Abstract
The purpose of this study was to determine the changes of oxidative response and exercise-induced muscle damage after two different resistance exercise protocols. Whether training with low or high intensity resistance programs cause alterations in the activities of lipid peroxidation, nitric oxide (NOx), and creatine kinase (CK) activity in human plasma was investigated. Twenty untrained males participated into this study. Ten of the subjects performed high intensity resistance (HR) exercise circuit and the rest of them performed low intensity resistance (LR) exercise circuit of 4 different exercises as a single bout. Venous blood samples were drawn pre-exercise, immediately after the exercise, and at the 6th, 24th, 48th and the72nd hours of post-exercise. Samples were analyzed for markers of muscle damage (CK), lipid peroxidation (MDA) and NOx. NOx production increased in HR group (p < 0.05). The MDA response to the two different resistance exercise protocol in this study caused a significant increase between pre and post-exercise values in both groups (p < 0.05). Also, there was a significant difference in the MDA level between the two groups in post-exercise values (p < 0.05) and higher values were observed in HR group. CK activities showed a significant increase in all post exercise values (p < 0.05) of both groups but there were no difference between HR and LR groups. These findings support that high intensity resistance exercise induces free radical production more than low intensity resistance exercise program.
Key points.
High intensity resistance exercise caused increases in NOx, MDA and CK levels.
Light intensity resistance exercises increased MDA and CK levels but did not affect NOx levels.
Damage arose during resistance exercises may be related to the level of resistance applied.
Key words: Anaerobic, intensity, lipid peroxidation, damage, blood
Introduction
An increase in macromolecule oxidation has been demonstrated following both aerobic and anaerobic exercise of sufficient intensity (Bloomer et al., 2006). The generation of reactive oxygen and nitrogen species (RONS), such as singlet oxygen (.O), superoxide radical (O2-), hydroxyl radical (.OH), and peroxynitrite (ONO2-) occur as a consequence of normal cellular metabolism and seem to be increased under psychological and physical stress conditions (Sen et al., 1994). In anaerobic exercise (e.g., resistance, isometric, eccentric, and sprint training) however, other pathways of RONS generation exist (Boomer et al., 2004) including ischemia-reperfusion, xanthine and NADPH oxidase production, prostanoid metabolism, phagocytic respiratory burst activity, disruption of iron containing proteins, and altered calcium homeostasis (Bloomer et al., 2006). The production of RONS via these pathways may result partly from eccentric muscle actions, which cause muscle injury (McHugh et al., 1999).
Resistance training is reported to have many benefits, such as weight control, prevention of osteoporosis, improvement of cardiovascular risk factors, and prevention of injury (Dinubile, 1991; Verrill and Ribisl, 1996). However excessive resistance training program may increase oxidative stress and cellular damage (Liu et al., 2005). There are two theories supporting the concept that resistance exercise could lead to an increase in the production of oxygen free radicals in active muscle sites. A widely supported hypothesis involves the ischemia-reperfusion injury (McBride et al., 1998). Intense muscle contractions can result in a temporary decrease in blood flow and oxygen availability and subsequent ischemia. Following contraction (muscle relaxation), reperfusion produces an abundant reintroduction of O2 and results in the formation of O2- radical. Mechanical stress is another hypothesis used to explain an increase in free radicals (Viitala et al., 2004). In particular, eccentric exercise, which includes high levels of force, was shown to result in muscle tissue damage. This initiates the inflammation process that eventually produces oxygen free radicals and lipid peroxidation.
Plasma malondialdehyde (MDA) levels during exercise have also been correlated with creatine kinase (CK), a marker of muscle damage (Kanter et al., 1988). There has been an attempt to establish a relationship between free radicals and muscle damage (McBride et al., 1998) Studies have shown that increased lipid peroxidation occurs in patients with muscular dystrophy (Foxley et al., 1991).
Nitric oxide (NOx) is a free radical produced in biological systems. While serving in various physiological periods such as the control of blood pressure, neurotransmission, learning and memory in low concentrations, it is a defendant cytotoxin at high concentration. Although the precise mechanism for altered synthesis or activity of NOx during exercise has not been completely elucidated, a number of studies have suggested that flow and shear stress to endothelium activates synthesis and release of NOx from the endothelial cell (Cooke et al., 1991; Lawson et al., 1997). Both flow stress and shear stress increase intracellular calcium concentration in the endothelial cell, which, in turn, leads to activation of constitutive NOx synthase (Node et al., 1997; Ohno et al., 1993). Flow-mediated release of NOx is also believed to be important for exercise-induced vasodilatation, as suggested by the work of Gilligan and co-workers (1994). This result suggests that shear stress during the exercise may increase production of NOx in normal subjects.
