Abstract
The present study aims to investigate the probiotic nature of Pediococcus acidilactici MTCC5101 by an in vitro assay of bacterial adherence to intestinal epithelial cells of human gastrointestinal (GI) tract using Caco-2 cell line. Further to assess the in vivo survival in the GI tract, oral feeding was carried out with the help of 10 healthy volunteers. The effect on wellness was assessed by studying blood biochemical parameters of the volunteers. The survival of the bacteria was assessed using PCR-based detection of P. acidilactici MTCC5101 in fecal samples. The probiotic nature of P. acidilactici MTCC 5101 was strengthened by its adherence to the intestinal epithelial Caco-2 cell line in the in vitro SEM observations. Oral feeding study for assessing the survival of bacteria in GI tract of volunteers showed the strain to be established in the GI tract which survived for about 2 weeks after feeding.
1. Introduction
Human intestinal microbiota is a metabolically active microbial environment that is relatively stable in the guts of healthy individuals [1]. The commensal intestinal flora of humans and animals includes the genera Lactobacillus, Pediococcus, and Lactococcus [2, 3]. Strains of these genera are frequently used on large scale as starter cultures in food industries because of their generally recognized as safe (GRAS) and probiotic status [4–6].
Probiotics are known to exert protective influence in the human intestine through various mechanisms and represent promising applications in prophylaxis and therapy. It has also been acknowledged by WHO that probiotic microorganisms when administered in adequate amounts confer a health benefit to the host [7]. Improvement of health status by probiotics can be explained by the fact that these bacteria reduce production of toxic substances, aid in production and absorption of vital nutrients, stimulate gastrointestinal activity as well as immunity, and reduce colonization of anaerobic pathogens by competitive exclusion [8, 9].
For all the health giving effects, the probiotic bacteria should also be capable of surviving while passage through the GI tract. It is essential for them to overcome highly acidic environment of stomach, digestive enzymes, and bile salts of the small intestine, which thus become important selection criterion for probiotic strains. Apart from this, adherence of bacterial cells to intestinal epithelial cells and/or mucus is considered to be a desirable feature of probiotics, as it promotes enhanced gut residence time, pathogen exclusion, and interaction with host epithelial and immune cells [10, 11].
Pediococcus acidilactici MTCC5101 is an acid and bile tolerant probiotic strain that secretes a potent antibacterial bacteriocin designated as Pediocin CP2. Pediocin CP2 exhibits a wide range of antimicrobial activity against Gram-positive, Gram-negative bacteria as well as fungi [12]. Genes encoding production of Pediocin CP2 are localized to the ped operon present on the 8.9 kb plasmid pCP289 of P. acidilactici MTCC5101 [13–15]. These properties make this sp. of Pediococcus an attractive prophylactic and therapeutic agent against pathogenic bacteria in GI tract. However, the final confirmation of its probiotic nature can come only from human trials. It is these trials that provide evidence of the survival of the probiotic strain in vivo and provide the required basis for a credible claim.
The present study aims to investigate the probiotic nature of P. acidilactici MTCC5101 by an in vitro assay of bacterial adhesion to intestinal epithelial cells of human GI tract using Caco-2 cell line. Further, to assess the in vivo survival in the GI tract, oral feeding was carried out with the help of 10 healthy volunteers. The effect on wellness was assessed by studying blood biochemical parameters of the volunteers.
2. Materials and Methods
2.1. Bacterial Strain Procurement and Maintenance
Pediococcus acidilactici MTCC5101, characterized in the laboratory of Department of Biotechnology, Punjabi University, Patiala [12], was used in the present study. It was revived and maintained at 37°C for 18–24 h under microaerophilic conditions in de Man, Rogosa, and Sharpe (MRS) broth [16].
2.2. In Vitro Adhesion Assay of Pediococcus acidilactici MTCC5101
The enterocyte-like Caco-2 cells were obtained from National Centre for Cell Science (NCCS), Pune. Cells were routinely grown in Dulbecco modified Eagle's minimal essential medium containing 25 mM glucose, 10% fetal bovine serum, and 1% antibiotic solution containing penicillin and streptomycin (Himedia) [15].
The adherence of pediococci to Caco-2 cells was examined as described previously by Bernet et al. [17] with a few modifications. For the adhesion assay, monolayers of Caco-2 cells were prepared on glass coverslips placed in six-well tissue culture plates seeded at a concentration of 106 cells/mL. Experiments were carried out at 37°C in a 5% CO2 atmosphere. The culture medium was changed every alternate day and monolayers at late postconfluence stage (after 15 days) were used for adhesion assay. To begin with the assay, monolayers were washed twice with phosphate-buffered saline (PBS, pH 7.2). 1 mL bacterial culture containing one million CFU/mL suspended in PBS was added to the monolayers on glass coverslips placed in tissue culture plates containing DMEM without antibiotic solution. The plates were incubated for 1 h in 5% CO2 at 37°C. After 1 h, the monolayers were washed five times with sterile PBS and fixed for SEM observations. The adhesion assay was repeated thrice.
2.3. Scanning Electron Microscopy (SEM)
For SEM analysis, Caco-2 cells cocultured with bacterial cells were fixed for 1 h with 2.5% glutaraldehyde in 0.1 M phosphate buffer (PB, pH 7.4) at room temperature. The cells were washed twice with PB and postfixed for 30 min with 2% osmium tetroxide in PB. After fixation, cells were again washed thrice with PB and then dehydrated in a graded series (30, 50, 70, 80, 90, and 100%) of ethanol [17]. Instead of critical point dryer, cells were treated with 100% hexamethyldisilazane for 10 min and coated with gold [18]. The specimens were examined with a JEOL JSM-6610LV scanning electron microscope at Indian Institute of Technology, Ropar, Punjab, India. A number of bacterial cells adhered onto Caco-2 cells were counted on various microscopic fields per coverslip while performing SEM. The values were represented as average number of bacterial cells adhered per 100 Caco-2 cells.
