Abstract
It is well known that strenuous eccentric exercise may result in muscle damage. We proposed that vigorous eccentric exercise (EE) would impair myoelectric activity of the biceps brachii. This study utilised a 7-day prospective time-series design. Ten healthy males performed a session of 70 maximal EE elbow flexion contractions. Analysis of surface electromyography activity (sEMG) was performed on the signals recorded during isometric contractions at 50% (IC50) and 80% (IC80) of maximum voluntary isometric torque (MVT), deriving RMS and MDF as sEMG parameters. Linear regression of the RMS and MDF time-series (20-s sustained IC50 and IC80) was used to extract intercepts and slopes of these signals on each day. Plasma creatine kinase activity (CK), MVT, arm circumference, subjective perception of soreness and elbow joint range of motion were also measured to assess effectiveness of EE to evoke muscle damage. CK increased over resting values until day 5 after EE, and remained significantly (p < 0.05) elevated even on day 7. MVT had decreased to 45% of its initial value by day 2 after EE, and remained significantly depressed for the following 6 days. In addition, muscle soreness and arm circumference increased, and range of motion decreased after EE. A significant shift of MDF intercept towards lower frequencies at both IC50 and IC80 was observed after EE in the exercised arm, and these values gradually recovered within the next 3 days during IC50. Although there were some changes in RMS values, these alterations were persistent in both control and exercised arms, and did not follow a consistent pattern. In conclusion, a prolonged reduction in MDF intercept was observed after EE, but this was not closely time-associated with the biochemical, anthropometric or functional markers of muscle damage. Compared to RMS, MDF was a more consistent measure to reflect changes in sEMG.
Key points.
EMG can be a useful tool to detect exercise-induced muscle damage,
MDF decreased after eccentric exercise,
This decrease could be related to a reduction in the recruitment of fast twitch fibres, and
Compared to RMS, MDF was a more consistent parameter to reflect the changes in EMG after eccentric exercise.
Key words: Eccentric exercise, creatine kinase, surface electromyography, median frequency, root mean square
Introduction
Eccentric exercise (EE) generates greater tension per active muscle fibre than concentric or isometric contractions, resulting in mechanical disruption of the muscle fibre (Clarkson and Sayers, 1999; Lieber and Friden, 2002). Delayed onset muscle soreness and impaired muscle function are the common consequences of excessive EE. Impaired glycogen resynthesis (O’Reilly et al., 1987), myofibrillar damage along the Z-band (Clarkson and Hubal, 2002), mitochondrial swelling and increased intramuscular pressures (Friden et al., 1983) are some of morphologic and metabolic signs of muscle alteration post-EE that are associated with muscle damage.
Exercise-induced muscle damage has been evaluated both directly (Miura et al., 2000; Stauber et al., 1990) and indirectly (Chen, 2003; Sayers et al., 2001). However, due to the invasive nature of direct studies, such as muscle biopsy, indirect methods of evaluating muscle damage have been preferred, and these have generally been utilized in human studies. Some examples of indirect methods have included measuring changes in plasma creatine kinase (CK) activity, perceived muscle soreness (SOR), maximum voluntary torque (MVT), inflammatory markers (in plasma and muscles), neuromuscular function (measured by electromyography, EMG), MRI signal intensity, and muscle oxygenation and blood flow (measured by ultrasound, plethysmography and near infrared spectroscopy) (For a review see Clarkson and Hubal, 2002). However, the accuracy and reproducibility of some of these muscle damage assessment techniques are somewhat uncertain, and further improvements and investigations on the application of these techniques are warranted.
Surface electromyography (EMG) is a technique for evaluating and recording physiologic properties of muscles at rest and during exercise. Electromyograms monitor and record neuromuscular action potentials as myoelectric signals. Two fundamental types of variables can represent EMG: frequency and amplitude. The underlying physiological processes associated with the excitation of motor units dictate the constituent frequencies that produce the generated myoelectric signal. Therefore, the frequency content of the recorded signal can be related to the numbers of units active as well as their constituent firing rates (Kamen and Caldwell, 1996). One popular measure of EMG frequency content is median frequency (MDF), the point at which the spectral power is divided into equal low- and high-frequency halves. MDF is a recommended variable for the study of muscle fatigue and damage (Felici et al., 1997; Merletti et al., 1995). On the other hand, EMG amplitude, which is usually presented as root mean square (RMS) of the signals, can also provide information about number and location of active motor units, recruitment of motor units and shape of motor unit action potentials (Felici et al., 1997).
