Abstract
This study addresses the hypothesis that the lack of anesthetic activity for (3α,5α)-3-hydroxypregn-16-ene-11,20-dione (Δ16-alphaxalone) is explained by the steroid Δ16 double bond constraining the steroid 20-carbonyl group to a position that prevents it from favorably interacting with γ-aminobutyric acid type A (GABAA) receptors. A series of Δ16 and Δ17(20) analogues of Δ16-alphaxalone was prepared to evaluate this hypothesis in binding, electrophysiological and tadpole anesthesia experiments. The results obtained failed to support the hypothesis. Instead, the results indicate that it is the presence of the C-21 methyl group in Δ16-alphaxalone, not the location of the constrained C-20 carbonyl group, which prevents Δ16-alphaxalone from interacting strongly with the GABAA receptor and having anesthetic activity. Consistent with this conclusion, a Δ17(20) analogue of Δ16-alphaxalone without a C-21 methyl group was found to be very similar to the anesthetic steroid (3α,5α)-3-hydroxypregnane-11,20-dione (alphaxalone) with regard to time of onset and rate of recovery from anesthesia when administered to mice by tail vein injection.
Introduction
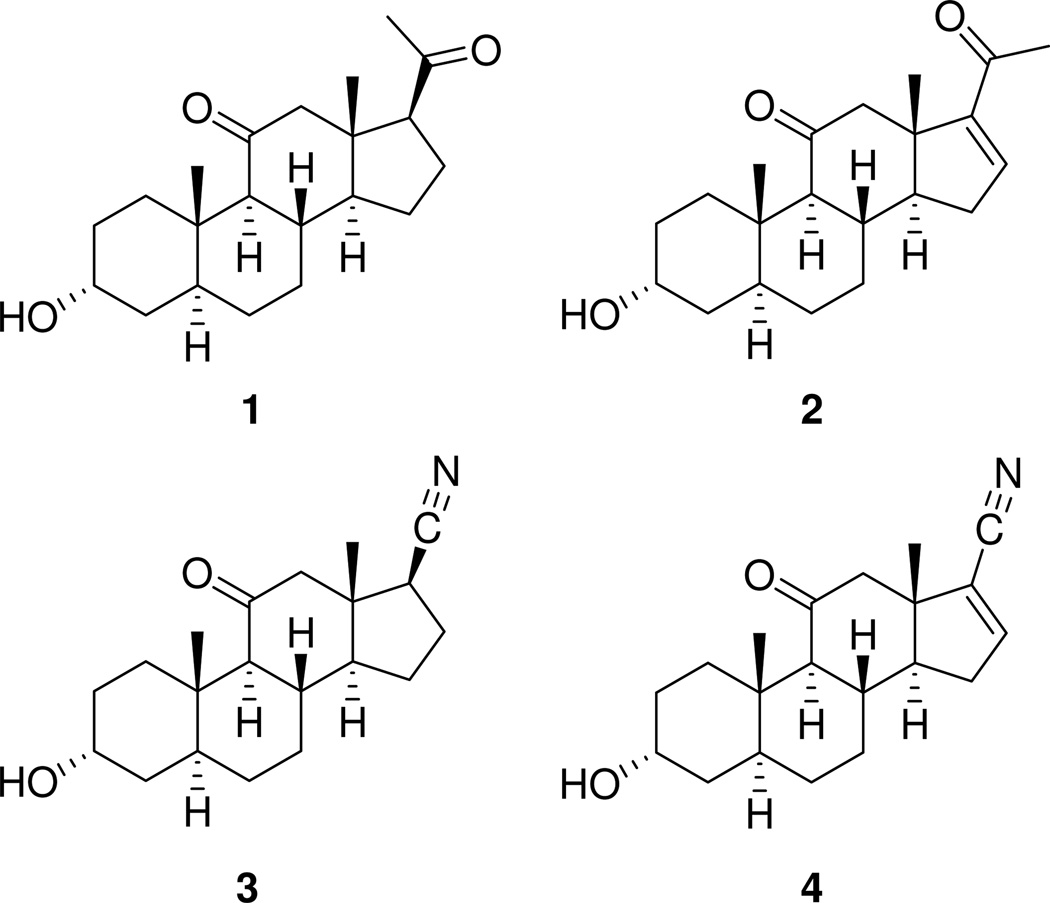
It is widely accepted that the intravenous anesthetic alphaxalonea (1, Chart 1) causes general anesthesia in humans because it allosterically increases chloride currents mediated by the inhibitory neurotransmitter γ-aminobutyric acid (GABA) acting at γ-aminobutyric acid type A (GABAA) receptors in the brain.1,2 By contrast, Δ16-alphaxalone (2), has greatly diminished allosteric activity at GABAA receptors and is not an intravenous general anesthetic in mammals.3,4 This striking effect that the Δ16 double bond has on anesthesia has attracted the attention of many investigators.5–20 Recently, we determined that the effect the Δ16 double bond has on anesthetic activity depends on the group attached at C-17.21 Thus, steroids 3 and 4 were shown to be similar to each other and to steroid 1 in their GABAergic and anesthetic actions. We proposed that the Δ16 double bond found in steroid 4 had minimal pharmacological effect because it did not displace the compound’s C-17 carbonitrile group to either side of a vector that passes through the mid-point of the C-14,C-15 bond and C-17. This proposal is consistent with results from prior studies which demonstrated that the orientation of hydrogen bond accepting groups on the steroid D-ring critically affects the activity of anesthetic steroids acting at GABAA receptors. 22,23
Chart 1.
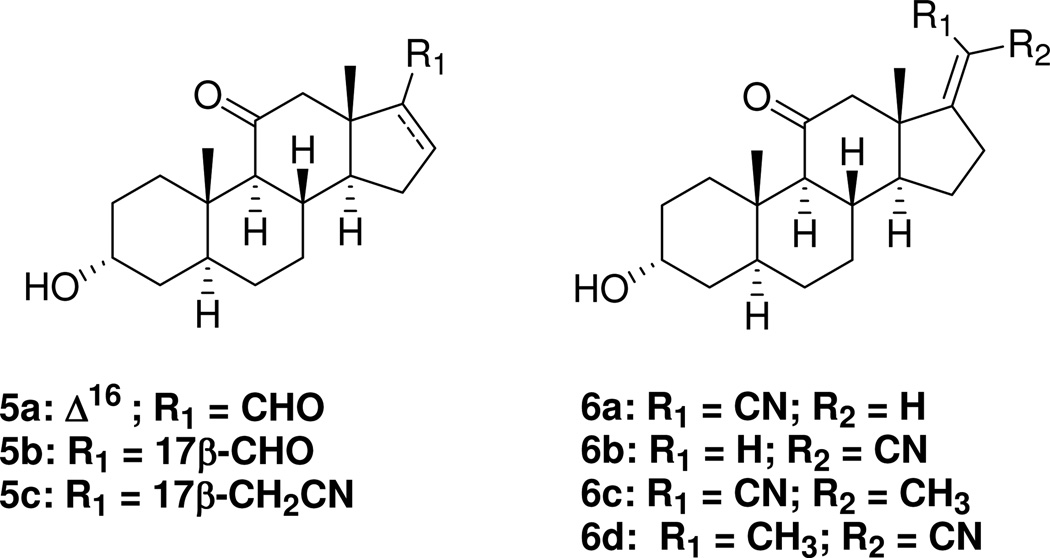
However, the previous results obtained with steroid 4 did not directly probe the structural features present in steroid 2 that prevent this compound’s C-20 carbonyl group from favorably interacting with the GABAA receptor. Is it, as has been proposed previously,20 that the carbonyl group of steroid 2 cannot favorably interact with the receptor because it is constrained to an unfavorable location in the steroid, or is it that the location of the carbonyl is allowed and another unsuspected structural feature, i.e., the presence of the constrained 21-methyl group in steroid 2, explains its low biological activity? To distinguish between these possibilities we synthesized and evaluated a series of Δ16 and Δ17(20) steroids (Chart 2). These analogues have allowed us to establish that constraints on the C-21 methyl group rather than constraints on the C-20 carbonyl group explain the low activity of steroid 2. Additionally, by comparing behavioral responses of steroid 6a with those of anesthetic steroid 1 in a mouse model of anesthesia, we identify steroid 6a as an analogue whose utility as an intravenous anesthetic merits further study.
Chart 2.