2.4. In Vivo Study Design
The study was approved by the Institutional Clinical Ethics Committee vide clearance no. ICEC/3/2011. Experiments were strictly performed according to the guidelines of Indian Council of Medical Research for conducting research on human trials. Ten healthy volunteers were selected after taking their informed consent. It was a controlled study consisting of parallel 6 weeks trials, with 4 weeks of intervention and 2 weeks wash-out period.
2.4.1. Preparation of Probiotic Buttermilk
The overnight grown P. acidilactici MTCC5101 cells were harvested by centrifugation (6000 rpm, 10 min) and resuspended slowly in PBS (pH 8.0). Cells at a concentration of 108–1010 cells/mL were added to fresh buttermilk as medium for oral feeding study (Verka Co. Ltd., Patiala, India). Cell counts were taken on a haemocytometer [19] and after thorough microbiological examination, cells at an average dosage level of 108–1010 cells/mL/day were fed to 10 healthy subjects in 1 mL buttermilk base, administered once a day for 4 weeks continuously [20]. Equivalent amount of buttermilk without P. acidilactici MTCC5101 was fed to a group of 3 control volunteers which served as negative controls. The subjects were asked to abstain from taking other fermented milk products and supplements with probiotics during the trial period. They were explained how to collect, store, and deliver the fecal samples. Participants were asked about changes in their wellbeing and/or had taken any kind of medication during study period.
2.4.2. Collection and Analysis of Fecal Samples
The fecal swab samples were collected using ear buds in 2 mL of sterile normal saline (0.85% NaCl) after feeding trials of 5, 10, 20, and 30 days [38]. The fecal suspension was cultured in MRS broth for bacterial enumeration for 18 h at 37°C. Cell pellets were harvested by centrifugation and were stored at −20°C for plasmid DNA isolation.
2.4.3. Molecular Identification of P. acidilactici MTCC5101 in Fecal Samples
A metagenomic approach was followed for the isolation of plasmid DNA from fecal samples [39]. Isolated DNA was stored at −20°C until further use and its quality, quantity, and purity were determined using agarose gel electrophoresis and absorbance measurements on spectrophotometer (UV mini-1240, UV-Vis spectrophotometer, Shimadzu Corporation). PCR was performed in PROGENE (Techne) using forward primer 5′-CTGCGTTGATAGGCCAGGT-3′ and reverse primer 5′-ACCTTGATGTCCACCAGTAGC-3′ which are specific for 323 bp fragment of pedA gene located on the cryptic plasmid pCP289 of P. acidilactici MTCC5101 [13]. The amplification program consisted of 1 cycle of 94°C for 3 min and 30 cycles of amplification (94°C for 1 min, 63°C for 1 min, and 72°C for 1 min).
2.4.4. Bacteriocin Activity Assay
Well diffusion assay was performed as per standard methodology of Sarkar and Banerjee [40]. MRS hard agar (1% w/v) was overlayed with MRS soft agar (0.75% w/v) preseeded with approximately one million cells of indicator Enterococcus faecalis. Wells were filled with heat inactivated supernatant of MRS grown fecal samples pertaining to the 30th day of intervention and plates were incubated at 37°C for 24 h.
2.5. Determination of Wellness Parameters
To check the effect of the probiotic on the wellness of test subjects, blood samples were drawn 2 weeks before and 2 weeks after the intervention. Wellness parameters such as total white blood cells (WBC) and red blood cells (RBC) counts, Hb (Haemoglobin), bleeding, and clotting time were recorded.
2.6. Statistics
Statistical analysis of data was performed using the Daniel's XL Toolbox, version 5.09. For the cell counts and hematological analysis, significant differences in the observed values were tested using one-way analysis of variance (ANOVA). When significant differences were found, multiple comparison/post hoc testing based on Bonferroni-Holm was carried out. Differences were considered statistically significant when P < 0.05.
3. Results
3.1. Adhesion of P. acidilactici MTCC5101 to Caco-2 Cells
Figures 1(a) and 1(b) represent SEM micrographs of untreated Caco-2 cells and P. acidilactici MTCC5101 with their typical tetrad arrangement. After examining the SEM images of cocultured P. acidilactici MTCC5101 with Caco-2 cells, it is clearly illustrated that the selected probiotic has a very high tendency to adhere to intestinal epithelium or Caco-2 cells at the mucosal surface. Adhesion of selected probiotic strain onto monolayers of Caco-2 cells was evaluated on different microscopic fields per coverslip and at an average 152 ± 33 cells adhered per 100 Caco-2 cells. A biofilm of adherent bacteria constituted of pediococci was observed. The bacteria were also found to adhere to each other (Figures 1(c) and 1(d)).
Figure 1.
