Abstract
Studies employing modified Biering-Sørenson tests have reported that low back endurance is related to the potential for developing low back pain. Understanding the manner in which spinal musculature fatigues in people with and without LBP is necessary to gain insight into the sensitivity of the modified Biering-Sørenson test to differentiate back health. Twenty male volunteers were divided into a LBP group of subjects with current subacute or a history of LBP that limited their activity (n = 10) and a control group (n = 10). The median frequency of the fast Fourier transform was calculated from bilateral surface electromyography (EMG) of the upper lumbar erector spinae (ULES), lower lumbar erector spinae (LLES) and biceps femoris while maintaining a prescribed modified Biering-Sørensen test position and exerting isometric forces equivalent to 100, 120, 140 and 160% of the estimated mass of the head-arms-trunk (HAT) segment. Time to failure was also investigated across the percentages of HAT. Fatigue time decreased with increasing load and differences between groups increased as load increased, however these differences were not significant. Significant differences in the EMG median frequency between groups occurred in the right biceps femoris (p ≤ 0.05) with significant pairwise differences occurring at 140% for the left biceps femoris and at 160% for the right biceps femoris. There were significant pairwise differences at 120% for average EMG of the right biceps femoris and at 140% for the right ULES, and right and left biceps femoris (p ≤ 0.05). The modified Biering-Sørensen test as usually performed at 100% HAT is not sufficient to demonstrate significant differences between controls and subjects with varying degrees of mild back disability based on the Oswestry classification.
Key points.
The results do not wholly support the modified Biering-Sørensen test utilizing resistance of 100% HAT to discern differences in fatigue in subjects with mild low back pain.
A greater activation of the biceps femoris by low back pain individuals probably contributed to the lack of significant differences in back fatigue times.
The possibility exists that subjects with more sophisticated strategies could yield higher fatigue times despite inferior neuromuscular fatigue and the existence of low back pain.
Key words: Endurance, electromyography, median frequency, back muscles, healthy subjects
Introduction
Poor neuromuscular endurance of low back musculature has been related to the potential for developing low back pain (Alaranta et al., 1995; Biering-Sørensen, 1984; Hultman et al., 1993; Mayer et al., 1995; Nelson et al., 1995; Smidt et al., 1983). Additionally, decreased trunk strength and endurance associated with a cyclical pattern of deconditioning through pain, avoidance and inactivity are noted as defining characteristics (Biering-Sørensen, 1984; Mayer and Gatchel, 1988).
There are numerous potential risk factors for developing back pain including poor back extensor endurance (Canadian Society for Exercise Physiology-CSEP, 2004) and identifying potential risk factors, such as poor lumbar extensor endurance may be important. The most widely reported fatigue test in the literature is the Biering-Sørensen test (Moreau et al., 2001). A modified Biering-Sørensen test to measure back fatigue is currently in use by the Canadian Society for Exercise Physiology in their Canadian Physical Fitness and Lifestyle Approach (CPAFLA) testing (CSEP, 2004).
Administration of the Biering-Sørensen test is inconsistently practiced in the literature, including differences in arm position, number of straps (or no straps) and conclusion criteria. These variations have been grouped together as modified Biering-Sørensen tests (Moreau et al., 2001). This test is generally considered safe for both healthy and clinical populations (Alaranta et al., 1994; 1995; Biering-Sørensen, 1984; Moffroid, 1997; Nordin et al., 1987; Mannion and Dolan, 1994; Peltonen et al., 1998). While forces required to maintain a horizontal position are well below forces of maximal voluntary isometric activations (MVIA) in healthy populations (Jørgensen and Nicolaisen, 1986; Mayer et al., 1995; Moffroid et al., 1993), they may rise to as much as 85% of a MVIA in a patient with chronic low back pain (Hultman et al., 1993). It has been suggested that performance of maximal activations in patients with low back pain could compromise safety (Moffroid et al., 1993). There is considerable range of mean fatigue times reported for the Biering-Sørensen test in the literature ranging from 84s to 180s in healthy males (Biering-Sørensen, 1984; Jørgensen and Nicolaisen, 1986; 1987; Hultman et al., 1993; Kankaanpaa et al., 1998a; Mannion and Dolan, 1994; Nicolaisen and Jørgensen, 1985; Sparto et al., 1997) and 80s-194s for males with low back pain (Biering-Sørensen, 1984; Jørgensen and Nicolaisen, 1987; Hultman et al., 1993; Nicolaisen and Jørgensen, 1985). The wide range of fatigue times may be related to the variety of modified protocols implemented in these studies as well as the degree of low back disability between individuals. Fatigue has been defined as a transient decrease in working capacity (Asmussen, 1979), loss of force output leading to reduced performance (Fitts and Metzger, 1993) or a decline in the force-generating capacity of the muscle (Degens and Veerkamp, 1994). Fatigue also may be experienced during prolonged submaximal intensity contractions without an apparent decrement in the targeted force. This type of fatigue may be defined as an acute impairment of performance that includes an increase in the perceived effort necessary to exert a desired force and an eventual inability to produce this force (Enoka and Stuart, 1992). All these definitions imply that the effects of fatigue can contribute to the risk factors associated with low back pain. The modified Biering-Sørensen test as employed by the Canadian Society for Exercise Physiology, Canadian Physical Fitness and Lifestyle Approach testing attempts to ensure standardization of testing and thus a valid assessment of back health (Albert et al., 2001).
Although the modified Biering-Sørensen test is generally considered a measure of low back function measuring overall lower back fatigue, activity of the biceps femoris and hip extensors have been argued to substantially contribute to fatigue times (Kankaanpaa et al., 1998B). Significant correlation has been observed between Biering-Sørensen fatigue times and EMG median frequency slopes of the biceps femoris (Moffroid et al., 1994; Moffroid, 1997). It would seem that more than just the erector spinae are involved in back fatigue.
Ng et al. , 1997 demonstrated that the multifidus has more activity than the iliocostalis lumborum during Biering- Sørensen testing. The multifidus fatigues at a faster rate than the iliocostalis lumborum during this test demonstrating a higher initial median frequency and normalized median frequency slope (Ng et al., 1997). Ng and Richardson, 1996 suggests that the modified Biering-Sørensen test with the use of EMG power spectral analysis may be a reliable method to measure the fatigue rate of the back muscles if cross-talk is minimized and adds that measuring the fatigue rate of the multifidus may be a useful clinical measure. Van Diėėn et al. (1993) observed that the multifidus muscle at the L5 level appeared to show the most consistent changes of the EMG power spectrum as a consequence of fatigue.
Maintaining a horizontal position during Biering-Sørensen test (referred to in this study as 100% of the head, arms and trunk {HAT} segments) results in higher fatigue times than at higher levels of resistance (Moffroid et al., 1993). With increased fatigue times, motivation, pain levels, and alternative muscle control strategies may play a larger role. According to the Canadian Society for Exercise Physiology, Canadian Physical Fitness and Lifestyle Approach manual (2004), the back extensor endurance test with the HAT as the resistance (modified Biering-Sørensen test) has been reported as a valid and reliable assessment of back extensor endurance, and found to be positively related to back health. The Canadian Society for Exercise Physiology, Canadian Physical Fitness and Lifestyle Approach manual (2004) indicates that this finding supports the use of back extensor endurance with HAT to differentiate levels of back health.
