often), 8 (nearly all the time), and 9 (all the time). In the entire sample, only one patient reported missing their medications “all the time” at their final visit, after they had self-discontinued medication. Among the rest of the sample, no subject reported a score higher than 3 (sometimes) at any visit, and the most common answer at each visit was 1 (never). Among study completers (9 patients with available response data on the medication adherence question), none reported a score higher than 3 (sometimes) at study completion, and 78% reported scores of 2 (rarely) or less.
We sought to verify adherence from the number of returned pills at each follow-up visit, but this proved difficult owing to inconsistent pill return rates. Among the entire sample (n = 23), 13 subjects (56%) were confirmed adherent, 2 subjects (9%) were confirmed as inconsistently adherent (both terminated early, by week 1), and 8 subjects (35%) were unverifiable based on not returning pills on a regular basis. Among the 12 study completers, 8 (67%) were confirmed adherent, and 4 (33%) were unverifiable.
No significant difference in adherence was observed between the treatment arms, either for completers or for the ITT sample (P > 0.05).
Depression Outcomes
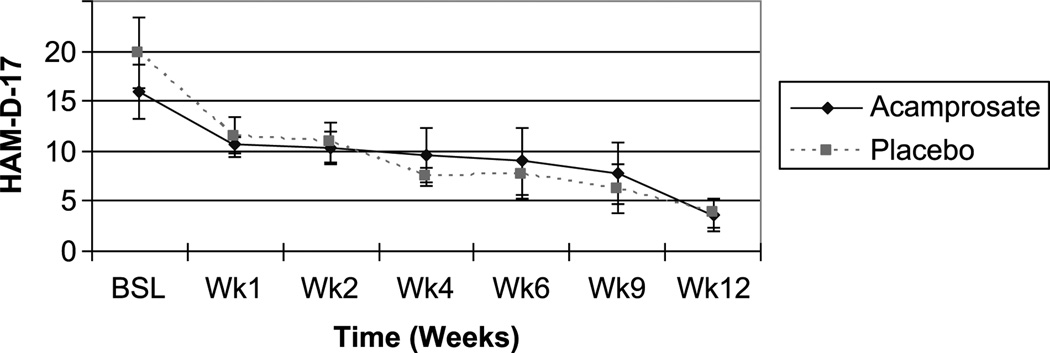
Both the acamprosate and placebo groups demonstrated significant improvement in the HAM-D-17 scores (P < 0.05 for both by nonparametric Wilcoxon signed rank test); the degree of improvement was not significantly different between the 2 treatment groups (Table 2). Depression response rates were 50% for the acamprosate group and 36% for the placebo group (not significant); and remission rates (defined as a final HAM-D-17 score of <8) were 42% and 36%, respectively (not significant) (Table 2). The time course of improvement in HAM-D-17 scores in study completers is illustrated in Figure 3. Scores did not separate between the acamprosate and placebo arms at any time point.
FIGURE 3.
Time course of depressive improvement based on AM-D-17 score (completers).
We also examined depressive outcomes based on the QIDSSR and CGI-S. The placebo group experienced a significant improvement in CGI-S scores, but the acamprosate group did not (Table 2). The difference in improvement between the groups was not significant, however. Both groups experienced improvement in the QIDS-SR scale, but the difference between the groups was not significant (Table 2).
Alcohol Use Outcomes
Prerandomization data were collected to establish baseline drinking severity in each treatment arm. In the acamprosate group, subjects reported 5 ± 2 drinks per drinking day, compared to 6 ± 4 for the placebo group (not significant). Those assigned to the acamprosate group reported 30% ± 34% days abstinent at baseline, whereas those assigned to placebo reported 52% ± 42% percent days abstinent (not significant) (Table 3).
TABLE 3.