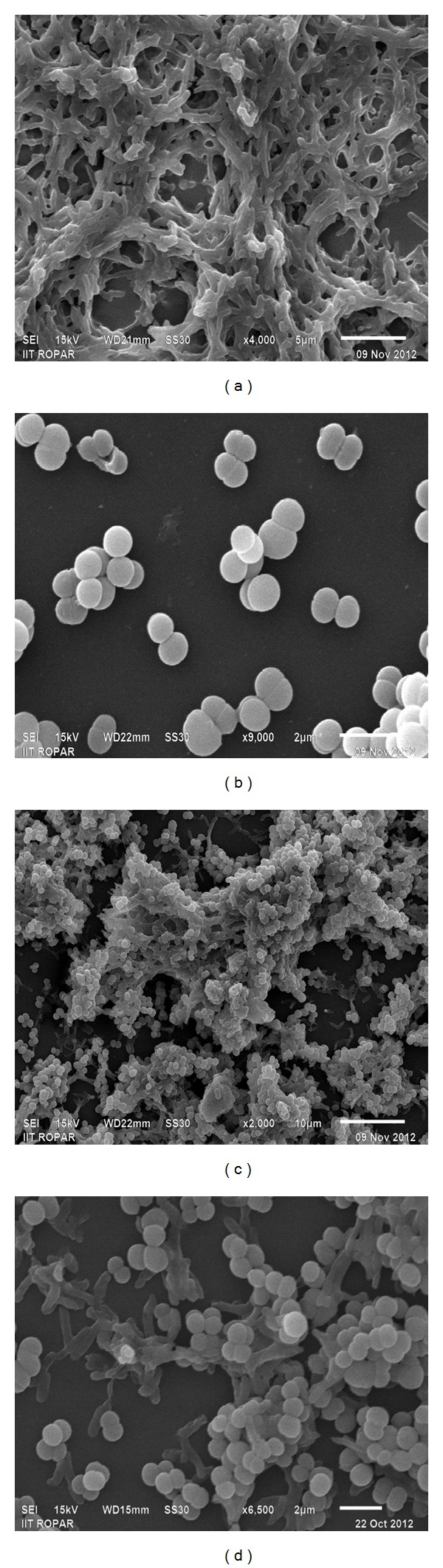
SEM micrographs of (a) untreated Caco-2 cells (magnification level 4,000x) (b) P. acidilactici MTCC 5101 (magnification level 9,000x), and (c) and (d) adherent P. acidilactici MTCC 5101 on Caco-2 cells (magnification level 2,000x and 6,500x).
3.2. Total LAB Count in Fecal Samples
In Table 1 the total cell counts of P. acidilactici MTCC5101 in buttermilk base are recorded. All the subjects have received oral doses of 108–1010 cells/mL/day of P. acidilactici in buttermilk base. The fecal swab samples cultured in MRS medium showed growth of mixed LAB with cell counts of 9.27 ± 1.01 cells/mL at the baseline (day 0) and 9.31 ± 0.98 cells/mL after 4 weeks of intervention. Similarly, fecal swab samples from control group showed cell counts of 10.17 ± 1.06 cells/mL at the baseline and 9.76 ± 0.33 cells/mL after 4 weeks (Table 2).
Table 1.
Viable cell counts of P. acidilactici MTCC5101 in buttermilk base.
| Food base | Bacterial counta (cells/mL) | |||
|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | |
| P. acidilactici MTCC5101/mL of buttermilk base | 9.9 × 108 | 1.01 × 109 | 1 × 1010 | 1.02 × 1010 |
aValues are expressed as cells/mL.
Table 2.
Total LAB counts enumerated in fecal samples.
| Interval (days) | Bacterial counts (cells/mL)a | |
|---|---|---|
| VC (n = 3) | VS (n = 10) | |
| At baseline (day 0) | 10.17 ± 1.06 | 9.27 ± 1.01 |
| After intervention (day 30) | 9.76 ± 0.33 | 9.31 ± 0.98 |
aValues are expressed as mean ± SD of log10 values.
VC: volunteer controls.
VS: volunteer subjects.
(n): no. of individuals.
3.2.1. Molecular Identification of P. acidilactici MTCC5101 in Fecal Samples
Results of the PCR analysis coincide with count data that indicates detectable levels of P. acidilactici MTCC5101 in all the volunteers after a 4-week intervention. Figures 2(a) and 2(b) depict agarose gel analysis of 323 bp fragment of partial pedA gene amplified from isolated whole LAB plasmids of 5th and 10th day's fecal samples. Intensity of the PCR amplicon is lesser in the 5th day's samples which show that the establishment of the probiotic strain requires a little longer time to persist and colonize in the human gut. The fragment of the expected size was obtained in nearly all 10th day's samples and the bands in agarose gels were more prominent as compared to 5th day which confirms that the pediococci have started gut colonization. A similar pattern is observed in the 20th and 30th day's fecal samples on 2% agarose (Figures 2(c) and 2(d)). The band intensity was more prominent as compared to 5th and 10th day samples which indicate that the strain has colonized in the GI tract of volunteers, hence proving that colonization progresses in a time-dependent manner.
Figure 2.
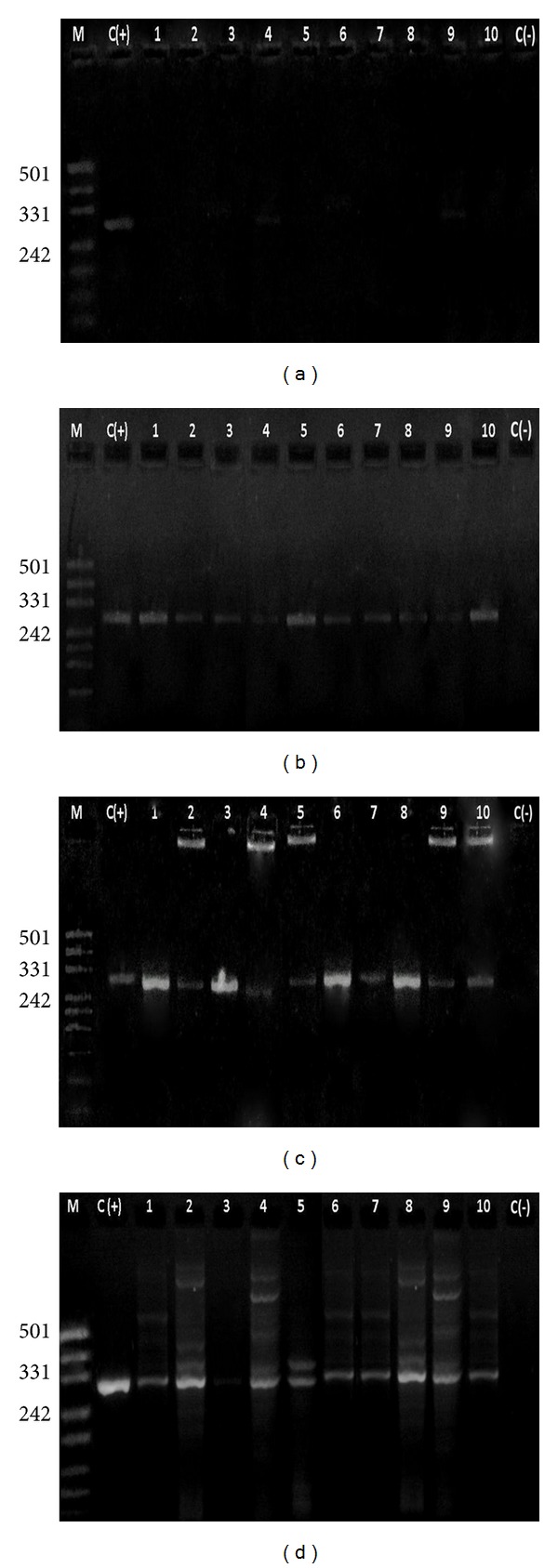
2% agarose gel showing 323 bp DNA fragment amplified using plasmids from fecal samples (a) M: marker pUC 19/Msp Digest, C(+): positive control, Lane 1–10: 5th day samples, C(−): negative control. (b) M: marker pUC 19/Msp digest, C(+): positive control, lane 1–10: 10th day's samples, C(−): Negative control. (c) M: Marker pUC 19/Msp Digest, C(+): Positive control, Lane 1–10: 20th day's samples, C(−): negative control. (d) M: marker pUC 19/Msp Digest, C(+): positive control, lane 1–10: 30th day's samples, C(−): negative control.