The analysis of EMG has been used to detect changes in the contractile properties of a muscle both during and after EE (Berry et al., 1990; Felici et al., 1997; McHugh et al., 2000). However, there are disagreements amongst different researchers who have studied the effects of EE on EMG signals. For instance, Komi and Viitasalo, 1977 and Berry and colleagues (1990) observed some increases in EMG activity after EE, while Day et al., 1998 did not find any significant change in this parameter. Similarly, Day and co-workers (1998) and Felici et al., 1997 observed significant decreases in mean and median frequency, while Berry and colleagues (1990) did not observe any consistent change in EMG frequency after EE. The reason for such divergence of findings is unknown, although they could be partially attributed to methodological differences in inducing muscle damage (and therefore the magnitude of morphological disruption) or to different methods of analysing data amongst previous studies. However, this issue needs to be further investigated to determine; (i) whether EE results in any changes of neuromuscular activity within exercised muscle? (ii) if there are any changes, what might be the possible mechanisms underlying these? and, (iii) can EMG be used as a tool to assess exercise induced-muscle damage? Therefore, in this study, the effects of EE on some of the physiological characteristics of the muscle assessed via surface electromyography were investigated. We hypothetized that MDF and RMS will shift to lower and higher values, respectively. These changes in MDF and RMS could be primarily due to the possible impairments in the function of fast-twitch fibres resulting from unaccustomed eccentric contractions.
Method
Subjects
Ten healthy males (age 25.4 ± 4.3 yr, body mass 73.1 ± 9.8 kg, stature 1.73 ± 0.07 m; mean ± SD), who had not participated in any regular upper body muscular exercise training (i.e. at least 2-times per week for more than 30-mins) for 12-months prior to commencing the study, took part in the experiment. The Human Research Ethics Committee of the University of Sydney approved this study. All subjects were informed of the purpose, nature, and potential risks of the investigation, and gave their written informed consent to participate. Subjects were in healthy physical condition with no signs or symptoms of neuromuscular disease. They were not under pharmacological treatments and followed a normal diet. All participants were requested to abstain from any exercise involving arm muscles for the duration of the study.
Study design
The exercise protocol used in this investigation was modified from previous studies that had been designed to induce muscle damage (Clarkson et al., 1992; Felici et al., 1997; Sbriccoli et al., 2001). Subjects were habituated to the equipment and performed some isometric contractions similar to those that they would perform during testing days. Testing sessions were performed over a 7-day period. Each subject’s biceps brachii of the non-dominant arm performed EE (Exercise), whereas the biceps brachii of the dominant arm was employed as non-exercise limb (Control).
Eccentric exercise protocol
On the first day, subjects performed two sets of 35 maximal voluntary EE contractions (5-s of contraction and 12-s of passive recovery) with the elbow of their non-dominant arm placed in an isokinetic strength machine. Subjects were requested and encouraged both verbally and visually (force output on a computer monitor), to maximally resist elbow extension movements in which the arm was forcibly extended from an elbow-flexed (on average 50°) to an elbow-extended (on average 170°) position at a preset angular velocity of 45 deg·s-1. The apparatus brought subjects’ arm back to the elbow-flexed position after each eccentric contraction. The two EE sets were separated by a 5-min recovery interval.
Resting assessments
Before and 30-min after the EE session on day 1 and for the next 6-days, the following measurements were made on Exercise and Control arms. It should be noted that Creatine Kinase (CK) activity was not assessed in all sessions for methodological reasons of minimising repeated phlebotomy, however the order of performing the measurements was the same within each session.
Plasma CK activity (CK): CK was measured on day 1 before and after EE, and on days 3, 5, and 7 at the beginning of each isometric exercise session. At each sampling time, about 5-ml of venous blood was withdrawn from the antecubital vein, centrifuged for 10-min to extract plasma samples, and analysed for CK activity within 24-hr. Plasma CK activity was assayed spectrophotometrically at 37°C using CK-NAC reagent kits (Thermo electron CORP., USA). Each plasma sample was assayed at least twice, until two assays were within 10% of the lower value and the mean of the two values was used for statistical analyses.
Elbow range of motion (ROM): Subjects were instructed to stand beside a whiteboard in a relaxed position with their investigated arm relaxed (extended position). At this time, an experienced investigator marked the locations of shoulder (Acromion), elbows (Olecranon) and wrist (Styloid process) on the whiteboard, and measured the resultant angles using a goniometer. The subject then flexed his forearm while the elbow and shoulder joints were kept constant with the assistance of another investigator. The new position of wrist was marked again on the whiteboard (flexed position) and the angle was measured. The difference between extended and flexed positions was taken into account as ROM. This was repeated 3-times and the average of the 3 ROM was used for statistical analyses.
Arm circumference (CIR): CIR was measured at 4, 6, 8, and 10-cm above the elbow joint, while allowing the arm to hang down by the side. The average of the three trials for ROM and CIR was used for statistical analyses.
Perception of muscle soreness (SOR): A subjective rating of SOR was performed during each session using a 7-point categorical scale, where 1 corresponded to “no pain ”and 7 to “very, very painful”. While standing, the subjects were instructed to palpate their upper arm during full range of motion biceps curls and then choose the number that corresponded to their perceived level of soreness (Sayers et al., 2000).
Exercising assessments
After subject placement, equipment set up and resting measurements (described above) were effected, the following exercise assessments were performed.
Isometric maximal voluntary contraction torque (MVT) was assessed on the Exercise and Control limbs with the subject’s elbow joint set to 90° and shoulder flexed at 45° using an isokinetic strength-assessment apparatus (Biodex System 2, Biodex, USA). Three 5-s repetitions were performed with 2-min of recovery between each maximal effort. The highest value was taken to represent the 100% MVT, and was employed for statistical analyses.