Alcohol-Related Outcomes (ITT Sample Based on LOCF)
| Acamprosate | Placebo | |
|---|---|---|
| Baseline (Before Randomization) | ||
| Drinks/drinking day, mean ±SD | 5 ±2 | 6± 4 |
| Drinks/wk, mean ± SD | 21 ± 12 | 24 ± 26 |
| Drinks/mo, mean ± SD | 86 ± 47 | 97 ± 103 |
| Percent days abstinent, mean ± SD | 30 ± 34 | 52 ± 42 |
| Treatment (After Randomization) | ||
| Days spent in treatment, mean ± SD | 34 ± 33 | 21 ± 17 |
| Drinks/drinking day, mean ± SD | 4 ± 2 | 4± 4 |
| Drinks/wk, mean ± SD | 15 ± 13 | 15 ± 21 |
| Drinks/mo, mean ± SD | 61 ± 53 | 61 ± 86 |
| Percent days abstinent, mean ± SD | 44 ± 45 | 62 ± 43 |
| Significance for change in drinks/drinking day | P = 0.058, Wilcoxon: Z = −1.60, P = 0.110 | P = 0.903 |
| Significance for change in drinks/wk | P = 0.005*, Wilcoxon: Z = −2.75, P = 0.006* | P = 0.270 |
| Significance for change in drinks/mo | P = 0.005*, Wilcoxon: Z = −2.75, P = 0.006* | P = 0.270 |
| Significance for change in Percent days abstinent | P = 0.163 | P = 0.325 |
All comparisons between the acamprosate and placebo arms were nonsignificant (P > 0.05).
Statistically significant, P < 0.05.
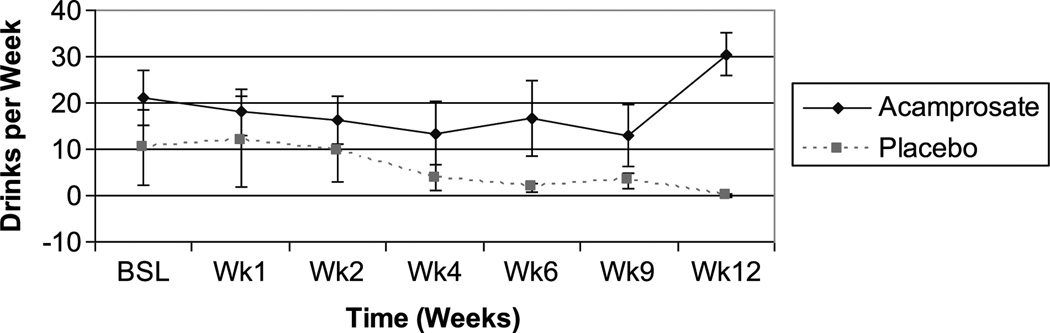
The acamprosate group reported a trend for a reduction in drinks per drinking day, and a significant improvement in drinks per week and drinks per month at study conclusion (Table 3), whereas those assigned to placebo reported no significant improvement in any alcohol use parameters; and there were no between-group differences (Table 3). The time course of improvement in drinks per week, based on study completers, suggested a slight trend to improvement in both groups over the first 4 weeks; but from week 6 onward, the attrition rate, particularly in the placebo group, resulted in the appearance of greater improvement in the placebo group (Fig. 4). This was driven largely by the discontinuation of 2 subjects that had been consuming 50 to 60 drinks per week. Acamprosate group completers reported a higher mean level of alcohol use than the placebo group by study end point (Fig. 4) despite the significant improvement based on last observation carried forward analysis. This was driven largely by 2 subjects who were drinking more than 30 drinks per week.
FIGURE 4.
Time course of alcohol use improvement based on rinks per week (completers).
The acamprosate group had a significant improvement in the Obsessive Compulsive Drinking Scale in all dimensions (obsessions, compulsions, and total); the placebo group also had significant improvement in these scales, except for the obsessiveness scale, in which there was only a trend to significance (Table 4).
TABLE 4.