The purpose of this paper was to compare trunk and hamstrings muscle activity in subjects with different degrees of back health (low back pain and no low back pain) and to investigate the effects of different percentages of HAT resistance added to the Canadian Society for Exercise Physiology modified Biering-Sørensen test for time to fatigue, median frequency and EMG.
Methods
Subjects
Twenty male volunteer subjects were recruited from the university population. These subjects were grouped into low back group (n = 10) and control groups (n = 10). Subjects were included in the low back pain group based on a self report of currently having low back pain or having a history of chronic or recurrent low back pain that limited activity. One of the researchers was a certified and practicing chiropractor who examined the subjects to ensure there was some degree of disability or pain and that conversely the controls did not have significant disability or pain. All subjects completed an Oswestry Low Back Pain Disability Questionnaire (Fairbank et al., 1980; Thomas et al., 1989) as well as a numeric pain scale. Subjects in the low back pain group had a mean age of 29.1 years (± 8.2) and mean mass of 79.7 kg (± 11.2) as compared to 24.7 years (± 2.9) and 81.9 kg (± 7.8) for controls. Table 1 reports subject characteristics and mean scores of the Oswestry Disability Index and 0-10 Pain scale. Oswestry Low Back Disability Index scores were 72% lower and pain scores 96% lower in the Control group than low back pain group. The low back pain group had an Oswestry mean score of 18.3% (± 11.8), which is clinically categorized as “mild disability ”as compared to control group that had an Oswestry of 5.1% (± 5.5), which is also considered “mild disability”. The mean pain score from the low back pain group was 3.43 (± 2.0) as compared to that of 0.1 (± 0.4) for controls. Using the Mann-Whittney Test, significant differences (p = 0.007) were found between Oswestry Low Back Disability Index scores between low back pain and Control groups and significant differences (p ≤ 0.001) in pain levels between low back pain and Control groups.
Table 1.
Comparison of p values for median frequency between low back pain (LBP) and controls at each percentage of HAT (head, arms, trunk segment).
| %HAT | LBP vs. Control | Between groups at each %HAT | |||
|---|---|---|---|---|---|
| 100% | 120% | 140% | 160% | ||
| Left ULES | .176 | .615 | .233 | .101 | .236 |
| Right ULES | .267 | .463 | .147 | .086 | .287 |
| Left LLES | .453 | .850 | .968 | .090 | .402 |
| Right LLES | .685 | .867 | .708 | .160 | .540 |
| Left biceps femoris | .132 | .631 | .099 | .037* | .415 |
| Right biceps femoris | .004* | .677 | .057 | .065 | .037* |
ULES: Upper lumbar erector spinae. LLES: Lower lumbar erector spinae.
* p ≤ 0.05
The experiment was explained to the subject and any questions or concerns were addressed and the subjects were informed that they could withdraw from the experiment at any time. A consent form was read and signed prior to experimentation. The Memorial University of Newfoundland Human Investigations Committee approved the study.
Prone back extension
The posture adopted for the test was a variation of the Bering- Sørensen test (Biering-Sørensen, 1984) as described and implemented by the Canadian Society for Exercise Physiology, Canadian Physical Fitness and Lifestyle Approach test (CSEP, 2004). The Beiring Sorensen test was originally described by the authors as having subjects lay prone on an examination table and maintain an unsupported trunk (from the upper border of the iliac crest) horizontally until they could no longer hold a horizontal position or for a maximum of 240 seconds. The buttocks and legs are fixed to the table with three, three inch canvas straps. Any variations from the described methods are known as modified Sorensen tests. Our tests differ from the original in numerous ways, as described in our methods, but most notably by having subjects exert force against a strain gauge, but also in that we did not define a default test duration of 240 seconds. All protocols were held to exhaustion (failure to maintain prescribed force). Subjects lay prone on a padded examination table, with the trunk of the body extended off the edge of the table at the level of the anterior superior iliac spine of the pelvis. The lower legs, thighs and mid-buttocks region were restrained from motion using wide straps attached to the examination table. A pad placed under the ankles prevented subjects from bracing against the table with their feet. A harness was attached around the trunk at the T4-5 level. The strain gauge was attached to this harness at a midline location of the trunk while the other end was attached to an anchor plate at floor level. The harness/strain gauge assembly was adjusted so the subject maintained a trunk orientation parallel with the floor. The trunk was supported against gravity during rest periods (Figure 1).
Figure 1.
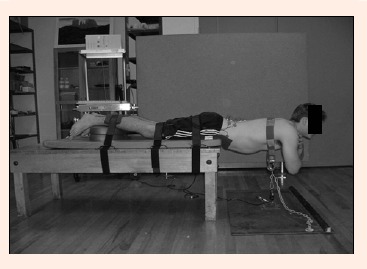
Posture for Biering-Sørensen test.
Definition of hat (head-arms-trunk segment)
Using the subject’s body mass and normative data derived through regression equations, (Zatsiorsky, 2002) the subject’s HAT mass was calculated. Using Zatsiorsky’s calculations, it was found that subjects’ HAT mass was 49.11% of their total body mass. HAT values were calculated based upon relative mass values from in vivo investigations by Zatsiorsky, 2002 of the inertial properties of 100 physically fit young males. These values are consistent with his regression equations, which are:
| Head and Neck: | y = 3.243 + 0.24x |
| Upper Arm (2): | y = -0.142 + 0.029x |
| Forearm (2): | y = 0.0165 + 0.0139x |
| Hand (2): | y = 0.109 + 0. 046x |
| Upper Trunk: | y = -0.078 + 0.0161x |
| Middle Trunk: | y = -2.222 + 0.194x |
| Lower Trunk: | y = -0.348 + 0.117x |
In these equations, x = the total body mass. The sum of the y values represents the mass of the HAT segment. The use of HAT-related values allowed for a normalized load condition across all subjects. These HAT-related loads, measured in Newtons, were equal to the HAT plus additional percentages of the HAT value of 10%, 20%, 30%, 40%, 50%, 60% and 70%. Since the segment was held in a horizontal orientation and the exertion was isometric, it was assumed that the resistance force vector was vertically oriented and acting through the centre of mass of the HAT segment.
Experimental design
The force displayed on the computer screen was calibrated so that 10% increments of HAT were visible to the subject for feedback. Repeated measures were taken over four sessions. Individual fatigue tests (test sessions) were separated by a minimum of 48 hrs and no longer than 96 hours. In each testing session, subjects were initially asked to perform a series of 3-5 repetitions of 2-5 s MVIA and then 7 randomly applied 2-5 s submaximal exertions of 100% -170% HAT in increments of 10%. The subjects viewed the computer screen and attempted to maintain the prescribed force (% of HAT).
There was a rest period of at least 2 minutes between exertions and a longer rest period of 5-10 minutes after all submaximal and maximal contractions were completed to minimize effects of muscle fatigue for the subsequent fatigue protocol (Behm et al., 2004). Subjects had to maintain the prescribed force for the submaximal exertions whereas they provided their greatest effort for the maximal exertions.