After a further 5-min of recovery, isometric elbow flexions were performed on the Exercise and Control limbs at 50% of MVT (IC50). The highest MVT from the first day before EE was used to set IC50 for all subsequent sessions. The rationale for the IC50 test was to set a constant muscle contraction force for all days before and after the EE stimulus. The 50% level was chosen after pilot testing to achieve a force that all subjects would be able to achieve after EE. During each session, subjects performed two isometric contractions at IC50, each lasting 20-s with 3-min of recovery between efforts.
Isometric elbow flexions were also assessed on the Exercise and Control arms at 80% of the MVT recorded for that particular session (IC80). The torque values for IC80, therefore, varied amongst days, depending on the MVT achieved for that day. Our rationale for the IC80 test was to assess EMG at a consistent level of effort before and after eccentric exercise-evoking muscle damage. Two contractions were performed at IC80 of equal duration and recovery intervals as for IC50. Participants could observe their effort to reach and maintain the average exercise intensity requested by the investigator (50%, 80%, or 100% of MVT).
IC50 and IC80 assessments were performed every day. On the first day only, an average of 10-min after the last IC80, subjects performed EE contractions (as described in ‘EE protocol’). Then, subjects were rested in a comfortable position for 30-min and the pre-EE assessments repeated. The experimental protocol measured one arm at a time and subjects were unaware which was going to be assessed first. However, on day one after the pre-EE assessments on Control and Exercise limbs, subjects performed eccentric contractions with their Exercise arm, then after 30-min passive rest, their Exercise and Control arms were assessed, respectively (post-EE assessments). The experimental sessions were set at the same period every day (in the mornings) for each subject, and room temperature was set between 23°C to 25°C for all subjects.
Electromyography
EMG signals were recorded from the biceps brachii muscle. The skin was prepared by shaving, abrading, and cleaning the recording area with alcohol. Bipolar electrodes (9-mm, square shape, Tyco Health Care, H49P Cloth Solid Gel ELEC C450) were fastened over the belly of the biceps brachii muscle, parallel to fibres, with a centre to centre distance of 35-mm. A passive reference electrode was placed on the dorsal surface of the wrist. Electrolyte gel was used to improve signal conduction between the skin and the electrodes. The electrode positions were selected and marked with a semi-permanent marker to assure standardized measurements from day to day. Electrode placement was preceded by abrasion of the skin to reduce the source impedance to <5 kΩ.
EMG was recorded using a Medelec Amplifier (MS6, input impedance of 500-MΩ// 30-pf, common mode rejection ratio of greater than 10,000:1 at 50-Hz). The raw signal was filtered (10-Hz to 1-kHz), monitored on a digital oscilloscope and digitized at 1000 Hz using a 12-bit analogue-to-digital (A/D) converter on a computer. Gain was adjusted to maximize resolution.
During each 20-s isometric contraction, the participants were asked to reach the required percentage of MVT in less than 2-s and maintain it for 18-s using real-time feedback displayed on a computer monitor. RMS and MDF are the two EMG-derived variables that have been used frequently in previous EMG studies (Felici et al., 1997; Linnamo et al., 2000; Hermann and Barnes, 2001; McHugh et al., 2001). Using purpose-built software (Bioproc2, Robinson G., University of Ottawa, Canada), RMS and MDF values were extracted at time epochs of 1-s.
Statistical analysis
For the EMG data, linear regression analyses of the time course of RMS and MDF (RMS and MDF against 1-s time epochs) were performed on the collected data (Merletti et al., 1990; Felici et al., 1997). To omit on- and off-transient phenomena associated with muscular exertion, the first and last 2-s of every contraction were discarded; therefore for each trial, there were 16-s of isometric effort (Figure 1). The axis intercepts (β0) and slopes (β1) of regression lines for RMS and MDF were used for statistical analyses. Prior to statistical analyses of RMS and MDF intercepts and slopes, a Chi-Square established that these were significantly different from cipher. Axis intercepts were employed as an index of muscle activation, while slopes represented the rate of fatigue in exercising muscles. The axis intercepts were assumed to be the indicative initial state of muscle activation, but because the first 2-s of each contraction had been discarded, this might slightly underestimate the “true ”intercept (Merletti et al., 1990).
Figure 1.

Sample plot of MDF vs. time during IC50 for Exercised arm before EE. The open (○) and filled (●) symbols denote the two muscle contractions performed. Also shown is the linear regression line for both contractions, combined.
A t-test was performed to test the null hypothesis of similarity of linear regression coefficients (β0, β1) for the 2 trials of IC50 and IC80 within each session. Figure 1 shows an example of such a test for two trials on the same arm. As there were no significant differences between trials, data from both trials were combined for subsequent analyses.