Secondary Outcome Measures (ITT Sample Based on LOCF)
| Acamprosate/Escitalopram (n = 12) | Placebo/Escitalopram (n = 11) | |||||
|---|---|---|---|---|---|---|
| Baseline (Mean ±SD) |
End Point (Mean ±SD) |
Significance | Baseline (Mean ±SD) |
End Point (Mean ±SD) |
Significance | |
| OCDS OBS | 12.7 ± 3.4 | 9.8 ± 3.7 | t = 2.95, P = 0.013*, Z = −2.68, P = 0.007* | 12.8 ± 4.8 | 10.0 ± 5.4 | t = 1.92, P = 0.084, Z = − 1.78, P = 0.075 |
| OCDS COMP | 16.7 ± 2.5 | 12.2 ± 4.3 | t = 3.25, P = 0.009*, Z = − 2.68, P = 0.008* | 16.5 ± 3.9 | 12.5 ± 6.0 | t = 2.40, P = 0.037*, Z = − 2.51, P = 0.012* |
| OCDS TOT | 29.6 ± 5.5 | 22.3 ± 7.4 | t = 3.14, P = 0.011*, Z = − 2.68, P = 0.008* | 29.3 ± 8.3 | 22.5 ± 11.1 | t = 2.21, P = 0.052, Z = − 2.08, P = 0.037* |
| Q-LESQ | 64.3 ± 14.7 | 77.1 ± 20.1 | t = − 3.61, P = 0.004*, Z = − 2.52, P = 0.012* | 58.2 ± 23.2 | 69.5 ± 24.7 | t = − 1.69, P = 0.122, Z = − 1.52, P = 0.128 |
| SF-36 Physical Function | 64.0 ± 19.8 | 81.0 ± 20.4 | t = − 2.64, P = 0.58, Z = − 2.20, P = 0.028* | 67.0 ± 39.0 | 95.3 ± 6.1 | t = − 1.66, P = 0.173, Z = − 2.02, P = 0.043 |
| SF-36 Role Physical | 35.0 ± 41.8 | 90.0 ± 13.7 | t = − 3.32, P = 0.029*, Z = − 1.84, P = 0.066 | 85.0 ± 22.4 | 90.0 ± 13.7 | t = − 0.54, P = 0.621, Z = − 0.58, P = 0.564 |
| SF-36 Body Pain | 63.4 ± 27.0 | 73.8 ± 19.1 | t = − 2.21, P = 0.091, Z = − 1.63, P = 0.102 | 71.8 ± 22.3 | 82.4 ± 22.9 | t = − 0.82, P = 0.461, Z = − 0.54, P = 0.593 |
| SF-36 General Health | 68.0 ± 7.4 | 76.6 ± 10.3 | t = − 2;.19, P = 0.094, Z = − 1.76, P = 0.078 | 65.6 ± 20.8 | 72.6 ± 23.1 | t = − 0.63, P = 0.562, Z = − 0.67, P = 0.500 |
| SF-36 Vitality | 49.0 ± 20.4 | 63.0 ± 24.9 | t = − 1.61,P = 0.184, Z = − 1.51, P = 0.131 | 38.0 ± 26.1 | 64.0 ± 15.6 | t = − 1.79, P = 0.148, Z = − 1.63, P = 0.104 |
| SF-36 Social Functioning | 57.5 ± 27.4 | 85.0 ± 22.4 | t = − 2.75, P = 0.051, Z = − 1.84, P = 0.066 | 65.0 ± 37.9 | 87.5 ± 17.7 | t = − 1.15, P = 0.313, Z = − 1.10, P = 0.276 |
| SF-36 Role Emotional | 33.3 ± 40.8 | 73.3 ± 27.9 | t = − 2.06, P = 0.109, Z = − 1.60, P = 0.109 | 46.7 ± 44.7 | 80.0 ± 29.8 | t = − 1.20, P = 0.298, Z = − 1.09, P = 0.276 |
| SF-36 Mental Health | 64.8 ± 15.3 | 80.8 ± 18.4 | t = − 1.65, P = 0.175, Z = − 1.49, P = 0.136 | 41.6 ± 20.5 | 72.0 ± 18.1 | t = − 2.32, P = 0.081, Z = − 1.75, P = 0.080 |
SF-36 results are only for completers with available data (placebo, 5; acamprosate, 5), as scores were obtained only at baseline visit and visit 12. All other results are for the ITT sample based on LOCF.
Statistically significant, P < 0.05.
All comparisons in changes in outcome measures between treatment groups (acamprosate vs placebo) were nonsignificant by independent samples t-test and by Mann-Whitney U test (P > 0.05).
COMP indicates compulsive subscale; OBS, obsessive subscale; OCDS, Obsessive Compulsive Drinking Scale; TOT, total OCDS score.
Association Between Changes in AUD Parameters and Antidepressant Response
We compared the changes in alcohol use parameters between depression responders and nonresponders in each treatment arm. In both treatment arms, antidepressant responders stayed longer in the study than nonresponders, and this difference reached significance for the acamprosate arm (Table 5). No significant differences were observed for changes in drinks per drinking day, per week, or per month, or for percent days abstinent between the antidepressant responders and the nonresponders. Linear regression revealed no significant association between change in HAM-D-17 score and change in any of the alcohol use parameters (P > 0.05). Logistic regression revealed no significant association between antidepressant response and change in any of the alcohol use parameters (P > 0.05).
TABLE 5.