Chemistry
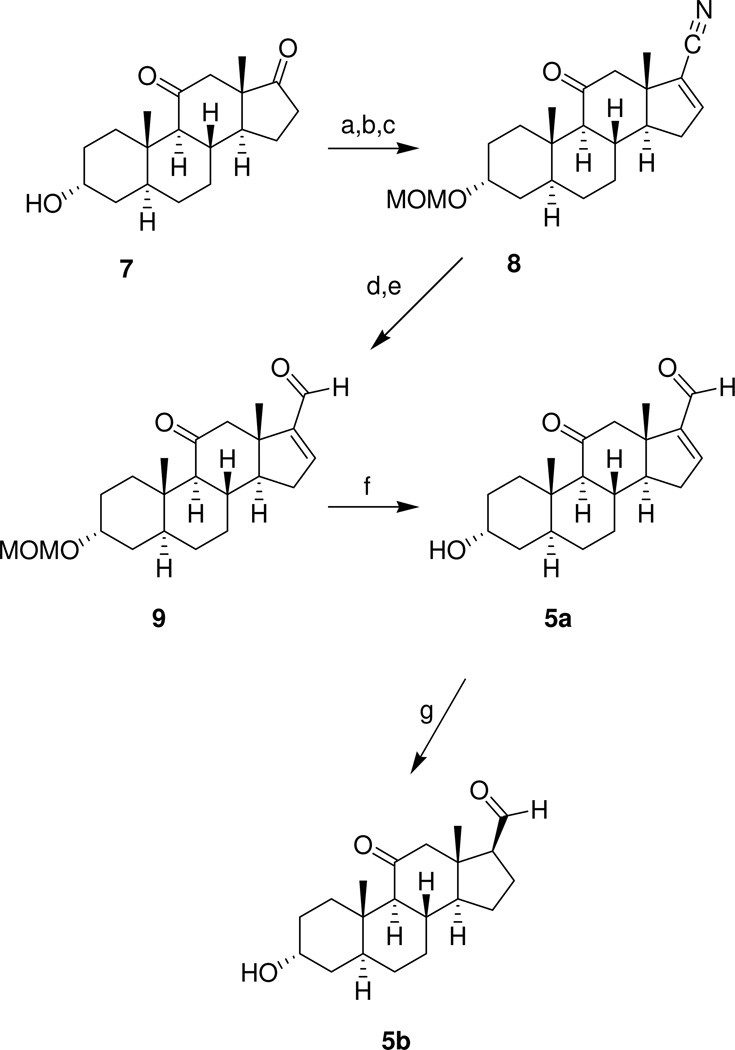
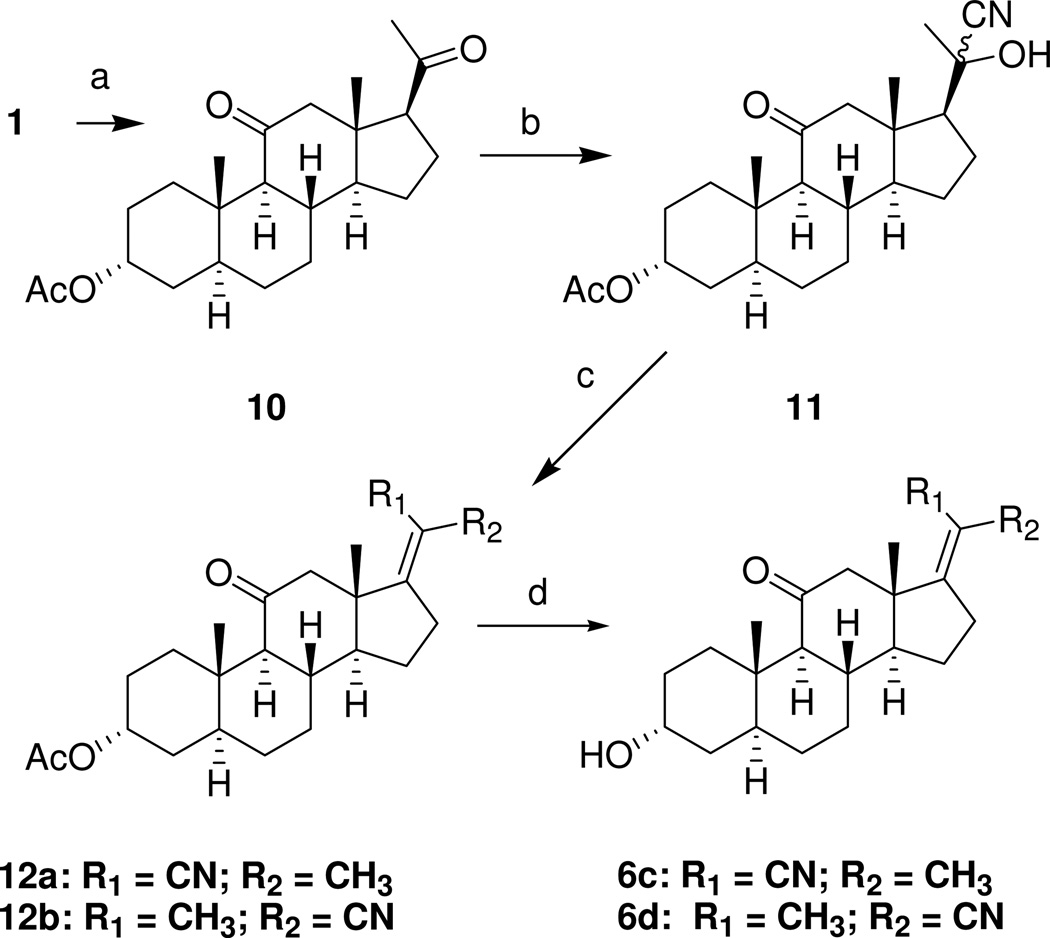
The steroids shown in Chart 1 were prepared using methods reported previously.21 Steroid 8 (Scheme 1) was prepared from (3α,5α)-3-hydroxyandrostane-11,17-dione (7) as described previously.21 Reduction of the carbonitrile group of steroid 8 with DIBALH also reduced the C-11 carbonyl group. This diol intermediate was not characterized and was instead oxidized using PCC to intermediate 9 in an overall yield of 48% for the two step procedure. Removal of the MOM protecting group gave analogue 5a in 85% yield. Hydrogenation of analogue 5a using Lindlar’s catalyst gave analogue 5b in 70% yield.
Scheme 1a.
aReagents: a) MOMCl, Hunig’s base, CH2Cl2; b) PhN(SO2CF3)2, KHMDS, THF, –78 °C; c) NaCN, CuI, Pd(PPh3)4, MeCN; d) DIBALH, toluene, CH2Cl2, –78 °C; e) PCC, CH2Cl2, f) EtOH, 6 N HCl; g) H2 (60 psi), Lindlar’s catalyst (5%), EtOAc.
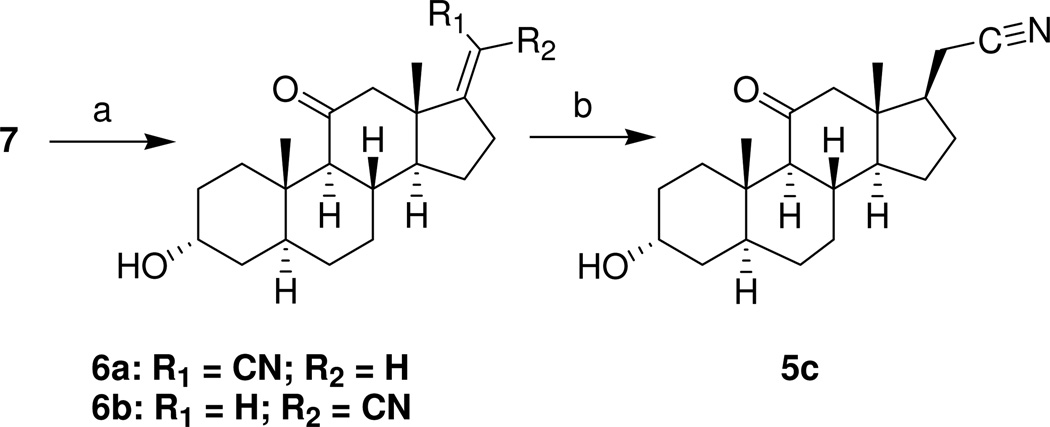
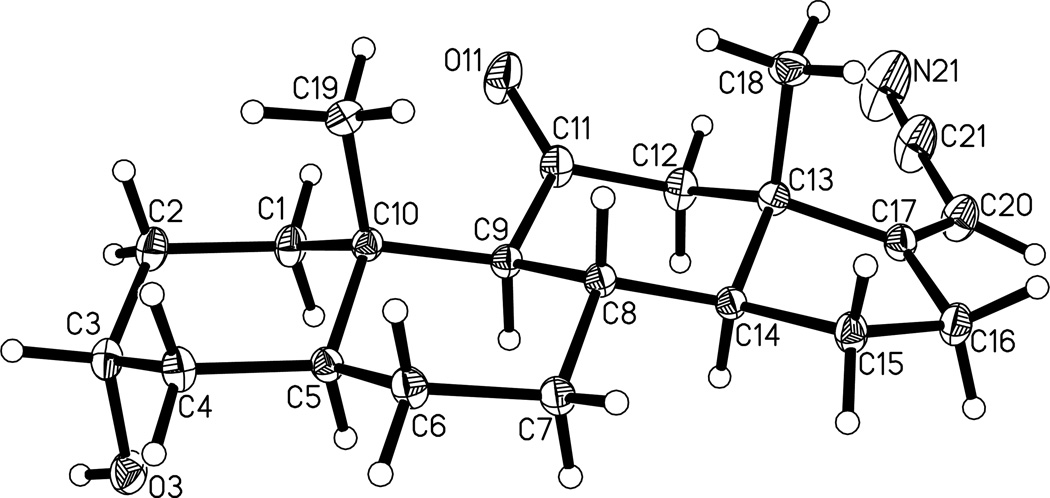
Analogues 6a (25%) and 6b (44%) were prepared as an isomeric mixture from steroid 7 (Scheme 2) by a Wittig-Horner reaction and separated by preparative TLC. The Z stereochemistry for the carbonitrile substituent in compound 6a was established by a crystal structure determination (Figure 1). Hydrogenation of steroid 6b gave analogue 5c in 57% yield.
Scheme 2a.
aReagents: a) NaH, diethyl(cyanomethyl)phosphonate, THF, 0 to 20 °C; b) H2 (60 psi), Pd/C, EtOAc/EtOH.
Figure 1.
Projection plot (50% thermal ellipsoids) of the X-ray crystal structure of steroid 6a.
Steroid 1 was used as starting material to prepare additional Δ17(20) analogues (Scheme 3). Steroid 1 was acetylated to yield steroid 10 and this steroid was used to prepare α-cyanohydrin diastereomers 11 following a literature procedure used for the preparation of similar steroids from other 20-ketosteroid precursors.24 After verifying by NMR that intermediate 11 was formed, it was immediately subjected to a dehydration reaction to yield Δ17(20) products 12a and 12b. Purification by recrystallizations and column chromatography yielded pure 12a (5.2%) and pure 12b (21%). Saponification of the 3α-acetoxy groups of steroid 12a and 12b gave the desired analogues 6c (82% yield) and 6d (82% yield), respectively. Comparison of the 1H NMR spectra of compounds 6a and 6b showed that the C-18 methyl group in compound 6a was shifted downfield relative to the C-18 methyl group of compound 6b due to a deshielding effect of the nearby nitrile group. Accordingly, for the steroid 6c, 6d double bond isomer pair, steroid 6c was assigned as the Z double bond isomer.