Subjects were then cued for the fatigue protocol and given standardized verbal encouragement during the effort. On each testing session, subjects would exert one randomly chosen force equivalent to their HAT mass plus a given percentage (0, 20, 40 or 60%) of that HAT mass until volitional failure. The test was terminated if the subject could not maintain the given force as displayed on the screen, or if their torso fell below parallel to the floor (a conclusion criterion only necessary when assessing the 100% HAT condition). The researchers monitored the subject’s position and would give an initial warning that the back position was not parallel. A second warning would result in termination of the test. Subjects used the visual feedback of a video monitor that demonstrated the target and actual forces. Electromyographic (EMG) signals, force and time to failure were all recorded.
Instrumentation
Surface EMG was collected using a bipolar differential collection system (ME3000P; Mega Electronics Ltd, Kuopio, Finland) utilizing 1cm diameter silver/silver electrodes spaced 1 cm apart. This was used to collect the electrical activities of 6 muscles in the trunk and thigh. Channels were sampled at 1000 Hz, band-pass filtered between 20 Hz and 500 Hz and amplified (differential amplifier: differential gain of 1000, common mode rejection ratio 130 dB, noise 1 µV). They were converted from analogue-to-digital (12-bit), and stored on computer for analysis. Signal amplification was done at the reference electrode site to minimize signal artifacts caused by movements and external noise.
Electrodes were placed bilaterally over the lumbosacral erector spinae (LSES) 2 cm lateral to the L5-S1 spinous processes and over the upper lumbar erector spinae (ULES) 6 cm lateral to the L1-L2, spinous processes. While a number of studies have used the L5/S1 configuration of surface EMG electrodes for examination of multifidus, (Vezina and Hubley-Kozey, 2000; Hermann and Barnes, 2001; Danneels et al., 2002), others suggest the intramuscular needle electrodes are necessary for accurate assessment (Stokes et al., 2003). For the present study, the EMG activity collected by the electrode arrangement is referred to as LSES as we expect we may have activity from more than just the multifidus. In the same way it is expected to emphasize the measurement of the multifidus at the lumbosacral junction with our narrow electrode placement, we expect to emphasize the longissimus thoracis with our placement of electrodes more lateral to the L1-L2 spinous processes. We are aware that we may also be interpreting signals from iliocostalis lumborum and multifidus and in this paper refer to the observed EMG activity as ULES. Electrodes were also placed bilaterally in the mid-belly of the biceps femoris. Reference electrodes were placed 5-10 cm away from the collecting electrodes for all collection arrays.
Bony landmarks and careful palpation was used to place electrodes in the same location. Both skin marking and measurement techniques enhanced the repeatability of electrode placement. The subjects’ skin was prepared prior to electrode placement by initially shaving local body hair, removing dead epithelial cells with very fine grade sandpaper and then cleansing the areas with an isopropyl alcohol swab.
Force exerted against the harness assembly placed at the T5/T6 level was collected through a Wheatstone bridge configuration strain gauge (Omega Engineering Inc. 55LCCA 250). The signal was converted from analogue-to-digital (MP100 analogue-to-digital: 12-bit; Biopac Systems Inc. Holliston, MA) and stored and analyzed through computer software. (Acqknowlege III, Biopac Systems Inc. Holliston, MA).
Data analysis and statistics
All signals were visually inspected during real time collection of EMG to ensure optimal signal quality. The median frequency was calculated using a Fast Fourier Transformation (FFT) algorithm and a Hamming window function. This was a data reduction option available from the MegaWin software (Mega Electronics Ltd, Kuopio, Finland) employed in the EMG data collection and analysis. A spectral estimate was calculated using a 1024 point moving window over the time from the initial marker flag representing the onset of activity to the final marker flag denoting the subject could no longer maintain the horizontal trunk position. The change in median frequency was calculated for the time period (Hz/sec) and employed as an estimate for muscular fatigue. Using the same time markers, the average amplitude of the EMG signal (aEMG) were also calculated. Descriptive statistics were reported for fatigue time, change in median frequency, and aEMG. These measures were compared across the conditions of 100%, 120%, 140% and 160% HAT using an ANOVA of a 2x4 (group x resistance) configuration (SPSS 12.0 for windows, SPSS Inc., US). Significance was set at p < 0.05 for all tests. Levene’s Test of Homogeneity was performed on force and EMG, to ensure reliability in EMG electrode placement. There were no significant differences between groups. A Bonferroni (Dunn) procedure was used to identify the differences among the percentage of HAT. Effect sizes (ES = mean change / standard deviation of the sample scores) were also calculated and reported (Cohen 1988). Cohen applied qualitative descriptors for the effect sizes (ES) with ratios of less than 0.41, 0.41-0.70 and greater than 0.7 indicating small, moderate and large changes respectively. Differences between groups for the Oswestry Low Back Disability Index and Pain Scales were analyzed with a 1 way ANOVA.
Intraclass correlation coefficients were calculated for extensor force, EMG of each muscle during each MVIA and each percentage of HAT. Reliability was assessed using an alpha (Cronbach) model intraclass correlation coefficient (Cohen 1988). The average force (N) output of the MVIA condition was compared between groups over the four sessions using an independent t-test.
Intraclass correlation coefficients were calculated for the EMG of MVIA’s and HAT for control and low back pain groups. The MVIA EMG and force ICCs were separately compared with a repeated measure 1 way ANOVA. The HAT EMG intraclass correlation coefficients were compared with a 2x8 (Group x HAT%) configuration ANOVA (SPSS 12.0 for windows, SPSS Inc., US) for each of the muscle groups. Differences were considered significant if they achieved an alpha level of p < 0.05. Bonferroni post-hoc tests were used to discriminate between individual and significant differences. Data in the text and figures include means and standard deviation (SD).
Results
Fatigue time
Figure 2 depicts the difference in fatigue time as resistance increases from 100% to 160% HAT. Expectedly, fatigue times decreased as resistance increased. The low back pain group had 4.5%, 34.2%, 40.6% shorter times at 120%, 140% and 160% of HAT respectively however no significant differences were detected between groups.
Figure 2.
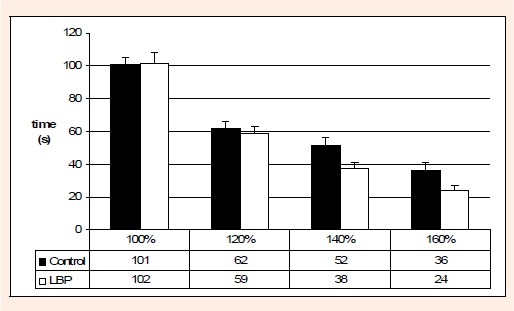
Comparison of mean endurance times (in seconds) between LBP and controls at given percentages HAT.