The aim of this study was to examine the effects of two different resistance exercise protocols in terms of volume and intensity on the markers of oxidative stress and muscle damage in the plasma of healthy, sedentary males.
Method
Subjects
Twenty healthy and untrained voluntary males (with an average age of 27.8 ± 2.8 yrs, height of 1.79 ± 0.07 m and a body weight with 75.9 ± 9.7 kg) participated in this study. They were randomly assigned to a high intensity resistance exercise group (HR, n: 10) and a low intensity resistance exercise group (LR, n: 10). All experimental procedures were approved by the ethical committee of Gazi University. All subjects were asked to give both verbal and written consent prior to participation.
Exercise protocol
The subjects were acquainted to 4 different exercises of the resistance exercise circuit (squat and leg extension for the lower extremities, latissimus dorsi pull and chest press for the upper extremities) and their single repetition maximum (1-RM) for each exercise was evaluated one week before the start of study. The intensities of the tests subjected to the two groups were defined in Table 1.
Table 1.
Programs of the high and low intensity resistance exercises.
| High intensity resistance exercise | Low intensity resistance exercise | ||||
|---|---|---|---|---|---|
| Load (%) | Repetitions | Set | Load | Repetition | Set |
| 95 | 2 | 3 | %35 | 20 | 3 |
| 90 | 4 | 3 | %30 | 23 | 3 |
| 85 | 6 | 3 | %25 | 26 | 3 |
| 80 | 8 | 3 | %20 | 30 | 3 |
Subjects participated to the resistance exercise circuit at 8.30 a.m. after an overnight fast and they had made no exercise for two days before this experiment. After a warm up on a cycle ergometer (15 min, 75 W) HR and LR groups performed their resistance circuit exercises. The recovery times between the different exercise stations were set as one minute.
Collection of blood samples
Venous blood samples were drawn by antecubital venipuncture before the bout, immediately after the bout (within 1 min.), and at the 6th, 24th, 48th and 72nd hours after training. The blood was immediately centrifuged at 1500 RCF for 10 min at 4°C, and the plasma was separated and stored in Eppendorf tubes at -70°C for subsequent use. Plasma samples were used for measurements of MDA, NOx level and CK activity.
Biochemical analysis
NOx Measurement: The NOx levels were measured in plasma as nitrites using the modified Griess reaction after converting nitrates to nitrites with vanadium chloride. Standard curves for sodium nitrite were prepared. Values were calculated with standard calibration plots for NaNO2 and NaNO3 as previously described (Green et al., 1982; Miranda et al., 2001).
Lipid peroxidation: Lipid peroxidation was quantified by measuring the formation of thiobarbituric acid reactive substances as described previously by Kurtel et al., 1992. Aliquots (0.5 ml) were centrifuged, and the supernatants were added to 1 ml of a solution containing 15 % (wt/vol) tricarboxylic acid, 0.375 % (wt/vol) thiobarbituric acid, and 0.25 N HCL. Protein precipiate was removed by centrifugation and the supernatants were transferred to glass test tubes containing 0.02 % (wt/vol) butylated hydroxytoluene to prevent further peroxidation of lipids during subsequent steps. The samples were then heated for 15 min at 100°C in a boiling water bath, cooled and centrifuged to remove the precipitant. The absorbance of each sample was determined at 532 nm. Lipid peroxide levels were expressed in terms of MDA equivalents using an extinction coefficient of 1.56x105 mol-1.
Creatine Kinase (CK): Plasma CK activity was tested from blood samples using Hitachi 912 biochemical device with Roche Diagnostic kit.
Statistics
Values are expressed as the mean ± SE. and were compared with ANOVA for repeated measures. Bonferroni test was used in order to learn which measurement time the difference comes from. Independent samples t-test was used for comparison of corresponding values between the groups. Pearson correlation was used for correlation among variables. Statistical significance was set at p < 0.05.
Results
NOx levels were siginificantly increased 6 hours after the exercise in HR group (p < 0.05). Such increase insisted on 24th and 48th hours (p < 0.05 for both), and then returned to the preexercise level at 72nd hour. However, NOx levels did not chanced after exercise in LR group (Figure 1).