3.2.2. Bacteriocin Activity Assay
To check for pediocin CP2 profile in the mixed fecal cell cultures of 30th day, standard well diffusion assay was carried out using E. faecalis as the indicator strain. After 24 h, definite zone of inhibition was seen in the plates that also confirms our previous findings on successful establishment of P. acidilactici MTCC5101 in GI tract of volunteers (Figure 3).
Figure 3.
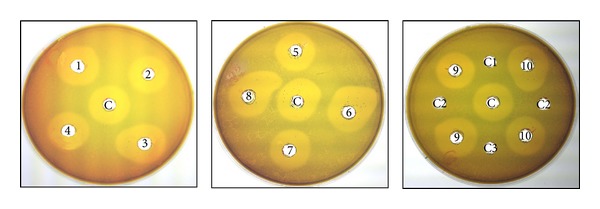
Well diffusion assay of cultured fecal samples of 30th day's against E. faecalis. C: P. acidilactici MTCC 5101; C1–C3: Fecal swab samples of volunteer controls; 1–10: Fecal swab samples of volunteer subjects.
3.3. Wellness Parameters Performed
Routine blood tests were performed to estimate the effect of probiotics on wellness parameters of volunteers. The tests include WBC and RBC counts, levels of Hb in blood, bleeding time, and clotting time (Table 3). Haematological survey was carried out before as well as after feeding trial to estimate the effect of probiotics on some of the wellness parameters of test subjects. Results indicated a small yet insignificant increase in the values of RBC counts and Hb levels of subjects. The findings confirm the safe oral consumption and health improvement capacity of probiotic strain.
Table 3.
Haematological analysis of healthy human subjects.
| Haematological tests | Interval | Response in volunteersa | |
|---|---|---|---|
| VC (n = 3) | VS (n = 10) | ||
| WBC counts (per mm3 of blood) | Before | 3.73 ± 0.02 | 3.71 ± 0.05 |
| After | 3.71 ± 0.03 | 3.70 ± 0.04 | |
| RBC counts (per mm3 of blood) | Before | 6.63 ± 0.02 | 6.56 ± 0.06 |
| After | 6.62 ± 0.02 | 6.66 ± 0.03 | |
| Hb (gm/dL) | Before | 0.98 ± 0.02 | 0.95 ± 0.03 |
| After | 0.98 ± 0.05 | 3.15 ± 4.4 | |
| Bleeding time (sec) | Before | 2.09 ± 0.03 | 2.04 ± 0.11 |
| After | 2.10 ± 0.03 | 2.04 ± 0.13 | |
| Clotting time (sec) | Before | 2.35 ± 0.08 | 2.34 ± 0.07 |
| After | 2.34 ± 0.07 | 2.35 ± 0.06 | |
aValues are expressed as mean ± SD of log10 values.
VC: volunteer controls.
VS: volunteer subjects.
(n): no. of individuals.
4. Discussion
Bacterial adhesion to epithelial cells in gut is initially based on nonspecific physical interactions between the two surfaces [41, 42]. After primary attachment to epithelial surface, secondary interactions between bacterial adhesins and complementary epithelial receptors play a key role in adhesion of bacterial cells to intestinal mucin and enterocytes [22, 43–47]. Since there is difficulty in studying bacterial adhesion in vivo, intestinal cell lines are widely used as in vitro models for assessment [48]. We have used a well-characterized cultured colon carcinoma Caco-2 cell line displaying typical features of enterocytic differentiation in the form of villi to study adhesion of P. acidilactici MTCC5101. A strong adherence of probiotic strains to intestinal epithelial cells has been reported previously in a number of studies (Table 4). In a recent study by Jensen et al. [49], it has been reported that the adhesion capacity of probiotics varies from species to species as a variation from 1% to 25% has been observed in case of 18 known probiotic lactobacilli and pediococci. The current study provides clear evidence that P. acidilactici MTCC5101 adheres strongly to villi of Caco-2 cells. These results further strengthened the claim of this strain for selection as a probiotics for human use.
Table 4.