Median frequency
Figure 3 illustrates differences in median frequency between Control and low back pain groups for each extensor muscle group. Median frequency decreased more as resistance increased from 100-160% HAT. Differences were observed only in the biceps femoris and only at higher percentages of HAT. Table 1 reports significant between group differences in the right biceps femoris. There were significant pairwise differences in the left biceps femoris at 140% HAT with 89% lower median frequency in controls. A significant pairwise difference was also evident in the right biceps femoris at 160% HAT with 77% lower median frequency in controls and significance was approached (p = 0.057) at 120% HAT with 107% lower median frequency in the control group.
Figure 3.
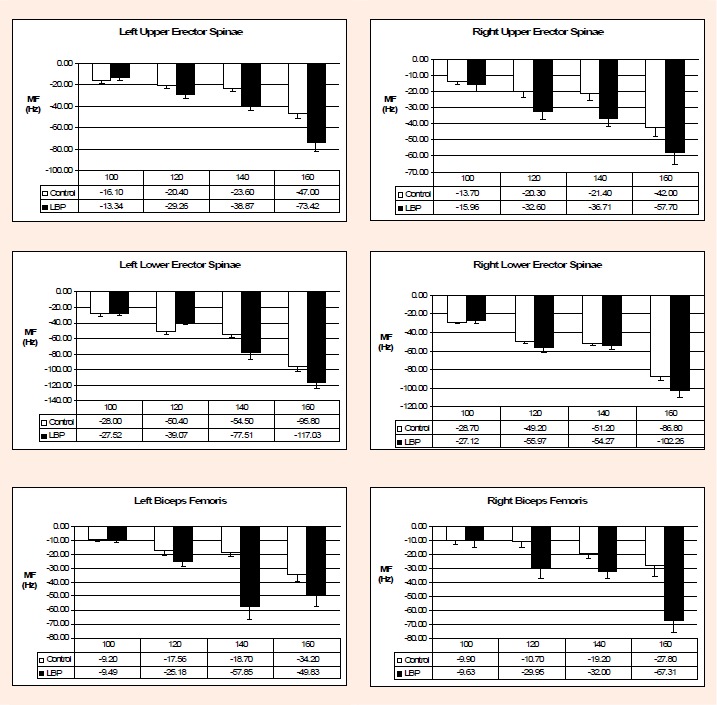
Change in MF for each extensor groups between LBP and controls. * p < 0.05.
Average EMG (aEMG)
For the control group, the aEMG consistently increased from 100% to 160% HAT. Table 2 reports aEMG means for each group across percentages of HAT. The aEMG was markedly increased in the control group between the 140% to 160% of HAT condition in all extensor muscle groups. In the low back pain group the 160% HAT condition only elicited marked changes in the left and right ULES, but failed to show marked differences in other muscles. Table 3 reports the interaction between groups and resistance for each muscle group. There was 54% less ULES aEMG in control group than in the LBP group. There was a significant difference at 140% HAT in the left biceps femoris with 86% lower aEMG in controls. The right biceps femoris demonstrated significant differences; with 65% lower aEMG in controls at 120% HAT and an 81% lower aEMG in controls at 140%.
Table 2.
Average EMG (μV) for each muscle at each percentage of HAT (head, arms, trunk segment). The asterisks signify significant differences between 140 and 160 % of HAT values for the muscle in that row.
| %HAT | 100 | 120 | 140 | 160 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Left ULES | Con | 24.60 | 15.88 | 33.80 | 21.77 | 32.9 | 66.51 | 159.3* | 143.41 |
| LBP | 10.79 | 19.95 | 6.21 | 67.23 | 78.53 | 56.82 | 152.3* | 183.71 | |
| Right ULES | Con | 31.10 | 35.24 | 45.40 | 41.94 | 30.2 | 46.68 | 172.9* | 142.94 |
| LBP | 14.24 | 30.45 | 35.46 | 51.25 | 84.48 | 30.76 | 217.4* | 331.06 | |
| Left LLES | Con | 14.20 | 15.25 | 23.00 | 17.54 | 15.84 | 37.93 | 74.3* | 50.91 |
| LBP | 3.46 | 16.67 | 15.71 | 39.95 | 11.10 | 32.42 | 29.80 | 49.64 | |
| Right LLES | Con | 12.50 | 17.43 | 22.20 | 18.20 | 13.5 | 47.31 | 93.5* | 94.20 |
| LBP | 2.01 | 21.03 | 18.48 | 28.76 | 50.31 | 68.26 | 53.51 | 108.52 | |
| Left biceps femoris | Con | 7.00 | 15.18 | 19.13 | 27.35 | 0.12 | 40.50 | 119.0* | 185.11 |
| LBP | 18.71 | 29.79 | 58.09 | 109.67 | 124.09 | 139.24 | 125.27 | 354.92 | |
| Right biceps femoris | Con | 19.60 | 24.74 | 16.80 | 36.05 | 23.10 | 45.06 | 129.7* | 186.95 |
| LBP | 13.73 | 28.33 | 65.76 | 63.05 | 183.51 | 359.86 | 53.17 | 144.73 | |
Con: Control. LBP: Low back pain. ULES: Upper lumbar erector spinae. LLES: Lower lumbar erector spinae
Table 3.
Comparison of p values for aEMG between LBP and Controls at each percentage of HAT (head, arms, trunk segment).
| %HAT | LBP vs. Control | Between groups at each %HAT | |||
|---|---|---|---|---|---|
| 100% | 120% | 140% | 160% | ||
| Left ULES | .355 | .112 | .235 | 1.128 | .926 |
| Effect size | .86 | 1.26 | .77 | .04 | |
| Right ULES | .088 | .283 | .648 | .009* | .703 |
| Effect size | .47 | .23 | 1.16 | .31 | |
| Left LLES | .095 | .161 | .607 | .744 | .071 |
| Effect size | .70 | .41 | .12 | .87 | |
| Right LLES | .118 | .251 | .737 | .186 | .402 |
| Effect size | .60 | .20 | .77 | .42 | |
| Left biceps femoris | .312 | .333 | .345 | .028* | .965 |
| Effect size | .77 | 1.42 | 3.06 | .03 | |
| Right biceps femoris | .269 | .636 | .05* | .018* | .336 |
| Effect size | .23 | 1.35 | 3.55 | .40 | |
Effect sizes are included with the following descriptors: <0.4: small effect, 0.4- 0.7: moderate effect, >0.7: large effect. LBP: Low back pain. ULES: Upper lumbar erector spinae. LLES: Lower lumbar erector spinae.
* p ≤ 0.05.
Reliability
Table 4 reports the intraclass correlation coefficients for all extensor muscles and compares the mean intraclass correlation coefficients of the six-extensor muscles for each %HAT and MVIA of Controls with that of the low back pain group. There was excellent correlation in all muscle groups in all % HAT in the control group, but much less homogeneity in the low back pain group compared to the control group.
Table 4.