Figure 1.
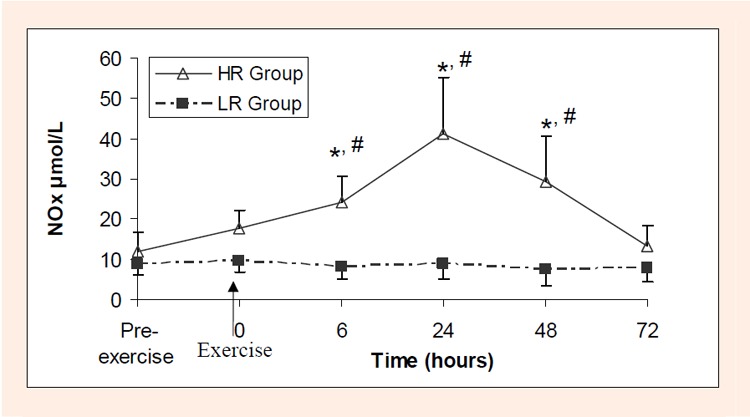
NOx (µmol/L) values before and after low (LR) and high resistance (HR) exercise.
* difference from value measured pre-exercise in HR group (p < 0.05)
# difference from corresponding value of LR group (p < 0.05)
MDA levels also rised immediately after cessation of the exercise in both HR and LR groups (p < 0.05 for both), but then, sharply declined through to the preexercise levels in 6 hours. MDA levels were not different from preexercise level in 6th, 24th, 48th and 72nd hours in both groups (Figure 2). Such sudden increase in MDA was significantly higher in HR group than LR group (p < 0.05) (Figure 2).
Figure 2.
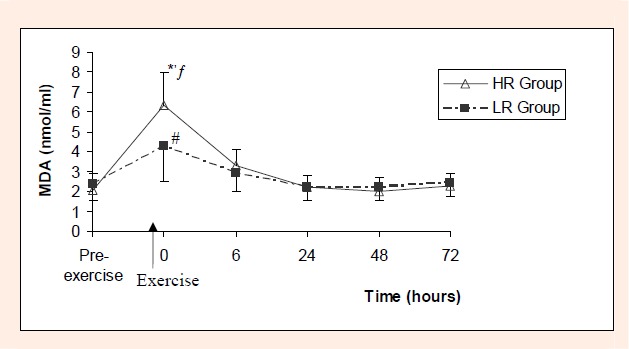
MDA (nmol/L) values before and after low (LR) and high resistance (HR) exercise.
* difference from value measured pre-exercise in HR group (p < 0.05)
ƒ difference from corresponding value of LR group (p < 0.05)
# difference from value measured pre-exercise in LR group (p < 0.05)
CK levels in HR group increased significantly immediately after the exercise and this increase continued for 48 hours (p < 0.05 for all). Then, CK levels returned to preexercise values in 72nd hour. A similar pattern was observed for CK also in LR group. There was no significant difference between corresponding CK values of two groups (Figure 3).
Figure 3.
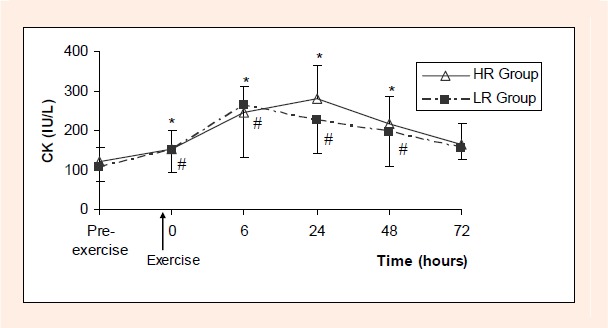
CK (IU/L) values before and after low (LR) and high resistance (HR) exercise.
* difference from value measured pre-exercise in HR group (p < 0.05)
# difference from value measured pre-exercise in LR group (p < 0.05).
A striking correlation was observed between CK and NOx levels in HR group (r = 0.557 p < 0.05). There was no correlation between other variables.
Discussion
This study examined the effects of different resistance exercise protocols on markers of muscle damage, lipid peroxidation and nitric oxide response in the plasma of the sedentary men. One of the primary findings of this investigation was the increase of NOx production in high resistance exercise (HR) group (p < 0.05). The response of the MDA to the two different resistance exercise protocols showed a significant increase between the values obtained before and immediately after the exercise in both groups (p < 0.05). Also there was a significant difference in the MDA level between the two groups in the values obtained immediately after cessation of the exercise (p < 0.05). CK activity increased significantly (p < 0.05) for all post exercise values in both HR and LR groups but there was no difference between them.