Comparison Between Change in Alcohol Use Parameters in Antidepressant Responders and Nonresponders to Each Intervention (ITT Sample Based on LOCF)
| Acamprosate | Placebo | |||
|---|---|---|---|---|
| Responders (Mean ± SD) |
Nonresponders (Mean ± SD) |
Responders (Mean ± SD) |
Nonresponders (Mean ± SD) |
|
| Days in treatment | 74 ± 22* | 29 ± 32* | 60 ± 30 | 32 ± 31 |
| Change in Drinks/drinking day | −1 ± 1 | −1 ± 1 | −1 ± 1 | 1 ± 2 |
| Change in drinks/wk | −4 ± 6 | −8 ±6 | 0 ± 0 | −7 ± 15 |
| Change in drinks/mo | −18 ± 25 | −32 ± 24 | −2 ± 2 | −28 ± 61 |
| Change in percent days abstinent | −1 ± 12 | −27 ± 42 | −1 ± 3 | 9 ± 18 |
Statistically significant difference between acamprosate responders and nonresponders: t = −2.84, P = 0.018; Mann-Whitney: U = 5.0, Z = −2.10, P = 0.036.
All other comparisons between responders and nonresponders were nonsignificant.
Quality-of-Life Measures
Scores on the Q-LESQ scale iproved for both groups but attained significance for the acamprosate group only (Table 4). Scores of the SF-36 improved in all dimensions for both treatment arms; significance was attained in the physical function and role physical dimensions for the acamprosate group (Table 4). In both scales, neither change differed significantly between the treatment arms.
Tolerability and Safety
Treatment was well tolerated. In the escitalopram/acamprosate group, 8 subjects reported adverse events; among which 6 symptoms were thought to be related to treatment (acamprosate and/or escitalopram): dry mouth (n = 1), nausea (n = 2), insomnia (n = 1), unspecified gastrointestinal upset (n = 2), diarrhea (n = 3), and headache (n = 1). Five subjects in the escitalopram/placebo group reported adverse events, of which 5 symptoms were thought to be related to treatment (escitalopram and/or placebo): dry mouth (n = 1), jaw tightening (n = 1), nausea (n = 1), headache (n = 1), and insomnia (n = 1).
No patients assigned to escitalopram/acamprosate attributed their discontinuation to adverse events. Two patients in the escitalopram/placebo group attributed discontinuation to adverse events (including insomnia, anxiety, and tension). Two patients in each treatment arm were lost to follow-up, and one patient in each arm did not specify reasons for discontinuing (Fig. 1).
No patients developed alcohol withdrawal symptoms at any time.
DISCUSSION
To our knowledge, there have been no prior studies of treatment of comorbid depression and alcohol use disorders (AUDs) with the combination of an antidepressant and acamprosate. We compared a combination of acamprosate plus escitalopram against escitalopram plus placebo in a small sample of adults with AUD and MDD, combined with a standard psychosocial intervention. Both groups had a significant improvement of their depressive symptoms. Whereas the fact that the placebo group was more depressed at baseline may have affected the results, our finding is consistent with several studies, showing that it is indeed possible to achieve improvement in depressive symptoms despite ongoing drinking.9,14,17,19 Thus, although MDD and AUD are often comorbid and more difficult to treat when occurring together,4,5 it seems possible to effectively treat depressive symptoms despite active problem alcohol use.
Depression response rates were somewhat greater in the acamprosate group than in the placebo group; and those assigned to acamprosate also experienced significant improvement in some alcohol use parameters, whereas those on placebo did not. Although the comparison between the treatment arms was not significant, our findings suggest the possibility of a stronger effect overall for acamprosate that might have shown separation from placebo in a larger sample, and also perhaps if the attrition rate had been more modest. Secondary outcome measures such as Q-LESQ and SF-36 tended to suggest somewhat of an advantage for the group receiving acamprosate and escitalopram, with more measures attaining significant improvement (Table 4); but the sample is small, and therefore, these findings need to be interpreted with caution.
Another notable finding is that although attrition rates were comparable in both treatment arms (Fig. 2), subjects in either treatment arm who experienced antidepressant response stayed in the study longer than those who did not; and this finding reached significance in the acamprosate group (Table 5). We speculate that a reduction in guilty feelings, increased motivation and energy, and improved mood and subjective sense of well-being may increase the chance of an eventual good outcome among patients with AUDs; and a more prolonged treatment relationship would be expected to confer greater alcohol use-related therapeutic opportunities. This finding is therefore encouraging.