Scheme 3.
aReagents: a) Ac2O, pyridine; b) KCN, pyridine, AcOH, EtOH, 0 to 20 °C; c) POCl3 pyridine; d) K2CO3, MeOH, reflux.
[35S]-tert-Butylbicyclophosphorothionate ([35S]-TBPS) Displacement Results
Compounds shown in Charts 1 and 2 non-competitively displaced [35S]-TBPS from the picrotoxin binding site on GABAA receptors with IC50 values given in Table 1. Compounds 1–4 are the reference compounds for this study and the binding results reported are those recently published.21 Steroid 5a is the unsaturated C-17 aldehyde analogue of steroids 2 and 4. Comparison of the IC50 values for [35S]-TBPS displacement potency for steroids 2 and 5a shows that changing the C-17 acetyl group to an aldehyde group results in a modest ~ twofold increase in displacement potency. Compound 5b, which lacks the Δ16 double bond, is about equal to steroid 5a as a displacer of [35S]-TBPS. Thus, it appears that neither the Δ16 double bond nor the C-21 methyl group has more than a ~ twofold effect on potency for [35S]-TBPS displacement. With regard to the effect of the Δ16 double bond, a comparison of the IC50 values for nitriles 3 and 4 leads to a similar conclusion. Comparison of the IC50 values for steroids 4 and 5a shows a ~ threefold difference in potency in favor of steroid 4.
Table 1.
Inhibition of [35S]-TBPS Binding by Steroids 1, 2 and Structural Analoguesa
| Compound | IC50 (nM) | nHill |
|---|---|---|
| 1b | 226 ± 24 | 1.10 ± 0.11 |
| 2b | 2,220 ± 260 | 1.24 ± 0.14 |
| 3b | 190 ± 18 | 1.14 ± 0.11 |
| 4b | 361 ± 58 | 1.00 ± 0.14 |
| 5a | 997 ± 187 | 1.50 ± 0.36 |
| 5b | 770 ± 98 | 0.97 ± 0.10 |
| 5c | 1,020 ± 204 | 0.94 ± 0.14 |
| 6a | 128 ± 11 | 1.44 ± 0.15 |
| 6b | 2,030 ± 810 | 0.90 ± 0.23 |
| 6c | 629 ± 89 | 1.48 ± 0.25 |
| 6d | 1,840 ± 480 | 1.46 ± 0.42 |
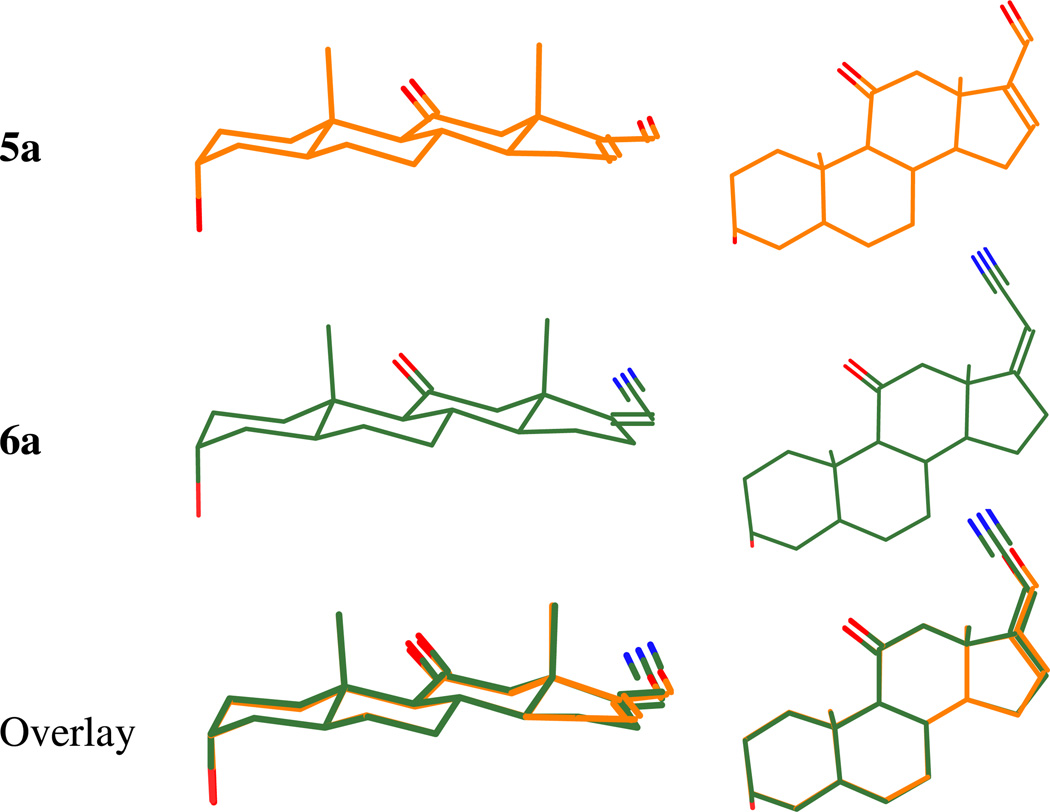
Steroid 6a is a close structural analogue of steroid 5a. The conformation of the steroid D-ring is nearly identical in both compounds and the 6a nitrile group lies along the axis of the atoms in the carbonyl group of steroid 5a (Figure 2). Additionally, the nitrile and carbonyl groups are both hydrogen bond acceptors. The [35S]-TBPS displacement results obtained with steroids 2 and 5a suggest that steroid 6a should have an IC50 value more similar to that of steroid 5a than to that of steroid 2 since steroid 6a does not have a C-21 methyl group. Indeed, this was found to be the case with compound 6a being ~ eightfold more potent than steroid 5a, but ~ 17-fold more potent than steroid 2. Surprisingly, steroid 6a was ~ twofold more potent than the anesthetic steroid 1.
Figure 2.
Molecular models showing steroids 5a (top) and 6a (middle) and their overlay (bottom). Left column, edge view. Right column, top view.
Comparison of the IC50 values of steroids 6a (Z isomer) and 6b (E isomer) indicates that interchanging the relative positions of the C-20 substituents (H, CN) has a large effect on [35S]-TBPS displacement potency. Steroid 6a was ~ 16-fold more potent at displacing [35S]-TBPS than steroid 6b. A comparison of the IC50 values for compounds 6a, 6b and 5c shows the effect that hydrogenation of the Δ16 double bond present in steroids 6a and 6b has on binding potency. The change in conformation of the D-ring and the loss of the steric restraint imposed by the Δ16 double bond increased the IC50 value of steroid 5c ~ eightfold relative to steroid 6a, and decreased the IC50 value ~ twofold relative to steroid 6b.
Steroid 6c (Z isomer) is an analogue of steroid 2 (the nitrile group replaces the carbonyl group) in its minimum energy conformation and steroid 6d (E isomer) is an analogue of steroid 2 in its high energy U-conformation (i.e., the conformation in which the relative positions of the carbonyl and C-21 methyl groups are interchanged). The compounds differ from the 6a and 6b analogues only by the presence of the C-21 methyl group in their structures. A comparison of steroids 6a and 6c shows displacement potency is decreased ~fivefold by the C-21 methyl group. By contrast, a comparison of steroids 6b and 6d shows no significant effect on displacement potency. Thus, the C-21 methyl group has a negative effect on the [35S]-TBPS displacement potency of the Z isomer (6c), but little effect on the [35S]-TBPS displacement potency of the E isomer (6d).
Electrophysiology Results
Each compound was evaluated for its ability to potentiate chloride currents mediated by 2 µM GABA at rat α1β2γ2L type GABAA receptors expressed in Xenopus laevis oocytes (Table 2). This concentration of GABA, on average, gates ~ 4% of the maximum response of a cell. However, the sensitivity to GABA of the receptors, which determines the degree of steroid potentiation that can be measured, varied from one batch of oocytes to another. Hence, it is not possible to confidently compare the absolute potentiation among analogues shown in Table 2, for which different oocyte batches were used. However, comparison of concentration-response data for individual compounds in the table can distinguish highly active from weakly active compounds. Highly active compounds cause increasing potentiation of the 2 µM GABA-mediated response as the concentration of the compound is increased (0.1, 1 and 10 µM). Compounds that are weak potentiators yield flat concentration–response data and frequently only augment GABA-mediated currents at the highest concentration tested (10µM).
Table 2.