A two-way analysis of variance (ANOVA) with repeated measures was used to test the main effect of arm (Control, Exercise) by time (day one to day seven) for all variables except for CK activity. When a significant arm-by-time interaction effect was observed, univariate ANOVA was performed for each arm. For CK, since blood was drawn only from one arm, a separate univariate ANOVA was performed without the arm main effect. We used SPSS (version 14) for the statistical analyses and statistical significance for a meaningful change was set at the 95% confidence level (p < 0.05). Values reported as mean ± the standard error of means (SE).
Results
Significant arm-by-day interaction effects were observed for MVT, ROM, CIR (at 6, 8 and 10 cm above elbow), and SOR (active, passive, flexed and extended). Therefore, further statistical analyses were performed on each arm separately, and these revealed that in the Exercised arm, there was a significant decrease in MVT immediately after EE (Figure 2), that remained lower than initial values for the following 5 days. ROM also decreased after EE and gradually returned towards initial levels over the following 5 days (Table 1). CIR significantly increased at all four measurement locations on the day two, and over the following 6 days (Table 1). Similarly, SOR increased after EE, and remained higher than pre-EE over the next 5 days (Table 1). CK also increased significantly after EE, and it remained higher than the initial values for the following days (Figure 3).
Figure 2.

MVT before and after EE sessions for Control (●) and Exercised (□) arms. On the X-axis, 1 PRE refers to the first session (before EE) and 1 POST refers to the session immediately after EE on day 1. Data are mean ± SE.
* denotes a value significantly different from Day 1 before EE (1-PRE), p < 0.05.
Table 1.
Summary of some physical signs and EMG parameters for the exercised arm. Data are means (±SE).
| DAY 1 PRE | DAY 1 POST | DAY 2 | DAY 3 | DAY 4 | DAY 5 | DAY 6 | DAY 7 | |
|---|---|---|---|---|---|---|---|---|
| ROM (deg) † | 156 (3) | 147 (4) * | 147 (4)* | 148 (4) | 149 (4)* | 151 (3) | 152 (3) * | 153 (3) |
| CIR (cm) † | 25.3 (.6) | 25.6 (.5) | 25.8 (.6)* | 25.9 (.6)* | 25.8 (.6)* | 26.1 (.5)* | 26.1 (.6) * | 25.9 (.6)* |
| SOR † | 1.0 (.0) | 1.7 (.3)* | 3.0 (.3)* | 3.4 (.5) * | 3.0 (.4)* | 2.4 (.4)* | 1.7 (.3) * | 1.5 (.2) |
| MDF IC50 slope (β1; Hz·s-1) | -.55 (.06) | -.62 (.08) | -.81 (.01) | -.57 (.08) | -.41 (.11) | -.42 (.08) | -.45 (.08) | -.59 (.04) |
| MDF IC80 slope (β1; Hz·s-1) | -.90 (.16) | -.54 (.09)* | -.89 (.10) | -.68 (.15) | -.65 (.11) | -.57 (.09) | -.69 (.10) | -.72 (.11) |
| RMS IC50 slope (β1) | 011 (.005) | .013 (.005) | .005 (.004)* | .002 (.003)* | -.003 (.004) | -.001 (.002)* | .004 (.002) | .002 (.002)* |
| RMS IC80 slope (β1) | .017 (.009) | .004 (.005) | .001 (.002) | .008 (.005) | .010 (.004) | .004 (.005) | -.001 (.006) | .005 (.004) |
Note: SOR refers to each subject’s perception of muscle soreness while arm was actively extended. ROM refers to active elbow range of motion. CIR refers to arm circumference at 8-cm above elbow. CIR at all anthropometrical landmarks (e.g. 4, 6, and 10-cm above elbow) followed a similar pattern, therefore, data was only presented from CIR at 8-cm. MDF refers to EMG median frequency. Derivation of regression intercept (β0) and slope (β1) are described in the text.
† indicates a significant interaction between main effects of arms × time (days);
* denotes significant (p<0.05) differences between day 1 pre-EE and other days.
Figure 3.
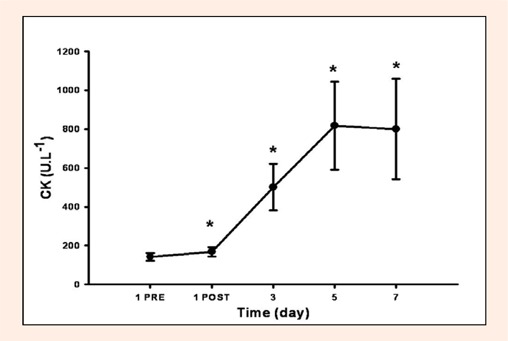
Blood-borne CK before and after EE session on Day 1. On the X-axis, 1 PRE refers to the first session (before EE) and 1 POST refers to the session immediately after EE on day 1. Data are mean ± SE.
* denotes a value significantly different from Day 1 before EE (1-PRE), p < 0.05.
During IC50, MDF intercept decreased significantly after acute EE, and was significantly lower than pre-EE values on day 3, but gradually returned towards the initial values over the next 2 days (Figure 4). No significant arm-by-day interaction effects were observed for MDF slope (Table 1), RMS intercept (Figure 5) and RMS slope (Table 1) during IC50.