Adhesion studies based on Probiotics.
| Organism(s) | Cells/cell line(s) | Adhesion index* | Reference |
|---|---|---|---|
|
Bifidobacterium breve (4, 5, 25) B. longum (4, 16, 18, 22) B. bifidum (7, 8) B. infantis (1) |
Caco-2 HT29-MTX |
B. breve (4): 205 ± 18 B. longum (16): 72 ± 13 B. bifidum (8): 35 ± 6 B. infantis (1): 161 ± 14 |
[17] |
|
| |||
|
Lactobacillus rhamnosus GG L. casei strain Shirota L. johnsonii La1 L. rhamnosus LC 705 B. lactis Bb12 |
Mucus from feces |
L. rhamnosus GG: 28.8% L. johnsonii La1: 27.3% B. lactis Bb12: 10.0% L. casei strain Shirota: 4.0% L. rhamnosus Lc705: 1.3% |
[21] |
|
| |||
|
L. rhamnosus GG B. animalis subsp. lactis Bb12 B. animalis IATA-A2 B. bifidum IATA-ES2 |
Caco-2 HT29-MTX |
HT29-MTX: 0.5–2.3% Caco-2/HT29-MTX: 0.6–3.2% |
[22] |
|
| |||
|
L. plantarum (9, 72, 75, 77, 90, 91) L. delbrueckii subsp. bulgaricus CH4 L. plantarum CSCC5276 |
HT-29 Caco-2 |
L. plantarum Lp91: 12.8 ± 1.56 | [23] |
|
| |||
| 163 Lactobacillaceae sp. | HT-29 HT-29 MTX |
HT-29
Pediococci: 12.51% ± 1.4% Lactobacilli: 4.8% ± 61.6% HT-29 MTX Pediococci: 13.5% ± 62.0% Lactobacilli: 10.3% ± 62.4% |
[24] |
|
| |||
| P. acidilactici MTCC 5101 | Caco-2 | P. acidilactici: 152 ± 33 | Present study |
*Adhesion is indexed as % adhesion or mean ± SD of the number of bacterial cells adhered per 100 cells of cell line used.
Oral consumption of probiotics has been advocated with prophylactic and curative properties that have been observed in case of intestinal disorders such as antibiotic-induced diarrheal disease, viral and bacterial diarrhea, lactose intolerance, and inflammatory bowel diseases [27, 29, 34, 50–55]. Recently, there has been accumulation of evidence from rigorous clinical studies on well-characterized probiotics having real health-promoting properties [56, 57].
Although minimum effective dose is not known exactly, usually an oral dose of 106 CFU/day or more than this has been followed in most studies (Table 5). Previous clinical and colonization studies prove successful intestinal colonization of probiotic bacteria and prevention of diseased condition. A comparative analysis of such in vivo clinical studies demonstrates that the persistence time of probiotic bacteria in gut varies from strain to strain (Table 5). The relative strain-specific persistence in vivo correlates accurately and significantly with in vitro outcomes as evident from a recent study on Lactobacillus plantarum [58]. Studies on probiotic Lactobacillus casei strain DN-114 001 and L. casei strain Shirota have proven the capacity of these strains to survive and colonize human gut [59, 60]. Persistence of probiotic strains in GI tract is also demonstrated by their bile and acid resistance properties, as shown in earlier study carried out on the present strain [12, 61–64].
Table 5.
Studies of oral dosage levels of probiotics in healthy volunteers and patients.
| Organism(s) | Subjects | Dose levels (CFU/mL/day) | Response/outcomes | Reference |
|---|---|---|---|---|
|
Lactobacillus paracasei subsp. paracasei (CRL-431) L. acidophilus |
Children and adults with diarrhea | 107-108 | Three 15 day trial periods; reduction in incidence of intestinal disorders | [25] |
| Lactobacillus GG | Healthy volunteers | 108–1010 | 1-week trial; effective gut colonization | [26] |
|
Bifidobacterium lactis BB-12 Lactobacillus GG |
Children with atopic eczema | 108-109 | 2-month trial; controlled allergic reactions | [27] |
| B. longum SBT2928 | Healthy volunteers | 1011 | 40-day trial; survival in the gut | [28] |
| Lactobacillus GG | Children with acute infectious diarrhea | 109 | Prophylactic; reduction in duration of diarrhea | [29] |
| L. casei subsp. rhamnosus Lcr35 | Healthy volunteers | 108–1012 | 1-week study; successful colonization of gut | [30] |
| L. reuteri ATCC 55730 | Healthy volunteers | 108 | 28-day trial; gut colonization; immune modulation | [31] |
|
B. animalis subsp. lactis BB-12 Streptococcus thermophilus |
Children with rotavirus diarrhea | 108 | Reduction in incidence of acute diarrhea and rotavirus shedding | [32] |
| B. lactis strain BB12 | Healthy breastfed infants | 106 | Prophylactic against acute diarrhea | [33] |
|
B. animalis subsp. lactis BB-12 L. reuteri ATCC 55730 |
Children with acute diarrhea | 107 | 12-week trial; fewer and shorter episodes of diarrhea | [34] |
|
L. delbrueckii subsp. bulgaricus
S. thermophilus |
Healthy volunteers | 107–109
(L. delbrueckii) 108–1010 (S. thermophilus) |
12-day trial; effective gut colonization | [35] |
|
B. animalis subsp. lactis BB-12 L. paracasei subsp. paracasei CRL-431 |
Healthy volunteers | 108–1011 | 7-week study; fecal recovery increases with increase in dose | [36] |
|
L. reuteri DSM 17938 L. rhamnosus GG |
Healthy volunteers | 109 | 3-week trial; increase in fecal recovery of viable lactobacilli | [37] |
| Pediococcus acidilactici MTCC 5101 | Healthy human volunteers | 108–1010 | 4-week trial; colonization and fecal recovery increases with time | Present study |
Survival in the GI tract depends on both the strain and the food matrix involved [65]. Fecal recovery of several probiotic strains has been demonstrated in different food matrices, including fermented milk and yoghurt [66, 67], fruit drinks [68], supplements [36, 69], and infant formula [65]. The survival of probiotics in human GI tract should lead to shedding of live cells in fecal samples which can be detected using quantitative methods like PCR [37, 70]. In the present parallel, controlled human intervention study, in vivo persistence and colonization of P. acidilactici MTCC5101 in GI tract provides a clear evidence for intimate interactions between the selected probiotic bacteria and intestinal mucosal surface. These interactions allow probiotic strain to persist in gut for a considerable time period, regardless of the dietary and physiological differences among individuals selected in the study. Furthermore, results indicate that buttermilk is a suitable carrier medium for P. acidilactici MTCC5101 strengthening the use of buttermilk as a probiotic product.