Modulation of Rat α1β2γ2L GABAA Receptor Function by Steroids 1, 2 and Structural Analogues.
| Compound | oocyte electrophysiologya | |||
|---|---|---|---|---|
| 0.1 µM | 1 µM | 10 µM | (gating) 10 µM | |
| 1b | 2.91 ± 0.57 | 4.70 ± 1.11 | 19.64 ± 4.04 | 0.11 ± 0.02 |
| 2b | 0.94 ± 0.04 | 0.97 ± 0.05 | 1.87 ± 0.14 | 0.08 ± 0.07 |
| 3b | 1.12 ± 0.03 | 4.59 ± 0.42 | 21.14 ± 2.14 | 0.14 ± 0.03 |
| 4b | 1.49 ± 0.44 | 4.07 ± 1.09 | 23.75 ± 3.61 | 0.21 ± 0.04 |
| 5a | 0.77 ± 0.06 | 1.57 ± 0.15 | 10.78 ± 0.64 | 0.02 ± 0.01 |
| 5b | 0.87 ± 0.02 | 1.44 ± 0.02 | 8.22 ± 0.14 | 0.04 ± 0.01 |
| 5c | 0.91 ± 0.03 | 1.13 ± 0.08 | 4.67 ± 0.14 | 0.01 ± 0.01 |
| 6a | 1.17 ± 0.04 | 5.06 ± 0.6 | 21.51 ± 7.07 | 0.17 ± 0.00 |
| 6b | 0.79 ± 0.05 | 0.77 ± 0.04 | 1.82 ± 0.21 | 0.02 ± 0.01 |
| 6c | 0.90 ± 0.05 | 1.52 ± 0.24 | 5.02 ± 0.62 | 0.04 ± 0.01 |
| 6d | 0.90 ± 0.01 | 0.86 ± 0.04 | 1.53 ± 0.02 | 0.05 ± 0.02 |
The GABA concentration used for the control response was 2 µM. Each compound was evaluated on at least four different oocytes at the concentrations indicated, and the results reported are the ratio of currents measured in the presence/absence of added compound. Gating represents direct current gated by 10 µM compound in the absence of GABA, and this current is reported as the ratio of compound only current/2 µM GABA current. Error limits are calculated as standard error of the mean (N ≥ 4). Methods were as reported previously. 25
These values are from the literature.21
As reported previously, steroid 1 is a strong potentiator and its Δ16 analogue 2 is not.21 Steroid 3 and its Δ16 analogue 4 are both strong potentiators.21 Steroid 5a, the Δ16 analogue in which the C-17 substituent is an aldehyde, yielded concentration-dependent potentiation, with a strong increment in potentiation at the highest concentration (10 µM). These results differ from those obtained with steroid 2, the Δ16 analogue containing the C-17 acetyl substituent, whose degree of measured potentiation increased minimally when the concentration was increased from 1 µM to 10 µM. As a potentiator, steroid 5a has a profile more similar to that of steroid 4 than to that of steroid 2. These results correlate well with the [35S]-TBPS displacement results where steroid 5a was found to be intermediate between steroids 2 and 4 for potency of [35S]-TBPS displacement.
Hydrogenation of the Δ16 double bond in steroid 5a had little effect on activity since steroids 5a and 5b similarly potentiated GABA-mediated currents when their concentrations were increased from 1 µM to 10 µM. The result is in striking contrast to the effect this structural difference has on the GABAergic actions of steroids 1 and 2, but similar to the effect it has on the GABAergic actions of steroids 3 and 4. All of these results correlate with those found for potency of [35S]-TBPS displacement (see Table 1).
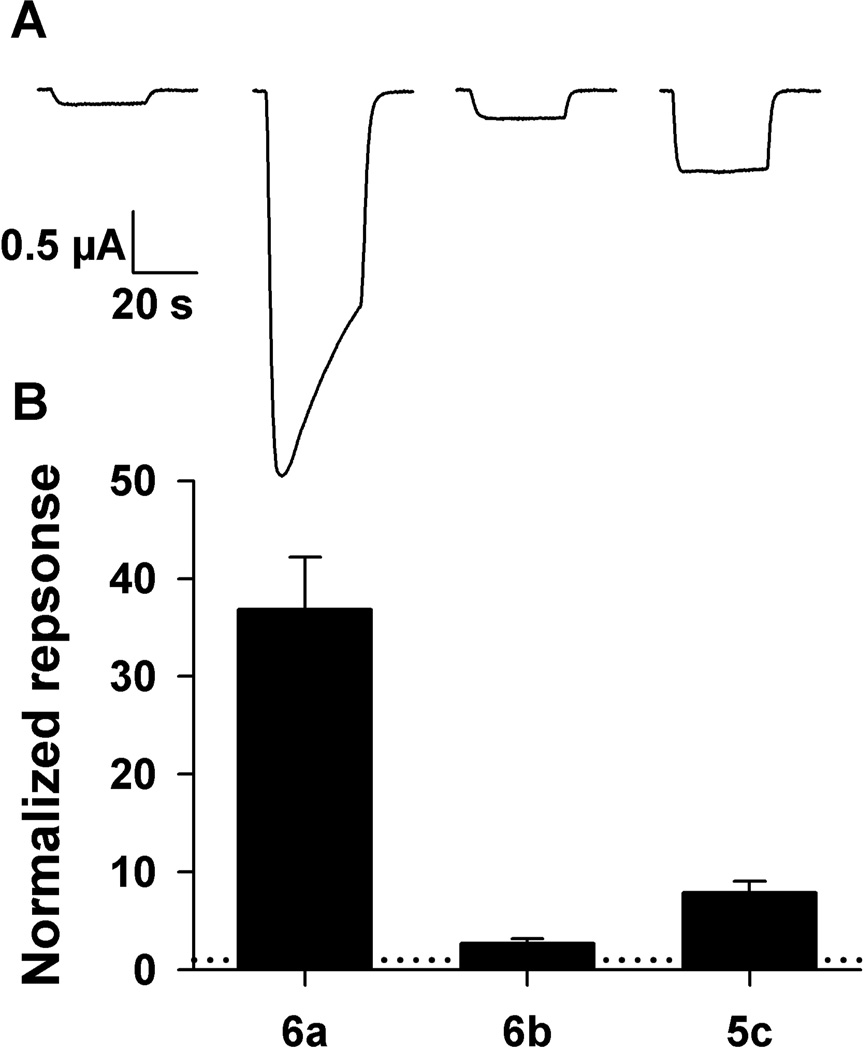
Table 2 qualitatively suggests that steroid 6a is a strong potentiator, steroid 6b is not and steroid 5c, the hydrogenation product of either 6a or 6b, has intermediate activity. In order to quantitatively distinguish enhancement differences for compounds 6a, 6b and 5c, these three compounds were directly compared at a concentration of 10 µM on the same oocytes (Figure 3). Steroid 6a strongly enhanced GABA-mediated currents and steroid 6b did not. The hydrogenation product, steroid 5c, enhanced currents more than steroid 6b, but less than steroid 6a. These functional results correlate with the order of the IC50 values for [35S]-TBPS displacement by these compounds.
Figure 3.
Direct quantitative comparison of steroids 6a, 6b and 5c at 10 µM on responses to GABA in Xenopus oocytes expressing α1β2γ2L GABAA receptor subunits. A. Responses of to 2 µM GABA alone (left trace) and to GABA co-applied with 10 µM each of 6a (second trace), 6b (third trace) and 5c (right trace). All responses are from the same oocyte. B. Summary of responses in which 6a, 6b and 5c were tested within oocytes, normalized to the response of GABA alone in each oocyte. The normalizing response is indicated with a dotted horizontal line at y = 1 (N = 4). Error bars are SEM.
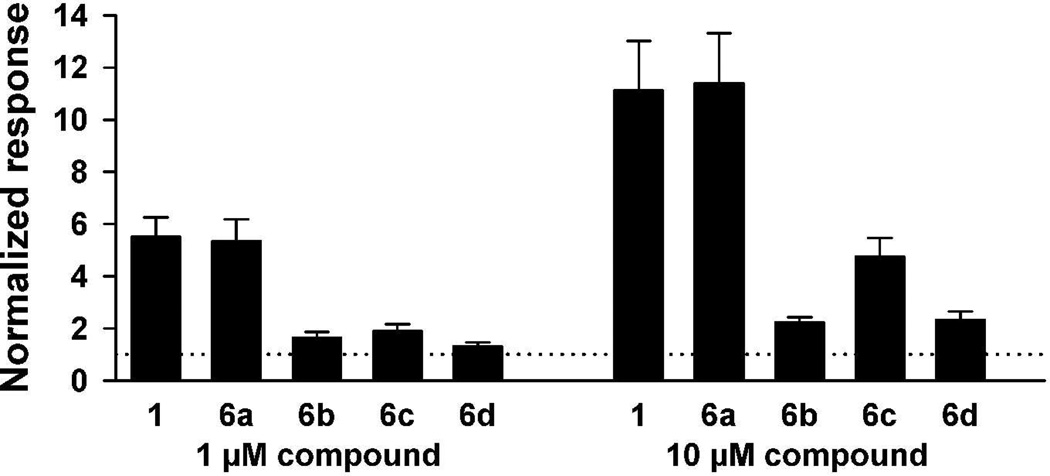
Neither steroid 6c nor steroid 6d, the two steroids containing the C-21 methyl group, was found to be a strong potentiator. To quantitate activity differences between these two steroids and to compare their electrophysiological activities to those of steroids 1, 6a and 6b, all five compounds were directly compared at a concentrations of 1 and 10 µM on the same oocytes (Figure 4). Steroid 6c was found to be two to threefold stronger than steroid 6d. These results again correlate with the IC50s of these two compounds for [35S]-TBPS displacement (see Table 1). Fig. 4 also shows that steroids 6c and 6d differ less from each other in their activities than do steroids 6a and 6b. Lastly, Figure 4 shows that steroids 1 and 6a potentiate to the same level when directly compared to each other on the same oocyte.
Figure 4.
Summary of normalized responses of oocytes to co-applied GABA (2 µM) plus 1 µM (left set of bars) or 10 µM (right set of bars) of the indicated compounds, tested within oocytes. The normalizing response of GABA alone is indicated by the horizontal dotted line at y = 1. Bars represent mean responses of 6 to 7 oocytes for each compound. Error bars are SEM.
Tadpole Loss of Righting Reflex (LRR) and Loss of Swimming Reflex (LSR) Results
The anesthetic effects of the compounds in tadpoles are summarized in Table 3. The results for steroids 1–4 were published previously.21 Unlike steroid 2, which did not cause LRR, steroid 5a does cause LRR with an EC50 of ~ 3 µM. Relative to steroid 4, steroid 5a is ~ threefold less potent at causing LRR, and only steroid 4 caused LSR. The concentration–response curve found for steroid 5a is very steep and unique among the compounds in this study. The reason for this phenomenon is not known. Steroid 5b, the compound produced by hydrogenation of steroid 5a, had LRR and LSR EC50 values comparable to those of steroid 5a. Steroid 5c was ~ fivefold less potent at causing LRR than steroid 3 and unlike steroid 3, it did not cause LSR.