Vascular formation of NOx is directly facilitated by increased shear stress (Cooke et al., 1991; Miller and Burnett, 1992). During a session of physical exercise, cardiac output increases and blood redistribute to the exercising muscles. The exercise-induced increase of blood flow elicits an increase in shear stress (Van Citters and Franklin, 1969), thereby providing a possible coupling between exercise and endogenous NOx formation. Although the role of endothelium-derived NOx in acute exercise has not been fully resolved, exercise training involving repetitive bouts of exercise over weeks or months up-regulates endothelial NOx bioactivity (Maiorana et al., 2003). We saw that NOx production increased in HR group (p < 0.05). There was a significant increase after the 6 hours of the exercise according to NOx results in HR group and this increase continued after 24 and 48 hours (Figure 1). In agreement with these findings, it has also been reported that NOx is increased in venous plasma after prolonged running, cycling (Jungersten et al., 1997) and after incremental cycling exercise to VO2 max (Node et al., 1997). In contrast, some other studies have also shown no change of NOx metabolism following an incremental treadmill test to exhaustion in healthy human subjects (Komiyama et al., 1997; Poveda et al., 1997; St Croix et al., 1999). In our study, there was no significant difference between measurement times in LR group (Figure 1). Our results suggest that the intensity of the resistance exercise increase NOx production, only when it was performed at high intensities. According to result of the correlations comparing the same measurement times, there was a positive correlation between CK and NOx in HR group (r = 0.557 p < 0.05)
Several different exercise models have been conducted to study the effect of acute physical activity on various oxidative stress indices and tissue damage markers and different results have been reported on different models (Alessio et al., 2000; Atalay et al., 1996; Khanna et al., 1999; Lovlin et al., 1987; McBride et al., 1998; Viitala et al., 2004; Simpson et al., 2005). Resistance training consists of repetitive, static muscle actions. These include concentric and eccentric muscle actions, which are considered as respectively, a low- or high- intensity resistance exercise protocol (Liu et al., 2005). Based on previous investigations, it was determined that the intensity of the exercise protocol used is a primary factor in creating a physiological environment for increased free radical production (Sahlin et al., 1992; Saxton et al., 1994). Few studies have assessed oxidative stress resulting from resistance exercise (McBride et al., 1998; Surmen-Gur et al., 1999). An increase in blood MDA was noted in 2 days following a full body resistance training protocol (McBride et al., 1998), whereas no change was reported in blood MDA 6 min following the performance of 20 eccentric/concentric actions with the knee extensors (Surmen-Gur et al., 1999). No change was also noted for TBARS following heavy full-body resistance exercise performed to failure. The differences in the protocols may have contributed to the discrepancy in the results. In this study both high and low intensity resistance exercise bouts resulted in significant increase in lipid peroxidation immediately after exercise (p < 0.05). But higher values were observed in HR group (Figure 2). Maughan et al., 1989 states that peak changes in MDA occur at 6 hours post exercise, while some studies have only examined immediate post exercise MDA values. Some of the investigations, which have examined resistance type exercise and free radical formation reported no increase in free radical formation (Ortenblad et al., 1997; Sahlin et al., 1992; Saxton et al., 1994). This may be a result of lighter loads and smaller amount of muscle tissue activation.
Intense muscle contractions associated with resistance exercise may result in ischemia-reperfusion at the site of the active muscles. The free radicals act as mediators of ischemia-reperfusion injury in skeletal muscles and result in muscle injury accompanied by increased amounts of CK. Kanter et al. (1993; 1988) have also shown that plasma MDA measurements correlate with CK activity during exercise. In this study, plasma MDA reached peak values immediately after the exercise in both groups, but plasma CK activity reached peak values at 24th hrs post exercise in HR group, 6th hrs post exercise in LR group and higher values were observed in HR group. But no difference was found between both groups according to same measurement times (Figure 3). The creatine kinase activity observed in this investigation showed that muscle tissue damage occurred well after the exercise in both resistance exercise protocols. The fact that the two types of exercise protocols predominantly used to study muscle damage, downhill running and high-force muscular contractions, show very different CK responses. For example, after downhill running, CK peaks about 12-24 hrs post-exercise, with increases in range from 100 to 600 IU (Byrnes et al., 1985; Clarkson and Hubal, 2002; Schwane et al., 1983), whereas after high-force eccentric exercise the increase does not begin until about 48 hrs post exercise, with peak activity (generally 2000-10000 IU) occurring about 4 to 6 days post exercise (Clarkson et al., 1992). The current findings confirm that the high-intensity whole body resistance exercise can result in the formation of free radicals. These free radicals may play a role in adaptation of the muscle tissues to the physiological stress caused by resistance exercise (Liu et al., 2005; Ramel et al., 2004). Ischemia-reperfusion during resistance exercise at the site of muscle, and post-exercise production of free radicals via oxidative burst from neutrophils, are key factors that must be taken to account while trying to decrease the muscle injury during this type of exercise (Pyne, 1994).