Comparison of intraclass correlation coefficients for back extensor musculature between groups.
| %HAT | Left ULES |
Right ULES |
Left LLES |
Right LLES |
Left BF |
Right BF |
Mean | p | |
|---|---|---|---|---|---|---|---|---|---|
| 100 | Control | .80 | .88 | .94 | .93 | .94 | .91 | .90 | .037* |
| LBP | .74 | .88 | .85 | .81 | .72 | .88 | .81 | ||
| 110 | Control | .81 | .74 | .92 | .90 | .95 | .93 | .88 | .044* |
| LBP | .60 | .79 | .75 | .72 | .83 | .86 | .76 | ||
| 120 | Control | .88 | .93 | .97 | .95 | .98 | .96 | .95 | .005* |
| LBP | .57 | .80 | .73 | .74 | .88 | .88 | .77 | ||
| 130 | Control | .85 | .91 | .96 | .94 | .98 | .93 | .93 | .008* |
| LBP | .37 | .71 | .63 | .68 | .86 | .84 | .68 | ||
| 140 | Control | .89 | .92 | .96 | .94 | .98 | .95 | .94 | .006* |
| LBP | .46 | .72 | .57 | .64 | .89 | .88 | .69 | ||
| 150 | Control | .89 | .92 | .97 | .94 | .98 | .97 | .95 | .011* |
| LBP | .03 | .61 | .33 | .44 | .88 | .86 | .53 | ||
| 160 | Control | .87 | .90 | .90 | .87 | .98 | .80 | .89 | .019* |
| LBP | .21 | .67 | .67 | .62 | .66 | .90 | .62 | ||
| 170 | Control | .84 | .90 | .95 | .91 | .98 | .95 | .92 | .025* |
| LBP | .45 | .31 | .67 | .65 | .92 | .92 | .65 | ||
| MVIA | Control | .92 | .96 | .96 | .93 | .99 | .96 | .95 | .021* |
| LBP | .36 | .52 | .72 | .72 | .93 | .94 | .70 | ||
| p | < .0001† | .002† | < .0001† | < .0001† | .001† | .043† |
HAT: head, arms, trunk segment. LBP: Low back pain. ULES: Upper lumbar erector spinae. LLES: Lower lumbar erector spinae. BF: biceps femoris
* p ≤ 0.05 between LBP and Controls Groups for each % HAT.
† p ≤ 0.05 between LBP and Controls Groups for the specific muscle group in that column.
Discussion
Back endurance as it relates to low back pain has received much attention. Currently, a modified Biering- Sørensen test is used as part of the Canadian Society for Exercise Physiology Canadian Physical Activity Fitness and Lifestyle Approach (CPAFLA) test. Many studies have demonstrated that differences in fatigue times are lower in those with low back pain than those without (Alaranta et al., 1995; Biering-Sørensen, 1984; Hultman et al., 1993; Mayer et al., 1995; Nelson et al., 1995; Smidt et al., 1983). This study however did not find such a clear distinction in those subjects identified with mild low back pain disability scores. The rigorous testing procedures outlined in our protocol may account for differences in overall fatigue times, but not in differences between groups. Differences in fatigue responses were observed through EMG evidence in select muscle groups at higher resistance of fatigue, but there were no differences at lower percentages of HAT. Further, fatigue time did not appear to be a sensitive measure to discern between mild low back pain and control groups.
There was no significant difference in the fatigue times between low back pain subjects and controls. These findings are similar to that of Biering-Sørensen, 1984(low back pain: 164s, controls: 195s), Sparto et al., 1997(low back pain with a mean of 109s), McKeon (2006) (low back pain: 15.3s, healthy males: 124.4s) and Hultman et al., 1993(low back pain: 134s, controls 150s). Kankaanpaa et al., 2005 also reported a lack of difference in paraspinal activation (EMG amplitude and mean power frequency) and relative fatiguability between low back pain participants and healthy males. In the current study the initial series of MVIA and submaximal exertions were performed by all subjects and therefore, should not have been a factor in the differences found between the groups. However even with adequate muscle recovery periods (Behm et al., 2004), the initial testing may account for lower fatigue times than found in most studies. The norms for the Canadian Physical Fitness and Lifestyle Approach back extension fatigue test indicate that both the low back pain (102s) and control (101s) subjects in the present study were situated in the 50th percentile (Payne et al. 2000). No subject in the low back pain group in this study reported recent severe bouts of low back pain within the past month, but all reported recurrent or chronic low back pain that was reported to affect their activity. Validated outcome measures and visual analogue pain scales, while significantly different between groups, did not convey a sense of severe pain or marked physical disability. However subjects with similar pain history and ranges of discomfort are likely characteristic of people that are candidates for back assessments.
Median frequency
Pairwise differences were only present at higher levels of resistance. Right biceps femoris demonstrated no difference in median frequency at 100%, but significant differences were evident at 120% and 160%. Significant differences were also found at the 140% HAT condition for left biceps femoris and right ULES. These findings may suggest that the lower resistance levels are not sufficient to delineate between groups, but as resistance increases, more extensor effort is required and the differences between groups occur primarily in the biceps femoris. Significant differences at the right ULES may also play a role. Whereas some studies have been able to delineate between healthy and low back pain subjects with a modified Biering-Sørensen test (Biering-Sørensen, 1984, Ng et al., 2002), questions arise regarding the reliability of the test. Van Diėėn and Heijblom (1996) reported that test retest errors between sessions could reach 20% but that similar to the increased discrimination in the present study at higher resistance levels, reliability increased with increased relative force. Luoto et al., 1995 indicated that the high incidence of low back pain in the 12-month follow-up in their study was implausible suggesting the reliability of the low back pain questionnaire was far from complete. Similarly, the limitation of the self-reported low back pain questionnaire (Oswestry) in the present study is discussed in further detail in the limitation section to follow. There is a vast spectrum of disability and pain associated with chronic low back pain individuals. The low back pain group heterogeneity in the present study might be considered a reflection of that population. The wide range of disabilities and pain levels would make it exceedingly difficult to accurately identify or predict low back pain with a single test utilizing a narrow range of resistance.
Average EMG
Differences in aEMG between groups were evident in the right ULES at 140% HAT. The only other significant differences occurred in the left biceps femoris at 140% HAT and in the right biceps femoris at 140 and 150% HAT. While the final product of force output through back extension is a composite of many synergistic muscles and recruitment strategies, it appears that the most marked differences in muscle recruitment between groups occurred in the biceps femoris at higher percentages of HAT. Numerically, the low back pain group had higher mean aEMG values for the right and left biceps femoris in 6 of the 8 measures. Conversely, the low back pain group had numerically lower aEMG values for the right and left LLES and ULES for 12 of the 16 measures. Hence there was statistically significantly greater biceps femoris activity in the low back pain group (left biceps femoris at 140% HAT, right biceps femoris at 120 and 140% hat) with a trend toward greater biceps femoris activity, which contrasts with lower low back pain LLES and ULES activity. These findings would suggest that the subjects with low back pain maintained similar back fatigue as controls due to a greater reliance on their hip extensor (biceps femoris) activity. It could be suggested that the test is not simply a test of back fatigue but also dependent upon either purposeful or automatic alterations in motor control strategies.