Figure 4.

MDF intercept coefficient (β0) on different days at IC50 (lower panel) and IC80 (upper panel) for Control (●) and Exercised (□) arms, respectively. Derivation of β0 is described in the text. On the X-axis, 1 PRE refers to the first session (before EE) and 1 POST refers to the session immediately after EE on day 1. Data are mean ± SE.
* denotes a value significantly different from Day 1 before EE (1-PRE), p < 0.05.
Figure 5.

RMS intercept coefficient (β0) obtained on different days at IC50 (lower panel) and IC80 (upper panel) for Control (●) and Exercised (□) arms, respectively. Derivation of β0 is described in the text. On the X-axis, 1 PRE refers to the first session (before EE) and 1 POST refers to the session immediately after EE on day 1. Data are mean ± SE.
* denotes a value significantly different from Day 1 before EE (1-PRE), p < 0.05.
There were significant arm-by-day interaction effect for MDF and RMS intercepts at IC80. Subsequently, a univariate ANOVA showed a significant decrease in MDF intercept after acute EE within the Exercised arm, which recovered afterwards (Figure 4). Although there was a significant arm-by-day interaction for RMS slope, the pattern of change was not consistent for either arm (Figure 5). No significant changes were observed for MDF and RMS slopes at IC80 (Table 1).
Discussion
The purpose of this study was to investigate the possible physiological changes within muscle assessed via surface electromyography after a session of heavy EE. The unique finding of this investigation was that EE-induced muscle damage revealed some significant alterations in surface EMG for up to seven days after exercise. Prolonged and significant decreases in MVT and ROM, and increases in CIR, SOR and CK were consistent with exercise-induced muscle damage. However, it is worthy to note that our perception of muscle damage was based on indirect measures such as CK, MVT, or physical signs, which are not categorical methods of determining muscle damage (e.g. muscle needle biopsy). Therefore, we have employed the term “muscle damage ”with some caution.
EMG activity after EE
In the present study, to track EMG parameters obtained day-to-day over seven days after EE, subjects were asked to perform isometric contractions at 50% of their MVT measured on the very first session (e.g. day 1 before-EE). That is, subjects were required to apply the same absolute amount of force to perform IC50 on every day of the 7-day trial. However, to elicit a higher level of muscle fibre recruitment, subjects also performed isometric contractions at 80% of their MVT obtained on each particular day. Therefore, the amounts of force to perform IC80 were different between sessions depending on subjects’ MVT during that session. We selected this approach to document possible changes of RMS and MDF at a constant level of force, as well as at a consistent level of effort. In this study, we did not normalize our EMG data based on daily EMG measures, because to do so would eliminate any changes that occurred between days. It is interesting to note that the 50% and 80% of MVT on days 2 and 3 often evoked similar isometric forces, because the subjects’ maximum force declined significantly on those days. With the above approach in mind, when we aimed to compare the day-to-day changes in EMG, IC50 might be relevant to describing muscle physiological responses at the same absolute levels of torque production. But, when wishing to study responses of a constant level of effort, IC80 might provide the best way to document muscle physiological adaptations during post-EE recovery.
Although our findings showed significant arm-by-day interaction in RMS regression intercepts during IC80 (Figure 5), the overall changes did not follow a consistent trend or pattern. Similarly, we did not observe a consistent pattern of changes in RMS intercept and slope at IC50 or in RMS slope at IC80 (Table 1). Therefore, it seemed that RMS did not provide reliable information about muscle recovery after damage. RMS is more susceptible to the day-to-day changes compared to MDF (Felici et al., 1997; Merletti et al., 1995). These results supported the findings of other authors who either did not find any significant changes in RMS after EE (Sayers et al., 2001), or their RMS data was not statistically linear, and they did not perform further statistical analyses on RMS (Felici et al., 1997).
MDF linear regression intercept decreased significantly after acute EE during both IC50 and IC80 within the Exercised arm and was also less than pre-EE on day 3 during IC50 (Figure 4). Although MDF had recovered by day 2 during IC80, there was a general trend of decrements over the next 5 days for this variable. This was also the case for MDF intercept at IC50 (Figure 4). These findings were in accord with the findings of some studies (Day et al., 1998; Felici et al., 1997), but not in line with the results of others (Berry et al., 1990; Komi and Viitasalo, 1977; McHugh et al., 2000). McHugh and co-workers (2000) reported that median frequency did not change after EE. Berry et al., 1990 did not observe any significant change in mean frequency after EE.