Conclusion
In conclusion, HR exercise caused increases in NOx, MDA and CK levels. Also, LR exercises increased MDA and CK levels but did not affect NOx levels. Damage arose during resistance exercises may be related to the level of resistance applied. More work is needed to aid our understandings of the potential role of resistance exercise in generating increased oxidative stress, especially since this form of anaerobic exercise is the one most widely prescribed as a component of a well rounded fitness program.
Biographies
Nevin ATALAY GUZEL
Employment
Assoc. Prof.
Degree
PhD
Research interests
Exercise, free radicals, muscle damage, L-carnitine.
E-mail: natalay@gazi.edu.tr
Serkan HAZAR
Employment
Assoc. Prof.
Degree
PhD
Research interests
Muscle damage, exercise type, training.
E-mail: hazarserkan@hotmail.com
Deniz ERBAS
Employment
Professor
Degree
PhD
Research interests
Nitric oxide.
E-mail: derbas@gazi.edu.tr
References
- Alessio H.M., Hagerman A.E., Fulkerson B.K., Ambrose J., Rice R.E., Wiley R.L. (2000) Generation of reactive oxygen species after exhaustive aerobic and isometric exercise. Medicine and Science in Sports and Exercise 32, 1576-1581 [DOI] [PubMed] [Google Scholar]
- Atalay M., Seene T., Hanninen O., Sen C.K. (1996) Skeletal muscle and heart antioxidant defenses in response to sprint training. Acta Physiologica. Scandinavica 158, 129-134 [DOI] [PubMed] [Google Scholar]
- Bloomer R.J., Goldfarb A.H. (2004) Anaerobic exercise and oxidative stress: a review. Canadian Journal of Applied Physiology 29, 245-263 [DOI] [PubMed] [Google Scholar]
- Bloomer R.J., Falvo M.J., Fry A.C., Schilling B.K., Smith W.A., Moore C.A. (2006) Oxidative stress response in trained men following repeated squats or sprints. Medicine and Science in Sports and Exercise 38, 1436-1442 [DOI] [PubMed] [Google Scholar]
- Byrnes W.C., Clarkson P.M., White J.S., Hsieh S.S., Frykman P.N., Maughan R.J. (1985) Delayed onset muscle soreness following repeated bouts of downhill running. Journal of Applied Physiology 59, 710-715 [DOI] [PubMed] [Google Scholar]
- Clarkson P.M., Nosaka K., Braun B. (1992) Muscle function after exercise-induced muscle damage and rapid adaptation. Medicine and Science in Sports and Exercise 24, 512-520 [PubMed] [Google Scholar]
- Clarkson P.M., Hubal M.J. (2002) Exercise-induced muscle damage in humans. American Journal of Physical Medicine & Rehabilitation 81, 52-69 [DOI] [PubMed] [Google Scholar]
- Cooke J.P., Rossitch E., Jr, Andon N.A., Loscalzo J., Dzau V.J. (1991) Flow activates an endothelial potassium channel to release an endogenous nitrovasodilator. The Journal of Clinical Investigation 88, 1663-1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinubile N.A. (1991) Strength training. Clinics in Sports Medicine 10, 33-62 [PubMed] [Google Scholar]
- Foxley A., Edwards R.H., Jackson M.J. (1991) Enhanced lipid peroxidation in Duchenne dystrophy muscle may be secondary to muscle damage. Biochemical Society Transactions 19, 180S. [DOI] [PubMed] [Google Scholar]
- Gilligan D.M., Panza J.A., Kilcoyne C.M., Waclawiw M.A., Casino P.R., Quyyumi A.A. (1994) Contribution of endothelium-derived nitric oxide to exercise-induced vasodilation. Circulation 90, 2853-2858 [DOI] [PubMed] [Google Scholar]
- Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. (1982) Analysis of nitrate, nitrite and 15N nitrate in biological fluids. Analytical Biochemistry 126, 131-138 [DOI] [PubMed] [Google Scholar]