The multifidus (a component of LLES activity in this study) has been reported to fatigue at a faster rate than the iliocostalis lumborum (Ng et al., 1997)(a component of ULES activity in this study) leading to the suggestion that the fatigue rate of the multifidus may be a useful clinical measure (Ng and Richardson, 1996). The iliocostalis and longissimus and multifidus muscles are arranged from lateral to medial and are contained within their own fascial compartment (Bogduk, 1980). The lumbar portions of the iliocostalis and longissimus attach to the mamillary, accessory and transverse processes of the lumbar vertebrae and apart from a small number of medial slips of the longissimus, the iliocostalis and longissimus do not have superior attachments in the lumbar spine (Macintosh et al., 1986). These muscles act at a distance having fibers that do not act in a plane parallel to compressive force, but are of a more posterior and caudal orientation and are well suited to resist anterior shearing forces. (McGill, 2002) The slips of the multifidus which attach distally at the sacral crest, interosseous sacroiliac ligament, thoracolumbar fascia and medial edge of the iliac crest span only two or three segments and attach to the posterior aspect of the spinous of each vertebrae. The extension torque creates more local compression and than does the iliocostalis and longissimus. The disparity in configuration of these muscles highlights why iliocostalis and longissimus are though to act as global stabilizers where as the multifidus is seen to impart stability on a more local level.
The lack of consistent differences in ULES and LLES activity in the present study with significantly greater biceps femoris EMG activity in the low back pain group would further suggest that not just back musculature are involved in maintaining the posture associated with the modified Biering-Sørensen test. Based on the results of this study, using aEMG of erector spinae muscles in low resistance modified Biering-Sørensen tests may not be ideal when attempting to evaluate healthy subjects from those with mild chronic or recurrent low back pain.
Muscle synergysm
Due to the synergism of muscles used in back extension; there are various motor control strategies that may be employed during a low intensity fatigue test to maintain a desired static posture. Motor unit substitution during fatigue protocols has been reported for a number of limb (Bawa et al., 2006, Kouzaki et al., 2004, Kouzaki and Shinohara, 2006) and trunk muscles (Westgaard and DeLuca, 1999). Kouzaki and Shinohara, 2006 reported that subjects with more frequent alternate muscle activity experience less muscle fatigue. Muscle substitution protects postural muscles from excessive fatigue when there is a demand for sustained low-level muscle activity (Westgaard and DeLuca, 1999). It is suspected that at higher intensities (larger percentages of HAT) there is less time for implementing a motor control strategy that coordinates load sharing across synergistic muscles. This may be the reason why fatigue time differences are more pronounced at 140% and 160% HAT. For an 80kg subject, 140% HAT is 540N or 87% of maximum for controls and 132% of maximum for the low back pain group. It is probable that at higher percentages of HAT that approach or exceed maximal values, there is less opportunity to employ alternative recruitment strategies.
In an isolated case, one of the control subjects had a higher fatigue time at 160% than at the 100% condition. When EMG data streams were reviewed, it was evident that he had developed a load sharing strategy between his lumbar extensors and biceps femoris, alternating bursts of activity in each muscle group thus creating “micro- rest periods”. This case highlights the idea that although the neuromuscular fatigue of the trunk and hip extensors contribute to fatigue time, motor control strategies may play an equal or superior role in the application of fatigue protocols.
Limitations
One of the most significant limitations of this study is having the subjects use self-report of low back pain to delineate control and low back pain groups. Although the differences in the pain and Oswestry scores were significant between groups, there was considerable variability in the scores within the low back pain group. Such variability may have reduced the discrimination between groups. Additionally, it should be noted that an Oswestry score of 18% classifies a subject as having only mild lower back disability. Although the relatively low levels of disability and pain are a likely cause for decreased differences between groups, it can be argued that clients with similar pain and disability characteristics are likely candidates for conservative care treatment and likely to present to kinesiologists or trainers for fitness appraisals.
Based on this limitation, it might be suggested that the present HAT-based protocol would specifically aid practitioners in classifying patients with varying degrees of mild back disability based on the Oswestry classification. For future studies, it is suggested that scores or other form of external assessment be used as grouping criteria groups independent of self classification as back pain sufferers or not. There were some limitations in the research design. Firstly we used a relatively small number of subjects with each group containing 10 subjects. Secondly, a series of maximal and submaximal tests were performed prior to the fatigue protocol. Although adequate recovery times were used, this could have potentially led to shorter fatigue times. Because this was done consistently on each session and for all subjects, it is not a factor influencing differences between groups.
Conclusion
According to the Canadian Society for Exercise Physiology, Canadian Physical Fitness and Lifestyle Approach manual (2004), the modified Biering-Sørensen back extensor endurance test with the HAT as the resistance has been reported as a valid and reliable assessment of back extensor endurance, and it has been found to be positively related to back health (Albert et al., 2001). The main finding of the present study indicates that the results do not wholly support the modified Biering-Sørensen test utilizing resistance of 100% HAT to discern differences in fatigue in subjects with mild low back pain. No significant differences in fatigue time between groups at 100% HAT or even at higher resistance levels are reported. A greater activation of the biceps femoris by low back pain individuals probably contributed to the lack of significant differences in back fatigue times. This finding suggests that the modified Biering- Sørensen back extensor endurance test may not entirely reflect back fatigue as alternative loads sharing strategies such as emphasizing hip extensor activity can prolong the test time. The possibility exists that subjects with more sophisticated strategies could yield higher fatigue times despite inferior neuromuscular fatigue and the existence of low back pain. Future research designs that evaluate motor control strategies during prone extension could yield important information for further design of assessment tools and rehabilitative procedures.
Acnowledgments
The Natural Science and Engineering Research Council (NSERC) supported this study.
Biographies

Mark J. PITCHER
Employment
Chiropractor; Vail Integrative Medical Group.
Degree
DC, MSc. (Kinesiology)
Research interests
Rehabilitation of the lumbar spine and pelvis. Techniques for assessment and enhancement of spinal stability.
E-mail: drpitcher@vailhealth.com

David G. BEHM
Employment
Professor; Memorial University of Newfoundland.
Degree
PhD
Research interests
Neuromuscular responses to acute and chronic activity.
E-mail: dbehm@mun.ca

Scott N. MacKINNON
Employment
Associate Professor at Memorial University of Newfoundland.