The possible reason for a decline in MDF after EE might be explained on a physiological basis. MDF represents information about conduction velocity of muscle fibres, the shape of motor unit action potentials, the mean firing rate of the individual motor units, the recruitment of motor units and the extent of superposition of action potentials from concurrently active motor units (Felici et al., 1997). Muscle fibre conduction velocity is higher for fast-twitch fibres (Andearssen and Arendt-Nielsen, 1987), which means that when fast twitch fibres are more active the MDF value will be higher. Friden et al., 1983 found that fast twitch fibres showed significant disruption at the myofibrillar Z-band after EE compared to the other types of muscle fibres (Friden et al., 1983). Therefore, fast twitch fibres are more susceptible to damage and fatigue (Berry et al., 1990), and consequently a shift towards greater recruitment of slow twitch motor units might be anticipated in order to decrease the stress on the susceptible fast-twitch fibres (McHugh et al., 2001). Therefore, a decrease in MDF could be the result of a preferential reduction in the recruitment of fast-twitch fibres. On the other hand, the changes in intra muscular pressure, as well as the changes in water content and blood volume of the muscle could have affected the EMG findings. Blood flow can affect characteristics of surface-recorded signals by imposing a low-pass filter medium. This tissue filtering can decrease the frequency content of the signal (Kamen and Caldwell, 1996). Additionally, an increase in blood flow generally increases local temperature, which can change spectral features of the EMG signals (Holewijn and Heus, 1992).
Some of the reasons for the dissimilar outcomes of our study compared to previous investigations might include dissimilar methodologies and different muscle groups that were employed to study the EE-induce muscle damage. In addition, different methods have been used to quantify EMG activity. For example, McHugh et al., 2000 obtained their MDF data from an MVT test, while MDF in the current study was derived from isometric contractions at pre-set percentages of subjects’ MVT. In voluntary exercise, e.g. where a MVT is performed, there is always some variation in the instantaneous force due to motivation and other factors. This may consequently increase the variance in EMG data and mask the effect of EE on EMG signal. Therefore, some changes in the EMG parameters during maximal contractions could be attributed to factors such as motivation (Linnamo et al., 2000). By obtaining the EMG data from IC50, which was a constant level of force based on the MVT of day 1, we minimized the effect of subject’s motivation on EMG acquisition. The EMG power spectrum has been shown to be reliable for measurements during isometric contractions with a given intensity, repeated over separate days (Linnamo et al., 2000). Additionally, the magnitude of muscle damage might have been relatively less in McHugh et al., 2000 compared to our study. Although they did not assess CK, the percentage of decrease in muscle strength after EE in the McHugh and colleagues’ (2000) study (10%) was less than ours (45%). A lower reduction in MVT (and probably a less magnitude of muscle damage) could be due to the lower level (60% of MVT) of EE intensity that they employed to induce muscle damage, compared to our study (on average 100% MVT). Further more, McHugh and colleagues (2000) assessed the myoelectric activity of hamstrings. Their subjects, therefore, sat on the EMG electrodes during the tests. Sitting on the electrodes during hamstring contractions might have changed the orientation of the electrodes to the motor point of the respective muscles (McHugh, 2000)
One of the methodological differences that could be observed between our study and that of Berry and co-workers (1990), is that their group employed mean frequency, resulting from 10 subsequent samples. Averaging the mean frequency values possibly smoothed their results. Besides, Berry et al., 1990 performed their EMG assessments while their subjects lifting their own legs off the ground. Although, the same assessments were performed before and after EE, it is not clear that this leg-lifting was equivalent to any known percentage of MVT. However, one can assume that the leg -lifting exercise would require a force level of much lower than 50% of MVT. The myoelectrical behaviour of muscles could be different during low vs. high intensity contractions (Felici, 1997). In other words, the higher force produced during EMG acquisition in our study compared to that of Berry et al., 1990, probably better revealed any physiological changes within the muscle.
In this study, we observed that MDF decreased over time during sustained isometric contractions (Figure 1). These decrements, which were shown as MDF slopes, were present in both Control and Exercised arms and at both intensities (IC50 and IC80). However, there were not any significant day-to-day changes amongst the slopes obtained from different arms and different intensities (Table 1). This suggests that in a sustained situation such as a 20-s isometric contraction, the rate of decrease in MDF, which could be also assumed as a rate of fatigue, was independent of EE-induced muscle damage. A possible mechanism for the decrease in MDF during prolonged contractions is the external accumulation of potassium ions (Mills and Edwards, 1984). An outward leakage of potassium resulting in an ionic imbalance around sarcolemma might slow the action potential and consequently decrease MDF (Day et al., 1998).
Kroon and Naije (1991) observed a significant increase in the slope of mean power frequency immediately after EE, which recovered gradually within the consequent few days. Although our results followed a similar pattern to those of Kroon and Naije (1991), the changes in regression slope coefficient observed in our study were not statistically significant (Table 1). The reasons for this disparity of findings are not clear. Kroon and Naije (1991) recruited five subjects, which is a relatively small group compared to our cohort (n = 10). This might have induced larger inter-subject variations in EMG parameters. They did not delete any of the EMG data obtained during isometric contractions, while we deleted the first and the last 2-s to skip the transition phenomenon that could affect the EMG outcomes. Finally, their subjects performed different numbers of eccentric contractions at 40% of their MVT before they become exhausted, while in our study, the subjects performed a constant number of contractions (2 sets of 35) at 100% MVT (on average). A lower EE intensity could, therefore, result in a lesser magnitude of muscle damage, and this might have affected the fibre recruitment and consequently the EMG signals after EE.