Abnormal blood biochemical parameters are an indicator of a number of clinical disorders. Oral consumption of probiotics has not been linked to any adverse subclinical effects on blood biochemistry so far [71, this study]. Probiotics have been reported to enhance absorption of essential vitamins and minerals from the diet into the body [72, 73]. The enhanced absorption of vitamins and minerals has led to improved haematological environment and gut health. A slight increase in RBC counts and Hb levels of volunteer subjects after oral feeding with probiotics strain was observed. Both in vitro models and in vivo studies have suggested the successful establishment of P. acidilactici MTCC5101 in human gut that is being proposed herein with the possibility of providing beneficial health effects to the host.
5. Conclusions
In conclusion, P. acidilactici MTCC5101 can survive passage through the human GI tract when administered orally in a buttermilk food base. Overall, results indicate that P. acidilactici MTCC5101 is a safe and potent probiotic strain with strong adhesive and health-improving characteristics. The findings suggest an opportunity for successful use of P. acidilactici MTCC5101 in functional food applications in future.
Conflict of Interests
The authors declare that they have no conflict of interests.
Acknowledgment
This work has been supported by Department of Science and Technology, Government of India (Major Research Project no. SR/SO/HS-38/2009).
References
- 1.Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136(1):65–80. doi: 10.1053/j.gastro.2008.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaughan EE, Heilig HGHJ, Ben-Amor K, de Vos WM. Diversity, vitality and activities of intestinal lactic acid bacteria and bifidobacteria assessed by molecular approaches. FEMS Microbiology Reviews. 2005;29(3):477–490. doi: 10.1016/j.femsre.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Zoetendal EG, Vaughan EE, de Vos WM. A microbial world within us. Molecular Microbiology. 2006;59(6):1639–1650. doi: 10.1111/j.1365-2958.2006.05056.x. [DOI] [PubMed] [Google Scholar]
- 4.Salminen S, von Wright A, Morelli L, et al. Demonstration of safety of probiotics—a review. International Journal of Food Microbiology. 1998;44(1-2):93–106. doi: 10.1016/s0168-1605(98)00128-7. [DOI] [PubMed] [Google Scholar]
- 5.Carr FJ, Chill D, Maida N. The lactic acid bacteria: a literature survey. Critical Reviews in Microbiology. 2002;28(4):281–370. doi: 10.1080/1040-840291046759. [DOI] [PubMed] [Google Scholar]
- 6.Borriello SP, Hammes WP, Holzapfel W, et al. Safety of probiotics that contain Lactobacilli or bifidobacteria. Clinical Infectious Diseases. 2003;36(6):775–780. doi: 10.1086/368080. [DOI] [PubMed] [Google Scholar]
- 7.Food and Agriculture Organization of the United Nations-World Health Organization Working Group. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food. Geneva, Switzerland: World Health Organization; 2002. Guidelines for the evaluation of probiotics in foods. [Google Scholar]
- 8.Ezendam J, van Loveren H. Probiotics: immunomodulation and evaluation of safety and efficacy. Nutrition Reviews. 2006;64(1):1–14. doi: 10.1111/j.1753-4887.2006.tb00168.x. [DOI] [PubMed] [Google Scholar]
- 9.Lebeer S, Vanderleyden J, de Keersmaecker SCJ. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nature Reviews Microbiology. 2010;8(3):171–184. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- 10.Collado MC, Isolauri E, Salminen S, Sanz Y. The impact of probiotic on gut health. Current Drug Metabolism. 2009;10(1):68–78. doi: 10.2174/138920009787048437. [DOI] [PubMed] [Google Scholar]
- 11.Lebeer S, Vanderleyden J, de Keersmaecker SCJ. Genes and molecules of Lactobacilli supporting probiotic action. Microbiology and Molecular Biology Reviews. 2008;72(4):728–764. doi: 10.1128/MMBR.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaur B, Balgir PP. Purification, characterization and antimicrobial range of bacteriocin obtained from an isolate of Pediococcus spp. Journal of Punjab Academy of Sciences. 2004;1(2):139–144. [Google Scholar]
- 13.Kaur B, Balgir PP. Pediocin CP2 gene localization to plasmid pCP289 of Pediococcus acidilactici MTCC 5101. Internet Journal of Microbiology. 2007;3(2) [Google Scholar]
- 14.Kaur B, Balgir PP. Biopreservative potential of a broad-range pediocin CP2 obtained from Pediococcus acidilactici MTCC 5101. Asian Journal of Microbiology, Biotechnology and Environmental Sciences. 2008;10(2):439–444. [Google Scholar]
- 15.Kumar B, Balgir PP, Kaur B, Mittu B, Chauhan A. In vitro cytotoxicity of native and rec-pediocin CP2 against cancer cell lines: a comparative study. Pharmaceutical Analytical Acta. 2012;1(6, article 316) [Google Scholar]
- 16.de Man JC, Rogosa M, Sharpe ME. A medium for the cultivation of Lactobacilli . Journal of Applied Bacteriology. 1960;23(1):130–135. [Google Scholar]
- 17.Bernet M-F, Brassart D, Neeser J-R, Servin AL. Adhesion of human bifidobacterial strains to cultured human intestinal epithelial cells and inhibition of enteropathogen-cell interactions. Applied and Environmental Microbiology. 1993;59(12):4121–4128. doi: 10.1128/aem.59.12.4121-4128.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braet F, de Zanger R, Wisse E. Drying cells for SEM, AFM and TEM by hexamethyldisilazane: a study on hepatic endothelial cells. Journal of Microscopy. 1997;186(1):84–87. doi: 10.1046/j.1365-2818.1997.1940755.x. [DOI] [PubMed] [Google Scholar]