Degree
PhD
Research interests
Human Performance in Harsh Environmental Environments (motion induced interruptions, fatigue, sickness as it relates to physical and cognitive performance in maritime environments) Maritime Evacuation, Escape and Rescue Detection of muscular fatigue and overuse injures. Modelling of Situation Awareness in Maritime Command and Control Settings
E-mail: smackinn@mun.ca
References
- Alaranta H., Hurri H., Heliovaara M., Soukka A., Harju R. (1994) Non-dynamometric trunk performance tests: reliability and normative data. Scandinavian Journal of Rehabilitation Medicine 26(4), 211-215 [PubMed] [Google Scholar]
- Alaranta H., Luoto S., Heliovaara M., Hurri H. (1995) Static back endurance and the risk of low-back pain. Clinical Biomechanics 10(6), 323-324 [DOI] [PubMed] [Google Scholar]
- Albert W.J., Bonneau J., Stevenson J.M., Gledhill N. (2001) Back fitness and back health assessment. Considerations for the Canadian physical activity, fitness and lifestyle appraisal. Canadian Journal of Applied Physiology 26(3), 291-317 [DOI] [PubMed] [Google Scholar]
- Asmussen E. (1979) Muscle fatigue. Medicine and Science in Sports and Exercise 11(4), 313-321 [PubMed] [Google Scholar]
- Bawa P., Pang M.Y., Olesen K.A., Calancie B. (2006) Rotation of motoneurons during prolonged isometric contraction in humans. Journal of Neurophysiology 96, 1135-1140 [DOI] [PubMed] [Google Scholar]
- Behm D.G. (2004) Force maintenance with submaximal fatiguing contractions. Canadian Journal of Applied Physiology 29(3), 274-290 [DOI] [PubMed] [Google Scholar]
- Behm D.G., Button D.C., Barbour G., Butt J.C., Young W.B. (2004) Conflicting Effects of Fatigue and Potentiation on Voluntary Force. Journal of Strength and Conditioning Research 18(2), 365-372 [DOI] [PubMed] [Google Scholar]
- Biering-Sørensen F. (1984) Physical measurements as risk indicators for low-back trouble over a one-year period. Spine, 9(2), 106-119 [DOI] [PubMed] [Google Scholar]
- Bogduk N. (1980) A reappraisal of the anatomy of the human lumbar erector spinae. Journal of Anatomy 131, 525-540 [PMC free article] [PubMed] [Google Scholar]
- Coste J., Delecoeuillerie G., Cohen de Lara A., Le Parc J.M., Paolaggi J.B. (1994) Clinical course and prognostic factors in acute low back pain: an inception cohort study in primary care practice. British Journal of Medicine 308(6928), 577-580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Society for Exercise Physiology-CSEP (2004) Canadian Society for Exercise Physiology: The Canadian Physical Activity, Fitness, and Lifestyle Appraisal. Ottawa, Ontario, Health Canada [Google Scholar]
- Cohen J. (1988) Statistical Power Analysis for the Behavioral Sciences. 2nd Edition Hillsdale NJ, L. Erbaum Associates Publishing [Google Scholar]
- Danneels L.A., Coorevits P.L., Cools A.M., Vanderstraeten G.G., Cambier D.C., Witvrouw E.E., De C.H. (2002) Differences in electromyographic activity in the multifidus muscle and the iliocostalis lumborum between healthy subjects and patients with sub-acute and chronic low back pain. European Spine Journal 11, 13-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degens H., Veerkamp J.H. (1994) Changes in the oxidative capacity and fatigue resistance in skeletal muscle. International Journal of Biochemistry 26(7), 871-878 [DOI] [PubMed] [Google Scholar]
- Enoka R.M., Stuart D.G. (1992) Neurobiology of muscle fatigue. Journal of Applied Physiology 72(5), 1631-1648 [DOI] [PubMed] [Google Scholar]
- Fairbank J.C., Couper J., Davies J.B., O’Brien J.P. (1980) The Oswestry low back pain disability questionnaire. Physiotherapy 66(8), 271-273 [PubMed] [Google Scholar]
- Fitts R.H., Metzger J.M. (1993) Mechanisms of muscular fatigue. Medicine and Science in Sports and Exercise (Principles of Exercise Biochemistry Second Edition, Basel Karger) 33, 248-268 [Google Scholar]
- Hermann K.M., Barnes W.S. (2001) Effects of eccentric exercise on trunk extensor torque and lumbar paraspinal EMG. Medicine and Science in Sports and Exercise 33(6), 971-977 [DOI] [PubMed] [Google Scholar]
- Hultman G., Nordin M., Saraste H., Ohlsen H. (1993) Body composition, endurance, strength, cross-sectional area, and density of MM erector spinae in men with and without low back pain. Journal of Spinal Disorders 6(2), 114-123 [PubMed] [Google Scholar]
- Johnson W.G., Baldwin M.L., Butler R.J. (1998) Back Pain and Work Disability: The Need for a New Paradigm. Industrial Relations 37(1), 9-34 [Google Scholar]
- Jørgensen K., Nicolaisen T. (1986) Two methods for determining trunk extensor endurance. A comparative study. European Journal of Applied Physiology 55(6), 639-644 [DOI] [PubMed] [Google Scholar]
- Jørgensen K., Nicolaisen T. (1987) Trunk extensor endurance: determination and relation to low-back trouble. Ergonomics 330(2), 259-267 [DOI] [PubMed] [Google Scholar]
- Kankaanpaa M., Laaksonen D., Taimela S., Kokko S.M., Airaksinen O., Hanninen O. (1998a) Age, sex, and body mass index as determinants of back and hip extensor fatigue in the isometric Sørensen back endurance test. Archives of Physical Medicine and Rehabilitation 79(9), 1069-1075 [DOI] [PubMed] [Google Scholar]
- Kankaanpaa M., Taimela S., Laaksonen D., Hanninen O., Airaksinen O. (1998B) Back and hip extensor fatigability in chronic low back pain patients and controls. Archives of Physical Medicine and Rehabilitation 79(4), 412-417 [DOI] [PubMed] [Google Scholar]
- Kankaanpaa M., Colier W.N., Taimel S. (2005) Back extensor muscle oxygenation and fatigability in healthy subjects and low back pain patients during dynamic back extension exertion Pathophysiology 7, 416-423 [DOI] [PubMed] [Google Scholar]
- Kouzaki M., Shinohara M. (2006) The frequency of alternate muscle activity is associated with the attenuation in muscle fatigue. Journal of Applied Physiology 101, 715-720 [DOI] [PubMed] [Google Scholar]
- Kouzaki M., Shinohara M., Masani K., Fukunaga T. (2004) Force fluctuations are modulated by alternate muscle activity of knee extensor synergists during low level sustained contractions. Journal of Applied Physiology 97, 2121-2131 [DOI] [PubMed] [Google Scholar]
- Luoto S., Heliovaara M., Hurri H., Alaranta H. (1995) Static back endurance and the risk of low back pain. Clinical Biomechanics 10(6), 323-324 [DOI] [PubMed] [Google Scholar]
- Macintosh J.E., Valencia F., Bogduk N., Munro R.R. (1986) The morphology of the human lumbar multifidus. Clinical Biomechanics 1, 196-204 [DOI] [PubMed] [Google Scholar]
- Mannion A.F., Dolan D. (1994) Electromyographic median frequency changes during isometric contraction of the back extensors to fatigue. Spine 19(11), 1223-1229 [DOI] [PubMed] [Google Scholar]