There are previous lines of research in animals and humans that have suggested beneficial effects of serotonergic manipulation as an effective approach to decreasing alcohol use. For example, the SSRI citalopram decreased interest, desire, craving, and liking for alcohol significantly over placebo in a small sample of nondepressed alcohol-dependent drinkers.43 Tiihonen et al44 found similar benefit in 62 alcoholic patients. Naranjo et al45 suggested that serotonergic drugs may work by interfering with neurobiologic mechanisms regulating ethanol intake and may thus modulate alcohol use. Effects of this sort may have occurred in our sample, as both treatment groups received an SSRI. The combination of an SSRI and acamprosate, with the combined serotonergic, antiglutamatergic, and GABA-ergic mechanisms, may collectively provide a stronger benefit than either treatment alone; but this and the respective contributions of each mechanism requires further investigation in larger samples.
Limitations of this study include a small sample size and a high attrition rate, reducing our power to detect treatment effects. The study was originally powered to detect a difference in a sample of 40 with 74% power, based on a 30% difference in alcohol consumption. Recruitment proved to be more challenging than expected; and for this reason, the study only randomized 23 subjects. The difference in observed changes in monthly drinking between the 2 groups was also more modest than expected, on the order of only approximately 8% (37% for placebo and 29%for acamprosate), and the standard deviations were very wide for both groups. Given the observed monthly drinking change and standard deviation in the placebo group, even if the acamprosate group had obtained a decrease to zero drinks per month with an assumed standard deviation of approximately 50 (total sobriety, representing a large effect size of approximately 0.85), it would have attained only 50% power with α = 0.05. To attain a power of 80% with this optimistic effect size, an ITT sample of at least 46 would be required.
Another important limitation is that acamprosate is approved for maintaining rather than inducing abstinence and is thought to dampen symptoms of prolonged alcohol withdrawal.25,26 In this study, we administered acamprosate before alcohol discontinuation. Subjects on acamprosate who continued drinking could therefore not necessarily be expected to experience maximal benefit from the drug. It is also theoretically possible that if acamprosate dampens response to alcohol, patients who are actively drinking might drink more to obtain the same effect. However, the improvement in some alcohol use measures suggests a possible clinical benefit over time even among people who start the drug while actively drinking.
In summary, we have found modest beneficial effects of acamprosate in combination with escitalopram in depressed individuals with AUD, although this is a small pilot study and the results must be considered preliminary. Given the dearth of studies of dual pharmacotherapy in this population, our encouraging findings merit further exploration in larger studies.
Acknowledgments