- 19.Berkson J, Magath TB, Hurn M. The error of estimate of the blood cell count as made with the hemocytometer. The American Journal of Physiology. 1939;128:309–323. [Google Scholar]
- 20.Elli M, Callegari ML, Ferrari S, et al. Survival of yogurt bacteria in the human gut. Applied and Environmental Microbiology. 2006;72(7):5113–5117. doi: 10.1128/AEM.02950-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouwehand AC, Kurvinen T, Rissanen P. Use of a probiotic Bifidobacterium in a dry food matrix, an in vivo study. International Journal of Food Microbiology. 2004;95(1):103–106. doi: 10.1016/j.ijfoodmicro.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Laparra JM, Sanz Y. Comparison of in vitro models to study bacterial adhesion to the intestinal epithelium. Letters in Applied Microbiology. 2009;49(6):695–701. doi: 10.1111/j.1472-765X.2009.02729.x. [DOI] [PubMed] [Google Scholar]
- 23.Duary RK, Bhausaheb MA, Batish VK, Grover S. Anti-inflammatory and immunomodulatory efficacy of indigenous probiotic Lactobacillus plantarum Lp91 in colitis mouse model. Molecular Biology Reports. 2012;39(4):4765–4775. doi: 10.1007/s11033-011-1269-1. [DOI] [PubMed] [Google Scholar]
- 24.Turpin W, Humblot C, Noordine M, Thomas M, Guyot J. Lactobacillaceae and cell adhesion: genomic and functional screening. PLoS ONE. 2012;7(5) doi: 10.1371/journal.pone.0038034.e38034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez S, Albarracin G, de Ruiz Pesce ML, et al. Prevention of infantile diarrhoea by fermented milk. Microbiologie, Aliments, Nutrition. 1990;8:349–354. [Google Scholar]
- 26.Saxelin M, Ahokas M, Salminen S. Dose response on the faecal colonisation of Lactobacillus strain GG administered in two different formulations. Microbial Ecology Health Diseases. 1993;6(3):119–122. [Google Scholar]
- 27.Isolauri E, Arvola T, Sutas Y, Moilanen E, Salminen S. Probiotics in the management of atopic eczema. Clinical and Experimental Allergy. 2000;30(11):1604–1610. doi: 10.1046/j.1365-2222.2000.00943.x. [DOI] [PubMed] [Google Scholar]
- 28.Fujiwara S, Seto Y, Kimura A, Hashiba H. Intestinal transit of an orally administered streptomycin-rifampicin-resistant variant of Bifidobacterium longum SBT2928: its long-term survival and effect on the intestinal microflora and metabolism. Journal of Applied Microbiology. 2001;90(1):43–52. doi: 10.1046/j.1365-2672.2001.01205.x. [DOI] [PubMed] [Google Scholar]
- 29.Szajewska H, Mrukowicz JZ. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. Journal of Pediatric Gastroenterology and Nutrition. 2001;33(4):S17–S25. doi: 10.1097/00005176-200110002-00004. [DOI] [PubMed] [Google Scholar]
- 30.de Champs C, Maroncle N, Balestrino D, Rich C, Forestier C. Persistence of colonization of intestinal mucosa by a probiotic strain, Lactobacillus casei subsp. rhamnosus Lcr35, after oral consumption. Journal of Clinical Microbiology. 2003;41(3):1270–1273. doi: 10.1128/JCM.41.3.1270-1273.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valeur N, Engel P, Carbajal N, Connolly E, Ladefoged K. Colonization and immunomodulation by Lactobacillus reuteri ATCC 55730 in the human gastrointestinal tract. Applied and Environmental Microbiology. 2004;70(2):1176–1181. doi: 10.1128/AEM.70.2.1176-1181.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saavedra JM, Bauman NA, Oung I, Perman JA, Yolken RH. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. The Lancet. 1994;344(8929):1046–1049. doi: 10.1016/s0140-6736(94)91708-6. [DOI] [PubMed] [Google Scholar]
- 33.Chouraqui J, van Egroo L, Fichot M. Acidified milk formula supplemented with Bifidobacterium lactis: impact on infant diarrhea in residential care settings. Journal of Pediatric Gastroenterology and Nutrition. 2004;38(3):288–292. doi: 10.1097/00005176-200403000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Weizman Z, Asli G, Alsheikh A. Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics. 2005;115(1):5–9. doi: 10.1542/peds.2004-1815. [DOI] [PubMed] [Google Scholar]
- 35.Mater DDG, Bretigny L, Firmesse O, et al. Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus survive gastrointestinal transit of healthy volunteers consuming yogurt. FEMS Microbiology Letters. 2005;250(2):185–187. doi: 10.1016/j.femsle.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Larsen CN, Nielsen S, Kæstel P, et al. Dose-response study of probiotic bacteria Bifidobacterium animalis subsp. lactis BB-12 and Lactobacillus paracasei subsp. paracasei CRL-341 in healthy young adults. European Journal of Clinical Nutrition. 2006;60(11):1284–1293. doi: 10.1038/sj.ejcn.1602450. [DOI] [PubMed] [Google Scholar]