- Jungersten L., Ambring A., Wall B., Wennmalm A. (1997). Both physical fitness and acute exercise regulate nitric oxide formation in healthy humans. Journal of Applied Physiology 82:760-764 [DOI] [PubMed] [Google Scholar]
- Kanter M.M., Lesmes G.R., Kaminsky L.A., La Ham-Saeger J., Nequin N.D. (1988) Serum creatine kinase and lactate dehydrogenase changes following an eighty kilometer race. Relationship to lipid peroxidation. European Journal of Applied Physiology and Occupational Physiology 57, 60-63 [DOI] [PubMed] [Google Scholar]
- Kanter M.M., Nolte L.A., Holloszy J.O. (1993) Effects of an antioxidant mixture on lipid peroxidation at rest and post exercise. Journal of Applied Physiology 74, 965-969 [DOI] [PubMed] [Google Scholar]
- Khanna S., Atalay M., Laaksonen D.E., Gul M., Roy S., Sen C.K. (1999) Alpha-lipoic acid supplementation: tissue glutathione homeostasis at rest and after exercise. Journal of Applied Physiology 86, 1191-1196 [DOI] [PubMed] [Google Scholar]
- Komiyama Y., Kimura Y., Nishimura N., Hara K., Mori T., Okuda K., Munakata M., Masuda M., Murakami T., Takahashi H. (1997). Vasodepressor effects of exercise are accompanied by reduced circulating ouabainlike immunoreactivity and normalization of nitric oxide synthesis. Clinical and Experimental Hypertension 19, 363-372 [DOI] [PubMed] [Google Scholar]
- Kurtel H., Granger D.N., Tso P., Grisham M.B. (1992). Vulnerability of intestinal interstitial fluid to oxidant stress. The American Journal of Physiology 263, 573-578 [DOI] [PubMed] [Google Scholar]
- Lawson D.L., Chen L., Mehta J.L. (1997) Effects of exercise-induced oxidative stress on nitric oxide release and antioxidant activity. The American Journal of Cardiology 80, 1640-1642 [DOI] [PubMed] [Google Scholar]
- Liu J.F., Chang W.Y., Chan K.H., Tsai W.Y., Lin C.L., Hsu M.C. (2005). Blood lipid peroxides and muscle damage increased following intensive resistance training of female Weightlifters. Annals of the New York Academy of Science 1042, 255-261 [DOI] [PubMed] [Google Scholar]
- Lovlin R., Cottle W., Pyke I., Kavanagh M., Belcastro A.N. (1987). Are indices of free radical damage related to exercise intensity? European Journal of Applied Physiology 56, 313-316 [DOI] [PubMed] [Google Scholar]
- Maiorana A., O’Driscoll G., Taylor R., Green D. (2003) Exercise and the nitric oxide vasodilator system. Sports Medicine 33, 1013-1035 [DOI] [PubMed] [Google Scholar]
- Maughan R., Donnellly A.E., Gleeson M., Whiting P.H., Walker K.A., Clough P.J. (1989) Delayed onset muscle damage and lipid peroxidation in man after a downhill run. Muscle & Nerve 12, 332-336 [DOI] [PubMed] [Google Scholar]
- McBride J.M., Kraemer W.J., Triplett-McBride T., Sebastianelli W. (1998). Effect of resistance exercise on free radical production. Medicine and Science in Sports and Exercise 30, 67-72 [DOI] [PubMed] [Google Scholar]
- McHugh M.P., Connolly D.A., Eston R.G., Gleim G.W. (1999). Exercise-induced muscle damage and potential mechanisms for the repeated bout effect. Sports Medicine 27, 157-170 [DOI] [PubMed] [Google Scholar]
- Miller V.M., Burnett J.C., Jr. (1992). Modulation of NO and endothelin by chronic increases in blood flow in canine femoral arteries. The American Journal of Physiology 263, 103-108 [DOI] [PubMed] [Google Scholar]
- Miranda K.M., Espey M.G., Wink D.A. (2001) A rapid simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5, 67-71 [DOI] [PubMed] [Google Scholar]
- Node K., Kitakaze M., Sato H., Koretsune Y., Katsube Y., Karita M., Kosaka H., Hori M. (1997) Effect of acute dynamic exercise on circulating plasma nitric oxide level and correlation to norepinephrine release in normal subjects. The American Journal of Cardiology 79, 526-528 [DOI] [PubMed] [Google Scholar]