Dr Janet Witte was supported by a Young Investigator Award from The National Association for Research on Schizophrenia and Depression (NARSAD). She has also received research support from Forest Laboratories and Bristol-Meyers Squibb and honoraria from Eli Lilly. Escitalopram, acamprosate, and matching placebo for this study were kindly donated by Forest Laboratories. Dr A. Eden Evins was supported by National Institutes of Health Grants K23DA00510 and K24DA030443. She has received research support from GlaxoSmithKline, Pfizer, and the Bowman Family Foundation; she has served in advisory/consultative relationships with Boehringer Ingelheim and Pfizer. Dr Maurizio Fava has received research support from Abbott Laboratories, Alkermes, Aspect Medical Systems, AstraZeneca, BioResearch, BrainCells, Inc, Bristol-Myers Squibb Company, Cephalon, Clinical Trial Solutions, LLC, Eli Lilly & Company, EnVivo Pharmaceuticals, Inc, Forest Pharmaceuticals, Inc, Ganeden, GlaxoSmithKline, J & J Pharmaceuticals, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, NARSAD, NCCAM, NIDA, NIMH, Novartis, Organon, Inc, PamLab, LLC, Pfizer, Inc, Pharmavite, Roche, Sanofi-Aventis, Shire, Solvay Pharmaceuticals, Inc, Synthelabo, and Wyeth-Ayerst Laboratories. He has served as an advisor and consultant to Abbott Laboratories, Affectis Pharmaceuticals AG, Amarin, Aspect Medical Systems, AstraZeneca, Auspex Pharmaceuticals, Bayer AG, Best Practice Project Management, Inc, BioMarin Pharmaceuticals, Inc, Biovail Pharmaceuticals, Inc, BrainCells, Inc, Bristol-Myers Squibb Company, Cephalon, Clinical Trials Solutions, LLC, CNS Response, Compellis, Cypress Pharmaceuticals, Dov Pharmaceuticals, Eisai, Inc, Eli Lilly & Company, EPIX Pharmaceuticals, Euthymics Bioscience, Inc, Fabre-Kramer, Pharmaceuticals, Inc, Forest Pharmaceuticals, Inc, GlaxoSmithKline, Grunenthal GmBH, Janssen Pharmaceutica, Jazz Pharmaceuticals, J & J Pharmaceuticals, Knoll Pharmaceutical Company, Labopharm, Lorex Pharmaceuticals, Lundbeck, MedAvante, Inc, Merck, Methylation Sciences, Neuronetics, Novartis,Nutrition 21,Organon, Inc, PamLab, LLC, Pfizer, Inc, PharmaStar, Pharmavite, Precision Human Biolaboratory, Prexa Pharmaceuticals, Inc, PsychoGenics, Psylin Neurosciences, Inc, Ridge Diagnostics, Inc, Roche, Sanofi-Aventis, Sepracor, Schering-Plough, Solvay Pharmaceuticals, Inc, Somaxon, Somerset Pharmaceuticals, Synthelabo, Takeda, Tetragenex, Trans-Form Pharmaceuticals, Inc, Transcept Pharmaceuticals, Vanda Pharmaceuticals Inc, Wyeth-Ayerst Laboratories. He has received speaking and publishing honoraria from Adamed, Co, Advanced Meeting Partners, American Psychiatric Association, American Society of Clinical Psychopharmacology, AstraZeneca, Belvoir, Boehringer-Ingelheim, Bristol-Myers Squibb Company, Cephalon, EliLilly&Company,ForestPharmaceuticals, Inc, GlaxoSmithKline, Imedex, Novartis, Organon Inc, Pfizer Inc, PharmaStar, MGH Psychiatry Academy/Primedia, MGH Psychiatry Academy/Reed-Elsevier, UBC, and Wyeth-Ayerst Laboratories. He holds equity in Compellis. He currently holds a patent for SPCD and a patent application for a combination of azapirones and bupropion in MDD and has received copyright royalties for the MGH CPFQ, SFI, ATRQ, DESS, and SAFER diagnostic instruments. Dr David Mischoulon has received research support for other clinical trials from Amarin (Laxdale), Bowman Family Foundation, Bristol-Myers Squibb, Cederroth, Lichtwer Pharma GmbH, Nordic Naturals, Ganeden, Swiss Medica, and Fisher-Wallace; he has received consulting and writing honoraria from Pamlab; he has received speaking honoraria from Bristol-Myers Squibb, Nordic Naturals, Pfizer, Pamlab, and Virbac as well as from Reed Medical Education (a company working as a logistics collaborator for the Massachusetts General Hospital Psychiatry Academy); he has received royalty income from Back Bay Scientific for PMS Escape, and from Lippincott Williams & Wilkins for the book, Natural Medications for Psychiatric Disorders: Considering the Alternatives (Editors: David Mischoulon and Jerrold F. Rosenbaum).
Footnotes
AUTHOR DISCLOSURE INFORMATION
The remaining authors (Ms Kate Bentley, Dr Paola Pedrelli, Dr Lee Baer, and Ms Alisabet Clain) have no conflicts of interest to disclose.