Table 3.
Effects of Steroids 1, 2 and Structural Analogues on Tadpole Righting and Swimming Reflexesa
| Compound | Tadpole LRR EC50 (µM) |
Tadpole LRR nHill |
Tadpole LSR EC50 (µM) |
Tadpole LSR nHill |
|---|---|---|---|---|
| 1b | 1.12 ± 0.14 | −3.38 ± 2.28 | 5.48 ± 0.11 | −33 ± 0c |
| 2b | > 10 | – | Noned | – |
| 3b | 0.72 ± 0.11 | −1.49 ± 0.26 | 5.48 ± 0.12 | −33 ± 0 |
| 4b | 1.04 ± 0.14 | −1.77 ± 0.38 | 5.48 ± 0.12 | −33 ± 0 |
| 5a | 3.22 ± 0.03 | −16 ± 1.8 | None | – |
| 5b | 3.98 ± 2.43 | −2.76 ± 3.73 | None | – |
| 5c | 3.58 ± 1.59 | −3.21 ± 5.34 | None | – |
| 6a | 1.44 ± 0.20 | −2.84 ± 0.77 | 5.48 ± 0.20 | −33 ± 0 |
| 6b | 9.15 ± 5.37 | −1.70 ± 1.15 | 17.3 ± 0.17 | −36 ± 0 |
| 6c | 2.71 ± 0.26 | −2.47 ± 0.64 | 5.48 ± 0.20 | −33 ± 0 |
| 6d | 2.09 ± 0.12 | −2.42 ± 0.24 | > 10 | – |
The methods are as reported previously. 25 Error limits are calculated as standard error of the mean (N = 10 or more animals at each of five or more different concentrations).
These values are from the literature.21
LSR typically has a very steep dose–response curve and the nHill values reflect the fact that at 3 µM (10 µM for compound 6b) all or nearly all animals had a swimming response and at 10 µM (30 µM for compound 6b) the animals did not.
None indicated that all animals had a swimming response at 10 µM of the test compound.
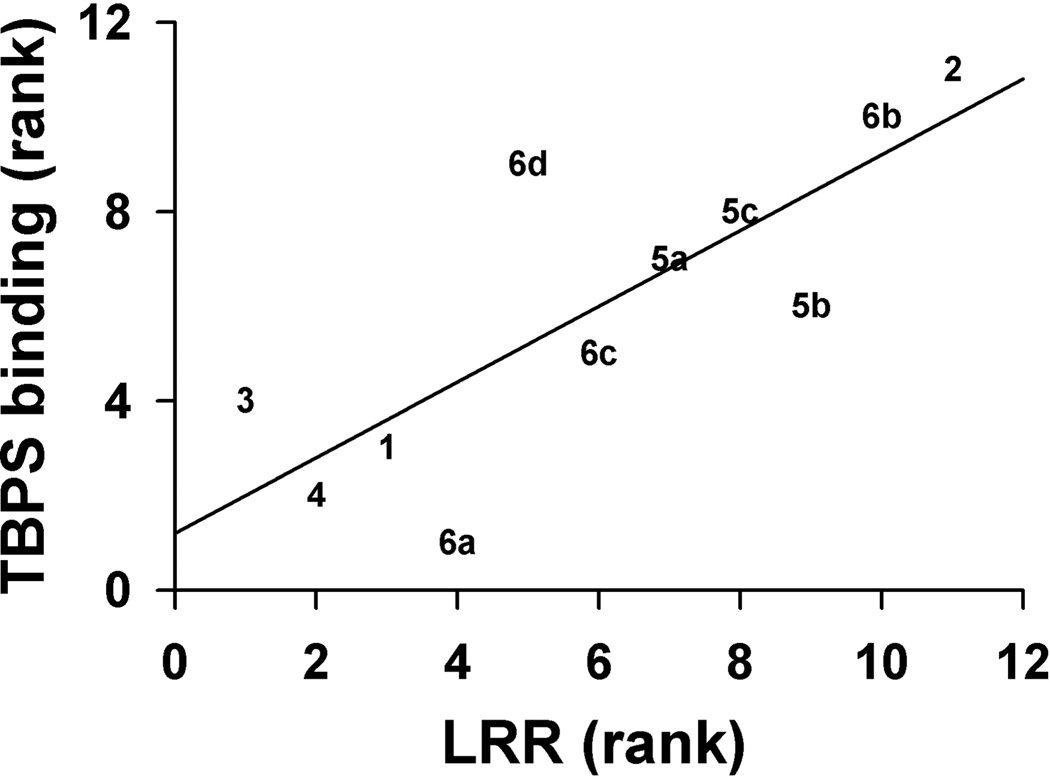
Steroid 6a is ~ eightfold and ~ threefold more potent than steroid 6b at causing LRR and LSR, respectively. Steroids 6c and 6d have similar tadpole LRR EC50 values, but only steroid 6c causes LSR with an EC50 below 10 µM. Comparison of tadpole LRR EC50 values for the Z isomers 6a and 6c shows that the presence of the C-21 methyl group in the steroid decreases the potency of the Z isomer for tadpole LRR slightly more than twofold. The same comparison for the E isomers 6b and 6d shows that the C-21 methyl group increases the potency of the E isomer slightly more than fourfold. Steroid 5c, the hydrogenation product of either steroid 6a or 6b, is less potent than steroid 6a and more potent than steroid 6b at causing tadpole LRR, and it does not cause LSR at concentrations ≤ 10 µM. Figure 5 shows a rank order correlation for the [35S-TBPS] IC50 values and tadpole LRR EC50 values for all 11 compounds. The correlation coefficient is 0.8 (p < 0.05) indicating a strong correlation between these parameters. Based on the [35S-TBPS] IC50 values, steroid 6a was less potent and steroid 6d was more potent at causing tadpole LRR than expected.
Figure 5.
A rank order correlation plot of the analogue [35S]-TBPS IC50 values with their corresponding tadpole LRR EC50 values. Compound numbers are used to represent data points on the plot. The correlation is significant, r = 0.8, (p < 0.05).
Anesthesia in Mice Results
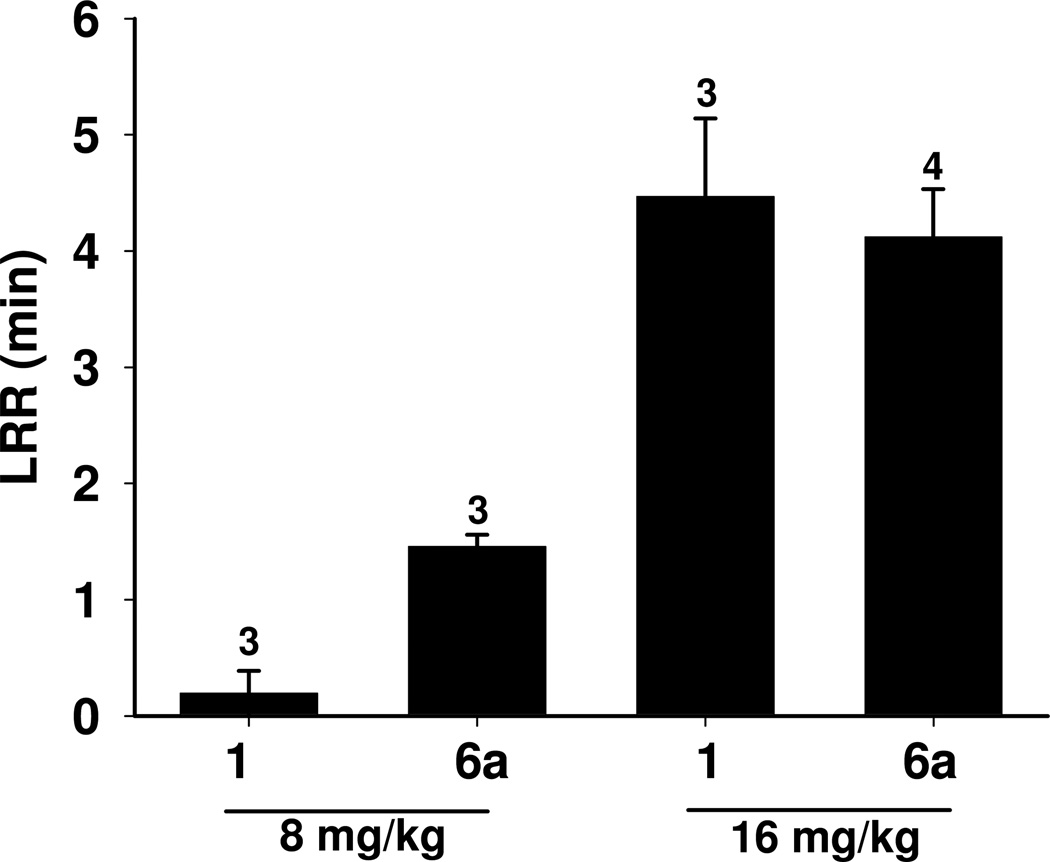
An assessment of the potency, rate of onset and rate of recovery of steroid 6a relative to these parameters for anesthetic steroid 1 was made using tail vein injections in mice. The duration of anesthesia, defined as loss of righting reflex, observed for the two steroids is shown in Figure 6. Steroid 1 caused very brief anesthesia at a dose of 8 mg/kg. At a dose of 16 mg/kg, steroid 1 caused an immediate loss of righting reflex that lasted for ~ 4 min. Recovery was characterized by a rapid progression over a period of 1–2 min from an initial return of leg movement followed by righting and subsequent walking around the cage.
Figure 6.