- Ohno M., Gibbons G.H., Dzau V.J., Cooke J.P. (1993) Shear stress elevates endothelial cGMP. Role of a potassium channel and G protein coupling. Circulation 88, 193-197 [DOI] [PubMed] [Google Scholar]
- Ortenblad N., Madsen K., Djurhuus M.S. (1997) Antioxidant status and lipid peroxidation after short-term maximal exercise in trained and untrained humans. The American Journal of Physiology 272, 1258-1263 [DOI] [PubMed] [Google Scholar]
- Poveda J.J., Riestra A., Salas E., Cagigas M.L., Lopez-Somoza C., Amado J.A., Berrazueta J.R. (1997) Contribution of nitric oxide to exercise-induced changes in healthy volunteers: effects of acute exercise and long-term physical training. European Journal of Clinical Investigation 27, 967-971 [DOI] [PubMed] [Google Scholar]
- Pyne D.B. (1994) Regulation of neutrophil function during exercise. Sports Medicine 17, 245-258 [DOI] [PubMed] [Google Scholar]
- Ramel A., Wagner K.H., Elmadfa I. (2004) Plasma antioxidants and lipid oxidation after submaximal resistance exercise in men. European Journal of Nutrition 43, 2-6 [DOI] [PubMed] [Google Scholar]
- Sahlin K., Cizinsky S., Warholm M., Hoberg J. (1992) Repetitive static muscle contractions in humans-a trigger of metabolic and oxidative stress? European Journal of Applied Physiology and Occupational Physiology 64, 228-236 [DOI] [PubMed] [Google Scholar]
- Saxton J.M., Donnelly A.E., Roper H.P. (1994) Indices of free-radical-mediated damage following maximum voluntary eccentric and concentric muscular work. European Journal of Applied Physiology and Occupational Physiology 68, 189-193 [DOI] [PubMed] [Google Scholar]
- Sen C.K., Packer L., Hanninen O. (1994) Exercise and oxygen toxicity. Amsterdam: Elsevier Science [Google Scholar]
- Schwane J.A., Johnson S.R., Vandenakker C.B., Armstrong R.B. (1983). Delayed-onset muscular soreness and plasma CPK and LDH activities after downhill running. Medicine and Science in Sports Exercise 15, 51-56 [PubMed] [Google Scholar]
- Simpson R.J., Wilson M.R., Black J.R., Ross J.A., Whyte G.P., Guy K., Florida-James G.D. (2005). Immune alterations, lipid peroxidation, and muscle damage following a hill race. Canadian Journal of Applied Physiology 30, 196-211 [DOI] [PubMed] [Google Scholar]
- St Croix C.M., Wetter T.J., Pegelow D.F., Meyer K.C., Dempsey J.A. (1999) Assessment of nitric oxide formation during exercise. American Journal of Respiratory and Critical Care Medicine 159, 1125-1133 [DOI] [PubMed] [Google Scholar]
- Surmen-Gur E., Ozturk E., Gur H., Punduk Z., Tuncel P. (1999) Effect of vitamin E supplementation on post-exercise plasma lipid peroxidation and blood antioxidant status in smokers: with special reference to haemoconcentration effect. European Journal of Applied Physiology and Occupational Physiology 79, 472-478 [DOI] [PubMed] [Google Scholar]
- Van Citters R.L., Franklin D.L. (1969) Cardiovascular performance of Alaska sled dogs during exercise. Circulation Research 24, 33-42 [DOI] [PubMed] [Google Scholar]
- Verrill D.E., Ribisl P.M. (1996) Resistive exercise training in cardiac rehabilitation. An update. Sports Medicine 21, 347-383 [DOI] [PubMed] [Google Scholar]
- Viitala P.E., Newhouse I.J., LaVoie N., Gottardo C. (2004) The effects of antioxidant vitamin supplementation on resistance exercise induced lipid peroxidation in trained and untrained participants. Lipids in Health and Disease 22, 3-14 [DOI] [PMC free article] [PubMed] [Google Scholar]