REFERENCES
- 1.Grant BF, Stinson SF, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Crum RM, Warner LA, et al. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the national Comorbidity Survey. Arch Gen Psychiatry. 1997;53:232–240. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- 3.Watkins KE, Paddock SM, Zhang L, et al. Improving care for depression in patients with comorbid substance misuse. Am J Psychiatry. 2006;163:125–132. doi: 10.1176/appi.ajp.163.1.125. [DOI] [PubMed] [Google Scholar]
- 4.Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug Alcohol Depend. 1995;39:197–206. doi: 10.1016/0376-8716(95)01160-4. [DOI] [PubMed] [Google Scholar]
- 5.Gilman SE, Abraham HD. A longitudinal study of the order of onset of alcohol dependence and major depression. Drug Alcohol Depend. 2001;63:277–286. doi: 10.1016/s0376-8716(00)00216-7. [DOI] [PubMed] [Google Scholar]
- 6.Litten R, Allen J. Pharmacotherapy for alcoholics with collateral depression or anxiety: an update of research findings. Exp Clin Psychopharmacol. 1995;3:1–7. [Google Scholar]
- 7.Weiss RD. Pharmacotherapy for co-occurring mood and substance use disorders. In: Westermeyer JJ, Weiss RD, Ziedonis DM, editors. Integrated Treatment for Mood and Substance Use Disorders. Baltimore, MD: The Johns Hopkins University Press; 2003. pp. 122–139. [Google Scholar]
- 8.Ziedonis DM, Krejci JA. Dual recovery therapy: blending psychotherapies for depression and addiction. In: Westermeyer JJ, Weiss RD, Ziedonis DM, editors. Integrated Treatment for Mood and Substance Use Disorders. Baltimore, MD: The Johns Hopkins University Press; 2003. pp. 90–121. [Google Scholar]
- 9.Pettinati HM. Antidepressant treatment of co-occurring depression and alcohol dependence. Biol Psychiatry. 2004;56:785–792. doi: 10.1016/j.biopsych.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Shaw JA, Donley P, Morgan DW, et al. Treatment of depression in alcoholics. Am J Psychiatry. 1975;132:641–644. doi: 10.1176/ajp.132.6.641. [DOI] [PubMed] [Google Scholar]
- 11.Ciraulo DA, Jaffe JH. Tricyclic antidepressants in the treatment of depression associated with alcoholism. J Clin Psychopharmacol. 1981;1:146–150. doi: 10.1097/00004714-198105000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Liskow BI, Goodwin DW. Pharmacological treatment of alcohol intoxication, withdrawal, and dependence: a critical review. J Stud Alcohol. 1987;48:356–370. doi: 10.15288/jsa.1987.48.356. Review. [DOI] [PubMed] [Google Scholar]
- 13.Mason BJ, Kocsis JH, Ritvo EC, et al. A double-blind, placebo-controlled trial of desipramine for primary alcohol dependence stratified on the presence or absence of major depression. JAMA. 1996;275:761–767. [PubMed] [Google Scholar]
- 14.McGrath PJ, Nunes EV, Stewart JW, et al. Imipramine treatment of alcoholics with primary depression: a placebo-controlled clinical trial. Arch Gen Psychiatry. 1996;53:232–240. doi: 10.1001/archpsyc.1996.01830030054009. [DOI] [PubMed] [Google Scholar]
- 15.Cornelius JR, Salloum IM, Ehler JG, et al. Fluoxetine in depressed alcoholics: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1997;54:700–705. doi: 10.1001/archpsyc.1997.01830200024004. [DOI] [PubMed] [Google Scholar]
- 16.Roy A. Placebo-controlled study of sertraline in depressed recently abstinent alcoholics. Biol Psychiatry. 1998;44:633–637. doi: 10.1016/s0006-3223(97)00509-x. [DOI] [PubMed] [Google Scholar]
- 17.Roy-Byrne PP, Pages KP, Russo JE, et al. Nefazodone treatment of major depression in alcohol-dependent patients; a double-blind, placebo controlled trial. J Clin Psychopharmacol. 2000;20:129–136. doi: 10.1097/00004714-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Pettinati HM, Volpicelli JR, Luck G, et al. Double-blind clinical trial of sertraline treatment for alcohol dependence. J Clin Psychopharmacol. 2001;21:143–153. doi: 10.1097/00004714-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Moak DH, Anton RF, Latham PK, et al. Sertraline and cognitive behavioral therapy for depressed alcoholics: results of a placebo controlled trial. J Clin Psychopharmacol. 2003;23:553–562. doi: 10.1097/01.jcp.0000095346.32154.41. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez-Avila CA, Modesto-Lowe V, Feinn R, et al. Nefazodone treatment of comorbid alcohol dependence and major depression. Alcoholism: Clinical and Experimental Research. 2004;28:433–440. doi: 10.1097/01.alc.0000118313.63897.ee. [DOI] [PubMed] [Google Scholar]
- 21.Muhonen LH, Lönnqvist J, Juva K, et al. Double-blind, randomized comparison of memantine and escitalopram for the treatment of major depressive disorder comorbid with alcohol dependence. J Clin Psychiatry. 2008;69(3):392–399. doi: 10.4088/jcp.v69n0308. [DOI] [PubMed] [Google Scholar]