Duration of anesthesia induced by tail vein injection of steroids 1 and 6a into mice at two doses. The steroids were dissolved in 22.5% aqueous 2-(hydroxypropyl)-β-cyclodextrin. Numbers of animals tested are given above the bars.
Steroid 6a was an anesthetic at a dose of 8 mg/kg. Loss of movement and righting reflex occurred in 10–25 sec and lasted for ~ 1.5 min. At a dose of 16 mg/kg, steroid 6a caused an immediate loss of righting reflex that lasted on average ~ 4 min. The behavioral pattern of recovery for mice injected with either dose of steroid 6a was not distinguishable from the pattern observed for the mice injected with 16 mg/kg of anesthetic steroid 1.
Discussion
Our recent study of steroid 4, the 17-carbonitrile analogue of steroid 2, confirmed an earlier hypothesis which stated that a change in the conformation of the steroid D-ring caused by introduction of the Δ16 double bond was not the reason for the reduction in GABAergic and anesthetic actions of steroid 2.21 The goal of this study was to gain an increased understanding of the other structural features of steroid 2 that are responsible for its diminished actions. We approached this problem by assuming that the rings of steroids 2 and 4 are superimposed when bound to the same binding site(s) on GABAA receptors. With this assumed alignment, it is easy to recognize that the C-20 carbonyl and C-21 methyl groups of steroid 2 are located to the left and right, respectively, of the nitrile group of steroid 4. Thus, the inactivity of steroid 2 could result from the fact that the C-20 carbonyl group, the C-21 methyl group, or both groups are placed in positions that prevent favorable interactions of steroid 2 with the receptor. We then prepared analogues to address these possibilities.
Steroid 5a allowed us to test the possibility that the C-21 methyl group of steroid 2 had a negative effect on the compound’s activity. We found that this was indeed the case with differences in activity being largest in the electrophysiological and tadpole bioassays. Compound 5b was made to address the possibility that the position of the aldehyde group in steroid 5a was in a favorable, not an unfavorable position. Because compounds 5a and 5b had very similar activities in all three bioassays we could not conclude that the steric restraint imposed by the Δ17(20) double bond had either a favorable or unfavorable effect. We could only conclude that it had a minimal effect on activity in the absence of a C-21 methyl group. Overall, these results suggest that it is the presence of the C-21 methyl group in steroid 2, not the location of the C-20 carbonyl group, which is responsible for the diminished pharmacological actions of steroid 2a. The result further implies that compounds having other hydrogen bond acceptor groups located where the carbonyl group of steroids 2 and 5a is located would have significant pharmacological activity provided that the new analogues did not have a C-21 methyl group.
To reinforce the above conclusions, we prepared analogues 6a–6d. Steroid 6a is a close structural analogue of steroid 5a, and steroid 6b is a close structural analogue of the high energy U conformation (i.e., the conformation of the steroid in which the positions of the carbonyl group and hydrogen atom on C-20 are interchanged) of steroid 5a. Based on the results obtained with steroid 5a, steroid 6a was expected to be more active than steroid 2. This was indeed the case as steroid 6a had activities comparable to those of anesthetic steroid 1. On the other hand, steroid 6b, which places the cyano group in the position of the 21-methyl group of steroid 2, had much lower activity than steroid 5a. Steroid 5c, in which the cyano group can freely rotate, has an activity intermediate between the activities of steroids 6a and 6b. These results reinforce the conclusion that the location of the 20-carbonyl group in steroid 2 is not the major structural reason for the inactivity of this steroid. The results further suggest that analogues which mimic the conformation of steroid 2 in its minimal energy conformation are likely to be more active that those that mimic the high energy U conformation of steroid 2.
Steroids 6c and 6d were made to probe the effect that adding a C-21 methyl group would have on the actions of steroids 6a and 6b. A comparison of the results obtained with steroids 6a–d clearly demonstrates that the presence of a C-21 methyl group negatively affects compound activity. The unfavorable effect of the C-21 methyl group is evident for both the Z stereoisomers (compare steroids 6a and 6c) and the E stereoisomers (compare steroids 6b and 6d).
Thus, the analogues prepared allowed us to achieve our goal of gaining a better understanding of why introducing a Δ16 double bond into anesthetic steroid 1 results in a major decrease of anesthetic activity. An additional outcome of the study was the identification of steroid 6a as an experimental intravenous steroid anesthetic. We have shown that steroid 6a is comparable in potency to anesthetic steroid 1 as an intravenous anesthetic in mice and, that like anesthetic steroid 1, this compound has a short onset of action. We also observed that mice anesthetized with either steroid 6a or steroid 1 displayed similar behaviors upon rapid recovery from anesthesia. It is hoped that steroid 6a also will have the other favorable anesthetic properties of anesthetic steroid 1. Future studies are planned to examine the effects of steroid 6a on heart rate, respiration and intracranial pressure.
Conclusion
We have shown that a previous hypothesis that explains the lack of GABAergic and anesthetic actions of Δ16-alphaxalone is inadequate. We determined that the C-21 methyl group location, not the previously proposed location of the C-20 carbonyl group, in Δ16-alphaxalone is likely the major reason for the loss of activity for this Δ16 steroid. We prepared a series of Δ17(20) analogues and identified anesthetic steroid 6a as a candidate for future investigation.
Experimental Section
General Methods
Solvents were either used as purchased or dried and purified by standard methodology. Extraction solvents were dried with anhydrous Na2SO4 and after filtration, removed on a rotary evaporator. Flash chromatography was performed using silica gel (32–63 µm) purchased from Scientific Adsorbents (Atlanta, GA). Melting points were determined on a Kofler micro hot stage and are uncorrected. FT-IR spectra were recorded as films on a NaCl plate. NMR spectra were recorded in CDCl3 at ambient temperature at 300 MHz (1H) or 74 MHz (13C). Purity was determined by combustion analysis for C,H and N (when present) and was performed by M-H-W Laboratories (Phoenix, AZ). Steroids 1 and 7 were purchased from Steraloids (Newport, RI). Steroids 2, 3 and 4 were prepared as described previously12.
(3α,5α)-3-Hydroxy-11-oxoandrost-16-ene-17-carboxaldehyde (5a)
Steroid 9 (110 mg, 0.31 mmol) dissolved in EtOH (8 mL) and 6 N HCl (2 mL) were stirred at room temperature for 6 h. The reaction was adjusted to basic pH by adding aqueous NaHCO3 and solvents were removed under reduced pressure to give a residue. Water was added and the product was isolated by extraction with CH2Cl2. The combined extracts were dried and concentrated to give a white solid, which was purified by passing through a short column of silica gel (eluted with 50% EtOAc in hexanes) to give product 5a as a white solid (83 mg, 85%): mp 164–167 °C; [α]D20 = +71.2 (c = 0.11, CHCl3); IR vmax 3392, 2922, 1702, 1677, cm−1; H NMR δ 9.70 (s, 1H), 6.85 (br s, 1H), 4.05 (br s, 1H), 2.98 (d, 1H, J = 12.6 Hz), 2.44 (d, 1H, J = 12.6 Hz), 1.02 (s, 3H), 0.84 (s, 3H);13C NMR δ 209.6, 189.1, 154.6, 152.3, 66.2, 66.0, 56.2, 53.6, 47.5, 39.1, 36.1, 35.3, 35.2, 32.4, 32.1, 30.9, 28.9, 27.8, 17.3, 10.9. Anal. (C20H28O3) C, H.
(3α,5α,17β)-3-Hydroxy-11-oxoandrostane-17-carboxaldehyde (5b)
A mixture of the unsaturated aldehyde 5a (31 mg, 0.1 mmol), Lindlar’s catalyst (60 mg) and EtOAc (10 mL) was hydrogenated in a Parr hydrogenation apparatus (H2, 60 psi) for 4 h. The reaction mixture was then passed through a short silica gel column eluted with EtOAc. After solvent removal, the product was isolated as a solidified foam with a low melting point that could not be accurately determined. Product 5b (26 mg, 70%) had: [α]D20 = +61.9 (c = 0.11, CHCl3); IR vmax 3391, 2921, 1706 cm−1; H NMR δ 9.71 (d, 1H, J = 1.9 Hz), 4.05 (br s, 1H), 1.01 (s, 3H), 0.71 (s, 3H);13C NMR δ 209.5, 203.2, 66.2, 64.3, 61.1, 56.1, 55.5, 47.8, 38.9, 36.3, 35.8, 35.2, 32.6, 30.8, 28.8, 27.8, 24.1, 21.5, 14.6, 10.9. Anal. (C20H30O3) C, H.
(3α,5α)-3-Hydroxy-11-oxopregnan-21-carbonitrile (5c)
A solution of steroid 6b (90 mg, 10.3 mmol) in EtOAc (45 mL) and EtOH was hydrogenated in the presence of Pd/C (10%, 10 mg) overnight at 60 psi. The next day additional Pd/C (10 mg) was added and the hydrogenation was continued for an additional 12 h. The catalyst was removed by filtration through a short column of silica gel eluted with CH2Cl2 and the solvent was removed to yield a white solid. Crystallization from Et2O/EtOAc/hexanes afforded product 5c (52 mg, 57%): mp 176–178 °C; [α]D20 = +24.1 (c = 0.25, CHCl3); IR vmax 3400, 2922, 2249, 1703 cm−1; H NMR δ 4.03 (1H, m), 1.00 (3H, s), 0.58 (3H, s); 13C NMR δ 209.9, 119.2, 66.4, 64.5, 55.4, 54.9, 46.5, 45.8, 39.1, 37.2, 35.9, 35.4, 32.7, 31.0, 29.0, 28.6, 27.9, 23.9, 17.7, 13.2, 11.1. Anal. (C21H31NO2) C, H, N.