Although, our findings showed significant arm-by-day interactions for RMS regression intercepts during IC80 (Figure 5), the overall changes did not follow a consistent trend or pattern. Similarly, we did not observe a consistent pattern of changes in RMS intercept and slope at IC50, as well as RMS slope at IC80. Therefore, it seems that RMS does not provide reliable information about muscle recovery after muscle damage. These results supported the findings of other authors who either did not find any significant changes in RMS after EE (Sayers et al., 2001), or their RMS data was not statistically linear, and they did not perform further statistical analyses on RMS (Felici et al., 1997). However, our findings were not in line with the increased RMS observed by Berry et al., 1990 and Kroon and Naije (1991) after EE. The added variance introduced by electrode repositioning influences amplitude more than frequency parameters such as MDF (Merletti et al., 1995; Felici et al., 1997). This could result in observing different findings among different studies.
Study limitation
Because of its anatomical position, we monitored biceps brachii as the only elbow flexor in this study. There are some other elbow flexors that take part in elbow flexion. For example, as compared to biceps brachii, the brachialis muscle has a larger cross sectional area and is the prime elbow flexor. Therefore, it is possible that the functional responsibilities of the elbow flexors have altered during isometric contractions performed in this study, and this might have a confounding effect on our findings.
Conclusion
We found a significant decrease in MDF during 50% and 80% of subject’s MVT after a session of EE that did not fully recover to pre-exercise values up to 3 days after EE. This decrease could be related to a reduction in the recruitment of fast twitch fibres due to damage to these fibres. We also observed that compared to RMS, MDF was a more consistent parameter to reflect the changes in EMG during recovery from muscle damage.
Acknowledgements
Authors are grateful to Mr. Ray Patton, Dr. Pat Ruell and Dr. Ché Fornusek for their technical support. Mr.Sirous Ahmadi is sponsored by the Iranian Ministry of Science, Research and Technology. Partial research funding was provided by a New South Wales Office of Science and Medical Research Program Grant.
Biographies

Sirous AHMADI
Employment
Rehabilitation Research Centre, Discipline of Exercise and Sports Science, Faculty of Health Sciences, University of Sydney.
Degree
BS, MA, PhD student
Research interests
Exercise-induced muscle damage, Muscle oxygenation and blood flow.
E-mail: Sahm8027@mail.usyd.edu.au

Peter J. SINCLAIR
Employment
Lecturer, Sport Knowledge Australia,Discipline of Exercise and Sports Science, Faculty of Health Sciences, University of Sydney.
Degree
PhD
Research interests
Computer modelling and biomechanics of sport.
E-mail: p.sinclair@ usyd.edu.au

Nasim FOROUGHI
Employment
Discipline of Exercise and Sports Science, Faculty of Health Sciences, University of Sydney.
Degree
BS, MA, PhD student
Research interests
Sports and clinical biomechanics, knee osteoarthritis.
E-mail: nfor3501@mail.usyd.edu.au

Glen M. DAVIS
Employment
Associate Professor, Rehabilitation Research Centre, Discipline of Exercise and Sports Science, Faculty of Health Sciences, University of Sydney.
Degree
PhD
Research interests
Exercise therapy and assistive technologies for populations of chronic disease and disability.
E-mail: G.Davis@usyd.edu.au
References
- Andearssen S., Arendt-Nielsen L. (1987) Muscle fibre conduction velocity in motor units of the human anterior tibial muscle: a new size principle parameter. Journal of Physiology 391, 561-571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C., Moritani T., Tolson H. (1990) Electrical activity and soreness in muscle after exercise. American Journal of Physiology, Medicine and Rehabilitation 69, 60-66 [DOI] [PubMed] [Google Scholar]
- Chen T.C. (2003) Effects of a second bout of maximal eccentric exercise on muscle damage and electromyographic activity. European Journal of Applied Physiology 89(2), 115-121 [DOI] [PubMed] [Google Scholar]
- Clarkson P., Hubal M. (2002) Exercise-induced muscle damage in humans. American Journal of Physiology, Medicine and Rehabilitation 81(Suppl. 11), S52-69 [DOI] [PubMed] [Google Scholar]
- Clarkson P., Nosaka K., Braun B. (1992) Muscle function after exercise-induced muscle damage and rapid adaptation. Medicine & Science in Sports & Exercise 24, 512-520 [PubMed] [Google Scholar]
- Clarkson P., Sayers S. (1999) Etiology of exercise-induced muscle damage. Canadian Journal of Applied Physiology 24, 234-248 [DOI] [PubMed] [Google Scholar]