- 22.Muhonen LH, Lahti J, Sinclair D, et al. Treatment of alcohol dependence in patients with co-morbid major depressive disorder—predictors for the outcomes with memantine and escitalopram medication. Subst Abuse Treat Prev Policy. 2008;3:20. doi: 10.1186/1747-597X-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence: a meta-analysis. JAMA. 2004;291:1887–1896. doi: 10.1001/jama.291.15.1887. [DOI] [PubMed] [Google Scholar]
- 24.Williams SH. Medications for treating alcohol dependence. Am Fam Physician. 2005;72:1775–1780. [PubMed] [Google Scholar]
- 25.Garbutt JC, West SL, Carey TS, et al. Pharmacological treatment of alcohol dependence: a review of the evidence. JAMA. 1999;281:1318–1325. doi: 10.1001/jama.281.14.1318. [DOI] [PubMed] [Google Scholar]
- 26.Mason BJ. Treatment of alcohol-dependent outpatients with acamprosate: a clinical review. J Clin Psychiatry. 2001;62(suppl 20):42–48. [PubMed] [Google Scholar]
- 27.Schwartz TL, Siddiqui UA, Raza S, et al. Acamprosate calcium as augmentation therapy for anxiety disorders. Ann Pharmacother. 2010;44:1930–1932. doi: 10.1345/aph.1P353. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez C, Bøgesø KP, Ebert B, et al. Escitalopram versus citalopram: the surprising role of the R-enantiomer. Psychopharmacology (Berl) 2004;174:163–176. doi: 10.1007/s00213-004-1865-z. [DOI] [PubMed] [Google Scholar]
- 29.Chen F, Larsen MB, Sánchez C, et al. The S-enantiomer of R,S-citalopram, increases inhibitor binding to the human serotonin transporter by an allosteric mechanism. Comparison with other serotonin transporter inhibitors. Eur Neuropsychopharmacol. 2005;15:193–198. doi: 10.1016/j.euroneuro.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 30.First MB, Spitzer RL, Gibbon M, Williams JBW. “Structured Clinical Interview for DSM-IV-TR Axis I Disorders—Patient Edition (SCID-I/P, 11/2002 revision) [Google Scholar]
- 31.Davis LL, Frazier E, Husain MM, et al. Substance use disorder comorbidity in major depressive disorder: a confirmatory analysis of the STAR*D cohort. American Journal of Addictions. 2006;15:278–285. doi: 10.1080/10550490600754317. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamilton M. Development of a rating scale for primary depressive illness. Br J Social Clin Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 34.Williams JB. A structured interview guide for the Hamilton depression rating scale. Arch Gen Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 35.Guidi J, Pender M, Hollon SD, et al. The prevalence of compulsive eating and exercise among college students: an exploratory study. Psychiatry Res. 2009;165:154–162. doi: 10.1016/j.psychres.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS) Clinician Rating (QIDS-C) and Self-Report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 37.Endicott J, Nee J, Harrison W, et al. Quality of life enjoyment and satisfaction questionnaire (Q-LES-Q): a new measure. Psychopharmacol Bull. 1993;29:321–326. [PubMed] [Google Scholar]
- 38.Guy W, editor. ECDEU Assessment Manual for Psychopharmacology, Revised. DHEW Pub. No. (ADM) 76-338. Rockville, MD: National Institute of Mental Health; 1976. [Google Scholar]
- 39.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 40.Anton RF, Moak DH, Latham P. The obsessive compulsive drinking scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res. 1995;19:92–99. doi: 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- 41.Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 42.Pettinati HM, Weiss RD, Miller WR, et al. COMBINE Monograph Series. Volume 2. Bethesda, MD: NIAAA; 2004. Medical Management Treatment Manual: A Clinical Research Guide for Medically Trained Clinicians Providing Pharmacotherapy as Part of the Treatment for Alcohol Dependence. DHHS Publication No (NIH) 04-5289. [Google Scholar]
- 43.Naranjo CA, Poulos CX, Bremner KE, et al. Citalopram decreases desirability, liking, and consumption of alcohol in alcohol-dependent drinkers. Clin Pharmacol Ther. 1992;51:729–739. doi: 10.1038/clpt.1992.85. [DOI] [PubMed] [Google Scholar]
- 44.Tiihonen J, Ryynänen OP, Kauhanen J, et al. Citalopram in the treatment of alcoholism: a double-blind placebo-controlled study. PharmacoPsychiatry. 1996;29:27–29. doi: 10.1055/s-2007-979538. [DOI] [PubMed] [Google Scholar]
- 45.Naranjo CA, Sellers EM, Lawrin MO. Modulation of ethanol intake by serotonin uptake inhibitors. J Clin Psychiatry. 1986;47(suppl):16–22. [PubMed] [Google Scholar]