[3α,5α,17(20)Z]-3-Hydroxy-11-oxopregn-17(20)-ene-21-nitrile (6a) and [3α,5α,17(20)E]-3-Hydroxy-11-oxopregn-17(20)-ene-21-nitrile (6b)
To a suspension of NaH (60% dispersion in oil, 0.55 mmol, 14 mg) in dry THF (5 mL) at 0 °C under N2, diethyl(cyanomethyl)phosphonate (0.6 mmol, 0.1 mL) was added dropwise. After disappearance of the sodium hydride, steroid 7 (147 mg, 0.48 mmol) in dry THF (10 mL) was added dropwise. This mixture was allowed to attain room temperature and stirred overnight. The reaction mixture was then poured into an aqueous solution of NH4Cl and the product was extracted with EtOAc. The combined extracts were washed with brine and dried. After solvent evaporation, the residue was purified on preparative TLC (4 plates) developed with EtOAc/hexanes (1:1) to obtain product 6b (65 mg) and an unseparated mixture of product 6a and unreacted steroid 7 (56 mg). The latter mixture was again treated as just described with NaH (7 mg, 60% dispersion in oil, 0.17 mmol) and diethyl(cyanomethyl)phosphonate (0.03 mmol, 0.2 mL) to convert the unreacted steroid 7 in the mixture to products 6a and 6b. Purification by preparative TLC provided product 6a (40 mg) and additional product 6b (5 mg).
Product 6a (40 mg, 25%) had: mp 219–221 °C (Et2O/hexanes), [α]D20 = +10.0 (c = 0.18, CHCl3); IR vmax 3391, 2922, 2215, 1704, 1636 cm−1; H NMR δ 5.15 (1H, t, J = 2.4 Hz), 4.02 (1H, br s), 3.16 (1H, d, J = 12 Hz), 0.99 (3H, s), 0.89 (3H, s);13C NMR δ 208.7, 175.7, 115.8, 89.4, 66.3, 64.6, 54.6, 53.3, 49.6, 39.0, 35.9, 35.7, 35.4, 32.7, 32.5, 30.9, 29.0, 27.8, 23.5, 18.2, 11.1. Anal. (C21H29NO2) C, H, N.
Product 6b (70 mg, 44%) had: mp 166–168 °C (Et2O/hexanes), [α]D20 = −5.9 (c = 0.36, CHCl3); IR vmax 3435, 2923, 2217, 1705, 1638 cm−1; H NMR δ 4.95 (1H, t, J = 2.7 Hz), 4.02 (1H, br s), 2.71–2.83 (2H, m), 0.99 (3H, s), 0.79 (3H, s);13C NMR δ 208.6, 177.7, 116.8, 89.2, 66.2, 64.7, 53.5, 53.2, 49.5, 39.0, 36.2, 36.0, 35.4, 32.5, 30.9, 30.7, 28.9, 27.8, 23.6, 19.2, 11.1. Anal. (C21H29NO2) C, H, N.
[3α,5α,17(20)Z]-3-Hydroxy-11-oxopregn-17(20)-ene-20-carbonitrile(6c)
Steroid 12a (20 mg, 0.053 mmol) and K2CO3 (25 mg) in MeOH (3 mL) were refluxed for 2 h. After cooling to room temperature, the MeOH was removed under reduced pressure to give a residue. Water (25 mL) was added and the product was extracted with CH2Cl2. Solvent removal gave a solid which was purified by column chromatography on silica gel (eluted with 50% EtOAc in hexanes) to give the product 6c (15 mg, 82%): mp 203–205 °C; [α]D20 = +4.0 (c = 0.08, CHCl3); IR vmax 3368, 2923, 2210, 1704, 1594, 1453 cm−1; H NMR δ 4.05 (br s, 1H), 3.26 (d, 1H, J = 12.4 Hz) 2.60–2.20 (m, 4H), 1.83 (br s, 3H), 1.01 (s, 3H), 0.88 (s, 3H); 13C NMR δ 208.9, 167.2, 118.5, 98.2, 66.2, 64.6, 54.9, 53.5, 49.0, 38.9, 35.8, 35.5, 35.2, 32.5, 31.1, 30.8, 28.9, 27.7, 23.2, 18.2, 18.0, 10.9. Anal. (C22H31NO2) C, H, N.
[3α,5α,17(20)E]-3-(Acetyloxy)-11-oxopregn-17(20)-ene-20-carbonitrile (6d)
Steroid 12b (60 mg, 0.053 mmol) was converted into steroid 6d using the procedure reported for the preparation of steroid 6c. Product 6d (45 mg, 82%) had: mp 186–188 °C; [α]D20 = −7.8 (c = 0.18, CHCl3); IR vmax 3468, 2924, 2210, 1704, 1639 cm−1 ; H NMR δ 4.02 (br s, 1H), 2.76 (d, 1H, J = 12.1 Hz), 2.69 (m, 2H), 2.56 (d, 1H, J = 12.4 Hz), 2.09 (m, 1H), 1.85 (t, 3H, J = 2 Hz), 0.98 (s, 3H), 0.84 (s, 3H); 13C NMR 8 209.1, 166.8, 119.6, 100.5, 66.0, 64.5, 55.1, 54.7, 49.2, 38.7, 35.7, 35.2, 33.2, 32.2, 30.7, 28.7, 27.6, 23.6, 17.0, 15.0, 10.8. Anal. (C22H31NO2) C, H, N.
(3α,5α)-3-(Methoxymethoxy)-11-oxoandrost-16-ene-17-carboxaldehyde (9)
To a cold (−78 °C) solution of steroid 8 (250 mg, 0.7mmol) in CH2Cl2 was added 1 M DIBALH in toluene (2.1 mL, 2.1 mmol) and the reaction was stirred at −78 °C for 90 min. The excess DIBALH was quenched by adding few drops of acetone and then 1 M HCl (15 mL), and the cooling bath was removed. The biphasic mixture was stirred at room temperature for 0.5 h. The CH2Cl2 layer was separated and the aqueous phase was extracted with CH2Cl2. The combined CH2Cl2 extracts were washed with brine, dried and concentrated to give a pale yellow oil. This material was subjected to oxidation without any purification or characterization.
The pale yellow oil dissolved in CH2Cl2 (10 mL) and PCC (862 mg, 4 mmol) were stirred at room temperature for 3 h and the brown solution was purified by column chromatography (silica gel, eluted with 30% EtOAc in hexanes) to give product 9 (120 mg, 48%): mp 111–114 °C; IR vmax 2923, 1704, 1681, 1594, cm−1; 1H NMR δ 9.69 (s, 1H), 6.85 (b s, 1H), 4.66 (d, 1H, J = 6.8 Hz), 4.63 (d, 1H, J = 6.9 Hz), 3.82 (s, 1H), 3.36 (s, 3H), 2.97 (d, 1H, J = 12.6 Hz), 2.43 (d, 1H, J = 12.6 Hz), 1.03 (s, 3H), 0.83 (s, 3H); 13C NMR δ 209.6, 189.1, 154.6, 152.3, 94.6, 71.4, 66.0, 56.3, 55.1, 53.6, 47.5, 39.8, 35.9, 35.2, 33.4, 32.4, 32.1, 31.6, 27.8, 26.0, 17.3, 11.1. Anal. (C22H32O4) C, H.
[3α,5α,17(20)Z]-3-(Acetyloxy)-11-oxopregn-17(20)-ene-20-carbonitrile (12a) and [3α,5α,17(20)E]-3-(Acetyloxy)-11-oxopregn-17(20)-ene-20-carbonitrile (12b)
The acetylated steroid 10 was prepared from steroid 1 using a standard acetylation procedure (pyridine/Ac2O). Steroid 10 (281 mg, 0.75 mmol), KCN (325 mg, 5 mmol), AcOH (0.8 mL), EtOH (3 mL) and water (0.2 mL) were stirred at 0 °C for 0.5 h and then allowed to warm to room temperature. Stirring was continued at room temperature for another 60 h. Water (50 mL) was added to the reaction mixture and the resulting white precipitate was filtered. The filter-cake was dried under high vacuum for 6 h. The NMR spectrum of this white solid showed that it was a mixture of diastereomeric cyanohydrins 11 and unreacted starting material. The product mixture was used without purification or further characterization.
The crude product mixture was dissolved in pyridine (3 mL) and POCl3 (0.8 mL) was added at room temperature and the reaction was stirred for 15 hours. It was then cooled to 0 °C and carefully quenched with water and the biphasic solution was extracted with CH2Cl2. The combined CH2Cl2 extracts were dried and concentrated to give a colorless oil. The crude product was purified by column chromatography (silica gel, 15– 35% EtOAc in hexanes).
The Z-isomer 12a (15 mg, 5.3%) eluted second from the column: mp 228–230 °C; IR vmax 2922, 2209, 1731, 1702, 1595 cm−1; 1H NMR δ 5.01 (br s, 1H), 3.27 (d, 1H, J = 12.6 Hz), 2.60–2.25 (m, 4H), 2.05 (s, 3H), 1.84 (b s, 3H), 1.03 (s, 3H), 0.89 (s, 3H); 13C NMR δ 208.7, 170.6, 167.1, 118.5, 98.2, 69.8, 64.4, 54.8, 53.5, 49.0, 39.9, 35.5 (2 × C), 32.3 (2 × C), 31.5, 31.1, 27.6, 25.8, 23.2, 21.5, 18.2, 18.0, 11.1. Anal. (C24H33NO3) C, H, N.
The E-isomer 12b (60 mg, 21%) eluted first from the column: mp 166–168 °C; IR vmax 2929, 2209, 1732, 1705 cm−1; 1H NMR δ 4.99 (br s, 1H), 2.78 (d, 1H, J = 12.4 Hz), 2.71 (m, 1H), 2.55 (d, 1H, J = 12.4 Hz), 2.25 (m, 1H), 2.03 (s, 3H), 1.87 (br s, 3H), 1.00 (s, 3H), 0.86 (s, 3H); 13C NMR δ 208.9, 170.5, 166.7, 119.7, 100.7, 69.7, 64.4, 55.2, 54.7. 49.2, 39.8, 35.4, 35.2, 33.2, 32.3, 32.2, 31.5, 27.5, 25.7, 23.6, 21.5, 17.0, 15.1, 11.0. Anal. (C24H33NO3) C, H, N.