- Day S.H., Donnelly A.E., Brown S.J., Child R.B. (1998) Electromyogram activity and mean power frequency in exercise-damaged human muscle. Muscle and Nerve 21, 961-963 [DOI] [PubMed] [Google Scholar]
- Felici F., Colace L., Sbriccoli P. (1997) Surface EMG modifications after eccentric exercise. Journal of Electromyography and Kinesiology 7(3), 193-202 [DOI] [PubMed] [Google Scholar]
- Friden J., Sjostrom M., Ekblom B. (1983) Myofibrillar damage following intense eccentric exercise in man. International Jounral of Sports Medicine 4, 170-176 [DOI] [PubMed] [Google Scholar]
- Hermann K.M., Barnes W.S. (2001) Effects of eccentric exercise on trunk extensor torque and lumbar paraspinal EMG. Medicine & Science in Sports & Exercise 33(6), 971-977 [DOI] [PubMed] [Google Scholar]
- Holewijn M., Heus R. (1992) Effects of temperature on electromyogram and muscle function. European Journal of Applied Physiology 65, 541-545 [DOI] [PubMed] [Google Scholar]
- Kamen G., Caldwell G.E. (1996) Physiology and interpretation of the electromyogram. Journal of Clinical Neurophysiology 13(5), 366-384 [DOI] [PubMed] [Google Scholar]
- Komi P., Viitasalo J. (1977) Changes in motor unit activity and metabolism in human skeletal muscle during and after repeated eccentric and concentric contractions. Acta Physiologica Scandinavica 100(2), 246-254 [DOI] [PubMed] [Google Scholar]
- Kroon G.W., Naieje M. (1991) Recovery of the human biceps electromyogram after heavy eccentric, concentric or isometric exercise. European Journal of Applied Physiology and Occupa-tional Physiology 63(6), 444-448 [DOI] [PubMed] [Google Scholar]
- Lieber R., Friden J. (2002) Mechanisms of muscle injury gleaned from animal models. American Journal of Physiology, Medicine and Rehabilitation 81(Suppl), S70-S79 [DOI] [PubMed] [Google Scholar]
- Linnamo V., Bottas R., Komi P.V. (2000) Force and EMG power spectrum during and after eccentric and concentric fatigue. Journal of Electromyography & Kinesiology 10(5), 293-300 [DOI] [PubMed] [Google Scholar]
- McHugh M.P., Connolly D.A., Eston Rg., Gartman Ej, Gleim Gw. (2001) Electromyographic analysis of repeated bouts of eccentric exercise. Journal of Sports Sciences 19(3), 163-170 [DOI] [PubMed] [Google Scholar]
- McHugh M.P., Connolly D.A., Eston R.G., Gleim G.W. (2000) Electromyographic analysis of exercise resulting in symptoms of muscle damage. Journal of Sports Sciences 18(3), 163-172 [DOI] [PubMed] [Google Scholar]
- Merletti R., Knaflitz M.A., De Luca C. (1990) Myoelectric manifestations of fatigue in voluntary and electrically elicited contractions. Journal of Applied Physiology 69, 1810-1820 [DOI] [PubMed] [Google Scholar]
- Merletti R., Lo Conte L., Sathyan D. (1995) Repeatability of Electrically-evoked Myoelectric Signals in the Human Tibialis Anterior Muscle. Jounral of Electromyography and Kinesiology, 5(2), 67-80 [DOI] [PubMed] [Google Scholar]
- Mills K., Edwards R. (1984) Muscle fatigue in myophosphorylase deficiency: power spectral analysis of the electromyogram. Electroencephalography & Clinical Neurophysiology 57(4), 330-335 [DOI] [PubMed] [Google Scholar]
- Miura H., Araki H., Matoba H., Kitagawa K. (2000) Relationship among oxygenation, myoelectric activity, and lactic acid accumulation in vastus lateralis muscle during exercise with constant work rate. International Journal of Sports Medicine 21(3), 180-184 [DOI] [PubMed] [Google Scholar]
- O’Reilly K., Warhol M., Fielding R., Frontera W., Meredith C., Evans W. (1987). Eccentric exercise-induced muscle damage impairs muscle glycogen repletion. Journal of Applied Physiology 63, 252-256 [DOI] [PubMed] [Google Scholar]
- Sayers S., Clarkson P., Lee J. (2000) Activity and immobilization after eccentric exercise: I. Recovery of muscle function. Medicine & Science in Sports & Exercise 32(9), 1587-1592 [DOI] [PubMed] [Google Scholar]
- Sayers S.P., Knight C.A., Clarkson P.M., Van Wegen E.H., Kamen G. (2001) Effect of ketoprofen on muscle function and sEMG activity after eccentric exercise. Medicine & Science in Sports & Exercise 33(5), 702-710 [DOI] [PubMed] [Google Scholar]
- Sbriccoli P., Felici F., Rosponi A., Aliotta A., Castellano V., Mazza C., Bernardi M., Marchetti M. (2001) Exercise induced muscle damage and recovery assessed by means of linear and non-linear sEMG analysis and ultrasonography. Journal of Electromyography & Kinesiology 11(2), 73-83 [DOI] [PubMed] [Google Scholar]
- Stauber W.T., Clarkson P.M., Fritz V.K., Evans W.J. (1990) Extracellular matrix disruption and pain after eccentric muscle action. Journal of Applied Physiology 69(3), 868-874 [DOI] [PubMed] [Google Scholar]