- Mayer T., Gatchel R., Betancur J., Bovasso E. (1995) Trunk muscle endurance measurement. Isometric contrasted to isokinetic testing in normal subjects. Spine 20(8), 920-926 [PubMed] [Google Scholar]
- Mayer T.G., Gatchel R.J. (1988) Functional restoration for spinal disorders: The Sports Medicine Approach. Philadelphia, Lea & Febiger; 628-641 [Google Scholar]
- McGill S. (2002) Low back disorders: evidence based prevention and rehabilitation. Champaign, IL, Human Kinetics; 62-63 [Google Scholar]
- McKeon M., Albert W., Neary P. (2006) Assessment of neuromuscular and haemodynamic activity in individuals with and without chronic lower back pain. Dynamic Medicine 31(5:6), doi: 10.1186/1476-5918-5-6. Avaliable form URL:http://www.dynamic-med.com/content/5/1/6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffroid M., Reid S., Henry S.M., Haugh L.D., Ricamato A. (1994) Some endurance measures in persons with chronic low back pain. Journal of Orthopedic Sports Physical Therapy 20(2), 81-87 [DOI] [PubMed] [Google Scholar]
- Moffroid M.T. (1997) Endurance of trunk muscles in persons with chronic low back pain: assessment, performance, training. Journal of Rehabilitation Research and Development 34(4), 440-447 [PubMed] [Google Scholar]
- Moffroid M.T., Haugh L.D., Haig A.J., Henry S.M., Pope M.H. (1993) Endurance training of trunk extensor muscles. Physical Therapy 73(1), 10-17 [PubMed] [Google Scholar]
- Moreau C.E., Green B.N., Johnson C.D., Moreau S.R. (2001) Isometric back extension endurance tests: a review of the literature. Journal of Manipulative Physiology and Therapy 24(2), 110-122 [DOI] [PubMed] [Google Scholar]
- Nelson B.W., O’Reilly E., Miller M., Hogan M., Wegner J.A., Kelly C. (1995) The clinical effects of intensive, specific exercise on chronic low back pain: a controlled study of 895 consecutive patients with 1-year follow up. Orthopedics 18(10), 971-981 [DOI] [PubMed] [Google Scholar]
- Ng J.K., Richardson C.A., Jull G.A. (1997) Electromyographic amplitude and frequency changes in the iliocostalis lumborum and multifidus muscles during a trunk holding test. Physical Therapy 77(9), 954-961 [DOI] [PubMed] [Google Scholar]
- Ng J.K., Richardson C.A. (1996) Reliability of electromyographic power spectral analysis of back muscle endurance in healthy subjects. Archives of Physical Medicine and Rehabilitation 77(3), 259-264 [DOI] [PubMed] [Google Scholar]
- Ng J.K., Kippers V., Parniapour M., Richardson C.A. (2002) EMG activity normalization for trunk muscles in subjects with and without back pain. Medicine and Science in Sports and Exercise 34(7), 1082-1086 [DOI] [PubMed] [Google Scholar]
- Nicolaisen T., Jørgensen K. (1985) Trunk strength, back muscle endurance and low-back trouble. Scandinavian Journal of Rehabilitation and Medicine 17(3), 121-127 [PubMed] [Google Scholar]
- Nordin M., Kahanovitz N., Verderame R., Parnianpour M., Yabut S., Viola K., Greenidge N., Mulvihill M. (1987) Normal trunk muscle strength and endurance in women and the effect of exercises and electrical stimulation. Part 1: Normal endurance and trunk muscle strength in 101 women. Spine 12(2), 105-111 [DOI] [PubMed] [Google Scholar]
- Payne N, Gledhill N, Katzmarzyk PT, Jamnik V. (2000) Health-related fitness, physical activity, and history of back pain. Canadian Journal of Applied Physiology 25(4), 236-249 [DOI] [PubMed] [Google Scholar]
- Peltonen J.E., Taimela S., Erkintalo M., Salminen J.J., Oksanen A., Kujala U.M. (1998) Back extensor and psoas muscle cross-sectional area, prior physical training, and trunk muscle strength—a longitudinal study in adolescent girls. European Journal of Applied Physiology 77(1-2), 66-71 [DOI] [PubMed] [Google Scholar]
- Pitcher Mark J., Behm D.G., MacKinnon Scott N. (2007) Reliability of Electromyographic and Force Measures During Prone Isometric Back Extension in Subjects With and Without Low Back Pain. Applied Physiology, Nutrition and Metabolism. (In press; ). [DOI] [PubMed] [Google Scholar]
- Smedley J., Inskip H., Cooper C., Coggon D. (1998) Natural history of low back pain. A longitudinal study in nurses. Spine 23(22), 2422-2426 [DOI] [PubMed] [Google Scholar]
- Smidt G., Herring T., Amundsen L., Rogers M., Russell A., Lehmann T. (1983) Assessment of abdominal and back extensor function. A quantitative approach and results for chronic low-back patients. Spine 8(2), 211-219 [DOI] [PubMed] [Google Scholar]
- Sparto P.J., Parnianpour M., Reinsel T.E., Simon S. (1997) The effect of fatigue on multijoint kinematics and load sharing during a repetitive lifting test. Spine 22(22), 2647-2654 [DOI] [PubMed] [Google Scholar]
- Spitzer W.O., LeBlanc F.E., Dupuis M. (1987). Scientific approach to the assessment and management of activity-related spinal disorders. A monograph for clinicians. Report of the Quebec Task Force on Spinal Disorders. Spine 12(Suppl. 7), S1-59 [PubMed] [Google Scholar]
- Straus B.N. (2002) Chronic pain of spinal origin: the costs of intervention. Spine 27(22), 2614-2619 [DOI] [PubMed] [Google Scholar]
- Stokes I.A., Henry S.M., Single R.M. (2003) Surface EMG electrodes do not accurately record from lumbar multifidus muscles. Clinical Biomechanics 18(1), 9-13 [DOI] [PubMed] [Google Scholar]
- Thomas A.M., Fairbank J.C., Pynsent P.B., Baker D.J. (1989) A computer-based interview system for patients with back pain. A validation study. Spine 14(8), 844-846 [DOI] [PubMed] [Google Scholar]
- Van Diėėn J.H., Toussaint H.M., Thissen C., van de Ven A. (1993). Spectral analysis of erector spinae EMG during intermittent isometric fatiguing exercise. Ergonomics 36(4), 407-414 [DOI] [PubMed] [Google Scholar]
- Van Diėėn J.H., Heijblom P. (1996) Reproducibility of isometric trunk extension torque, trunk extensor endurance, and related electromyographic parameters in the context of their clinical applicability. Journal of Orthopaedic Research 14, 139-143 [DOI] [PubMed] [Google Scholar]
- Vezina M.J., Hubley-Kozey C.L. (2000) Muscle activation in therapeutic exercises to improve trunk stability. Archives of Physical Medicine and Rehabilitation 81(10), 1370-1379 [DOI] [PubMed] [Google Scholar]
- Waddell G. (2004) The Back Pain Revolution. Edinburgh New York, Churchill Livingstone; 123-156 [Google Scholar]
- Westgaard R.H., DeLuca C.J. (1999) Motor unit substitution in long duration contractions of the human trapezius muscle. Journal of Neurophysiology 82, 501-504 [DOI] [PubMed] [Google Scholar]
- Williams D.A., Feuerstein M., Durbin D., Pezzullo J. (1998) Health care and indemnity costs across the natural history of disability in occupational low back pain. Spine 23(21), 2329-2336 [DOI] [PubMed] [Google Scholar]
- Zatsiorsky V.M. (2002) Kinetics of Human Motion. Human Kinetics; Champaign, IL: 76-142 [Google Scholar]