[35S]-TBPS Binding Methods
The methods used were as described previously.25
Xenopus Oocyte Electrophysiological Methods
Receptor expression and whole-cell recordings were carried out as described previously.25
Tadpole Behavioral Methods
The methods used were as described previously.25
Mouse Behavioral Methods
Anesthetic evaluations were performed in 7–8 weeks old BALB/C mice, weighing approximately 20 g. Steroids were dissolved in 22.5% (w/v) 2-hydroxypropyl-β-cyclodextrin solution (Sigma-Aldrich) at a concentration of 1.6 mg/ml or 3.2 mg/ml and injected through the tail vein in volumes of 5µl/g body weight. Doses (8 or 16 mg/kg) were calculated according to body weight. Animals were placed prone as soon as they stopped moving. Loss of LRR was defined as inability of mice to right themselves within 5 seconds after being placed in a prone position. Sleep time was defined as the time from when the mice displayed LRR until they were able to right themselves. Animals were placed on a warming blanket during the time that they were anesthetized. Each dose was administered to three or four mice and the results are presented as mean ± standard error of the mean.
Supplementary Material
Acknowledgement
This work was supported by NIH Grant GM47969 (D.F.C., A.S.E., C.F.Z.) The X-ray crystal structure determination was made possible by NSF Shared Instrument Grant No. CHE-0420497. The authors wish to thank Dr. Lihai Chen and Jacqui McDonough for their assistance with the mouse anesthesia experiments.
Footnotes
Supporting Information Available: Elemental analyses results for steroids 5a–c, 6a–d, 9 and 12a,b. X-ray crystallographic date for steroid 6a. This material is available free of charge via the Internet at http://pubs.acs.org. The crystallographic files for steroid 6a have also been deposited at The Cambridge Crystallographic Data Centre (CCDC 815191.)
Abbreviations: alphaxalone, (3α,5α)-3-hydroxypregnane-11,20-dione; GABA, γ-aminobutyric acid; GABAA, γ-aminobutyric acid type A; Δ16-alphaxalone, (3α,5α)-3-hydroxypregn-16-ene-11,20-dione; [35S]-TBPS, [35S]-tert- butylbicyclophosphorothionate; LRR, loss of righting reflex; LSR, loss of swimming reflex.
References
- 1.Hosie AM, Wilkins ME, Smart TG. Neurosteroid binding sites on GABAA receptors. Pharmacol Ther. 2007;116:7–19. doi: 10.1016/j.pharmthera.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Lambert JJ, Cooper MA, Simmons RD, Weir CJ, Belelli D. Neurosteroids: endogenous allosteric modulators of GABAA receptors. Psychoneuroendocrinology. 2009;34(Suppl 1):S48–S58. doi: 10.1016/j.psyneuen.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson RM, Davis B, Pratt MA, Sharpe HM, Tomich EG. Action of some steroids on the centtral nervous system of the mouse. II. Pharmacology. J Med Chem. 1965;8:426–432. doi: 10.1021/jm00328a004. [DOI] [PubMed] [Google Scholar]
- 4.Phillipps GH. Structure-activity relationships in steroidal anaesthetics. New York: Churchill Livingstone; 1974. pp. 32–47. [DOI] [PubMed] [Google Scholar]
- 5.Mavromoustakos T, Yang DP, Makriyannis A. Effects of the anesthetic steroid alphaxalone and its inactive Δ16-analog on the thermotropic properties of membrane bilayers. A model for membrane perturbation. Biochim Biophys Acta. 1995;1239:257–264. doi: 10.1016/0005-2736(95)00153-t. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence DK, Gill EW. Structurally specific effects of some steroid anesthetics on spin-labeled liposomes. Mol Pharmacol. 1975;11:280–286. [PubMed] [Google Scholar]
- 7.Lee AG. The interactions between phosphatidylcholines and anaesthetic steroids. Biochem Pharmacol. 1979;28:91–95. doi: 10.1016/0006-2952(79)90275-2. [DOI] [PubMed] [Google Scholar]
- 8.Makriyannis A, Fesik SW. Effects of anesthetics on sulfate transport in the red cell. J Neurosci Res. 1980;5:25–33. doi: 10.1002/jnr.490050105. [DOI] [PubMed] [Google Scholar]
- 9.Makriyannis A, Fesik S. Mechanism of steroid anesthetic action: interactions of alphaxalone and Δ16-alphaxalone with bilayer vesicles. J Med Chem. 1983;26:463–465. doi: 10.1021/jm00358a001. [DOI] [PubMed] [Google Scholar]
- 10.O'Leary TJ, Ross PD, Levin IW. Effects of anesthetic and nonanesthetic steroids on dipalmitoylphosphatidylcholine liposomes: a calorimetric and Raman spectroscopic investigation. Biochemistry. 1984;23:4636–4641. doi: 10.1021/bi00315a019. [DOI] [PubMed] [Google Scholar]
- 11.Fesik SW, Makriyannis A. Geometric requirements for membrane perturbation and anesthetic activity. Conformational analysis of alphaxalone and Δ16-alphaxalone and 2H NMR studies on their interactions with model membranes. Mol Pharmacol. 1985;27:624–629. [PubMed] [Google Scholar]
- 12.Makriyannis A, Siminovitch DJ, Das Gupta SK, Griffin RG. Studies on the interaction of anesthetic steroids with phosphatidylcholine using 2H and 13C solid state NMR. Biochim Biophys Acta. 1986;859:49–55. doi: 10.1016/0005-2736(86)90316-0. [DOI] [PubMed] [Google Scholar]
- 13.Makriyannis A, DiMeglio CM, Fesik SW. Anesthetic steroid mobility in model membrane preparations as examined by high-resolution 1H and 2H NMR spectroscopy. J Med Chem. 1991;34:1700–1703. doi: 10.1021/jm00109a024. [DOI] [PubMed] [Google Scholar]
- 14.Makriyannis A, Yang DP, Mavromoustakos T. The molecular features of membrane perturbation by anaesthetic steroids: a study using differential scanning calorimetry, small angle X-ray diffraction and solid state 2H NMR. Ciba Found Symp. 1990;153:172–184. doi: 10.1002/9780470513989.ch10. discussion 185–189. [DOI] [PubMed] [Google Scholar]
- 15.Ueda I, Tatara T, Chiou JS, Krishna PR, Kamaya H. Structure-selective anesthetic action of steroids: anesthetic potency and effects on lipid and protein. Anesth Analg. 1994;78:718–725. doi: 10.1213/00000539-199404000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Mavromoustakos T, Yang DP, Makriyannis A. Topography of alphaxalone and Δ16-alphaxalone in membrane bilayers containing cholesterol. Biochim Biophys Acta. 1994;1194:69–74. doi: 10.1016/0005-2736(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 17.Mavromoustakos T, Theodoropoulou E, Yang DP. The use of high-resolution solid-state NMR spectroscopy and differential scanning calorimetry to study interactions of anaesthetic steroids with membrane. Biochim Biophys Acta. 1997;1328:65–73. doi: 10.1016/s0005-2736(97)00078-3. [DOI] [PubMed] [Google Scholar]
- 18.Mavromoustakos T. Drug-membrane interactions an example with the pair of anesthetic steroids alphaxalone and Δ16-alphaxalone. Epitheorese Klin Farmakol Farmakokinet Int Ed. 1998;12:15–22. [Google Scholar]
- 19.Tatara T, Ueda I. Role of interfacial water in structure-selective anesthetic action of steroids. Progr. Anesth. Mech. 2000;6:603–608. [Google Scholar]
- 20.Bolger MB, Wieland S, Hawkinson JE, Xia H, Upasani R, Lan NC. In vitro and in vivo activity of 16,17-dehydro-epipregnanolones: 17,20-bond torsional energy analysis and D-ring conformation. Pharm Res. 1996;13:1488–1494. doi: 10.1023/a:1016019327120. [DOI] [PubMed] [Google Scholar]
- 21.Bandyopadhyaya AK, Manion BD, Benz A, Taylor A, Rath NP, Evers AS, Zorumski CF, Mennerick S, Covey DF. Neurosteroid analogues. 15. A comparative study of the anesthetic and GABAergic actions of alphaxalone, Δ16-alphaxalone and their corresponding 17-carbonitrile analogues. Bioorg Med Chem Lett. 2010;20:6680–6684. doi: 10.1016/j.bmcl.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akk G, Covey DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S. Mechanisms of neurosteroid interactions with GABAA receptors. Pharmacol Ther. 2007;116:35–57. doi: 10.1016/j.pharmthera.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veleiro AS, Burton G. Structure-activity relationships of neuroactive steroids acting on the GABAA receptor. Curr Med Chem. 2009;16:455–472. doi: 10.2174/092986709787315522. [DOI] [PubMed] [Google Scholar]
- 24.Sarett LH. A new method for the preparation of 17α-hydroxy-20-ketopregnanes. J Am Chem Soc. 1948;70:1454–1458. doi: 10.1021/ja01184a047. [DOI] [PubMed] [Google Scholar]
- 25.Jiang X, Manion BD, Benz A, Rath NP, Evers AS, Zorumski CF, Mennerick S, Covey DF. Neurosteroid analogues. 9. Conformationally constrained pregnanes: structure-activity studies of 13,24-cyclo-18,21-dinorcholane analogues of the GABA modulatory and anesthetic steroids (3α,5α)- and (3α,5β)-3-hydroxypregnan-20-one. J. Med. Chem. 2003;46:5334–5348. doi: 10.1021/jm030302m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.