- 37.Dommels YEM, Kemperman RA, Zebregs YEMP, et al. Survival of Lactobacillus reuteri DSM 17938 and Lactobacillus rhamnosus GG in the human gastrointestinal tract with daily consumption of a low-fat probiotic spread. Applied and Environmental Microbiology. 2009;75(19):6198–6204. doi: 10.1128/AEM.01054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gossling J, Slack JM. Predominant gram-positive bacteria in human feces: numbers, variety, and persistence. Infection and Immunity. 1974;9(4):719–729. doi: 10.1128/iai.9.4.719-729.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atashpaz S, Khani S, Barzegari A, et al. A robust universal method for extraction of genomic DNA from bacterial species. Microbiology. 2010;79(4):538–542. [PubMed] [Google Scholar]
- 40.Sarkar PK, Banerjee S. Antibacterial activity of lactic acid bacterial isolates obtained from natural habitats. Journal of Food Science and Technology. 1996;33(3):231–233. [Google Scholar]
- 41.Oliveira R. Understanding adhesion: a means for preventing fouling. Experimental Thermal and Fluid Science. 1997;14(4):316–322. [Google Scholar]
- 42.Hermansson M. The DLVO theory in microbial adhesion. Colloids and Surfaces B. 1999;14(1–4):105–119. [Google Scholar]
- 43.Beachey EH. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surfaces. Journal of Infectious Diseases. 1981;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- 44.Dalton HM, March PE. Molecular genetics of bacterial attachment and biofouling. Current Opinion in Biotechnology. 1998;9(3):252–255. doi: 10.1016/s0958-1669(98)80055-4. [DOI] [PubMed] [Google Scholar]
- 45.Knight SD, Berglund J, Choudhury D. Bacterial adhesins: structural studies reveal chaperone function and pilus biogenesis. Current Opinion in Chemical Biology. 2000;4(6):653–660. doi: 10.1016/s1367-5931(00)00144-7. [DOI] [PubMed] [Google Scholar]
- 46.Otto K, Elwing H, Hermansson M. The role of type 1 fimbriae in adhesion of Escherichia coli to hydrophilic and hydrophobic surfaces. Colloids and Surfaces B. 1999;15(1):99–111. [Google Scholar]
- 47.Viswanathan VK, Koutsouris A, Lukic S, et al. Comparative analysis of EspF from enteropathogenic and enterohemorrhagic Escherichia coli in alteration of epithelial barrier function. Infection and Immunity. 2004;72(6):3218–3227. doi: 10.1128/IAI.72.6.3218-3227.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lehto EM, Salminen S. Adhesion of two Lactobacillus strains, one Lactococcus and one Propionibacterium strain to cultured human intestinal Caco-2 cell line. Bioscience and Microflora. 1997;16(1):13–17. [Google Scholar]
- 49.Jensen H, Grimmer S, Naterstad K, Axelsson L. In vitro testing of commercial and potential probiotic lactic acid bacteria. International Journal of Food Microbiology. 2012;153(1-2):216–222. doi: 10.1016/j.ijfoodmicro.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez SN, Cardozo R, Apella MC, Oliver G. Biotherapeutic role of fermented milk. Biotherapy. 1994;8(2):129–134. doi: 10.1007/BF01878496. [DOI] [PubMed] [Google Scholar]
- 51.Schiffrin EJ, Rochat F, Link-Amster H, Aeschlimann JM, Donnet-Hughes A. Immunomodulation of human blood cells following the ingestion of lactic acid bacteria. Journal of Dairy Science. 1995;78(3):491–497. doi: 10.3168/jds.S0022-0302(95)76659-0. [DOI] [PubMed] [Google Scholar]
- 52.Fukushima Y, Kawata Y, Hara H, Terada A, Mitsuoka T. Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. International Journal of Food Microbiology. 1998;42(1-2):39–44. doi: 10.1016/s0168-1605(98)00056-7. [DOI] [PubMed] [Google Scholar]
- 53.Gaon D, Garmendia C, Murrielo NO, et al. Effect of Lactobacillus strains (L. casei and L. acidophillus strains cerela) on bacterial overgrowth-related chronic diarrhea. Medicina. 2002;62(2):159–163. [PubMed] [Google Scholar]
- 54.Blum S, Schiffrin EJ. Intestinal microflora and homeostasis of the mucosal immune response: implications for probiotic bacteria? Current Issues in Intestinal Microbiology. 2003;4(2):53–60. [PubMed] [Google Scholar]
- 55.Hart AL, Lammers K, Brigidi P, et al. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004;53(11):1602–1609. doi: 10.1136/gut.2003.037325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Vrese M, Stegelmann A, Richter B, Fenselau S, Laue C, Schrezenmeir J. Probiotics—compensation for lactase insufficiency. The American Journal of Clinical Nutrition. 2001;73(2):421S–429S. doi: 10.1093/ajcn/73.2.421s. [DOI] [PubMed] [Google Scholar]
- 57.Messaoudi S, Kergourlay G, Rossero A, et al. Identification of Lactobacilli residing in chicken ceca with antagonism against Campylobacter. International Microbiology. 2011;14(2):103–110. doi: 10.2436/20.1501.01.140. [DOI] [PubMed] [Google Scholar]
- 58.van Bokhorst-van de Veen H, van Swam I, Wels M, Bron PA, Kleerebezem M. Congruent strain specific intestinal persistence of Lactobacillus plantarum in an intestine-mimicking in vitro system and in human volunteers. PLoS ONE. 2012;7(9) doi: 10.1371/journal.pone.0044588.e44588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oozeer R, Leplingard A, Mater DDG, et al. Survival of Lactobacillus casei in the human digestive tract after consumption of fermented milk. Applied and Environmental Microbiology. 2006;72(8):5615–5617. doi: 10.1128/AEM.00722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tuohy KM, Pinart-Gilberga M, Jones M, Hoyles L, McCartney AL, Gibson GR. Survivability of a probiotic Lactobacillus casei in the gastrointestinal tract of healthy human volunteers and its impact on the faecal microflora. Journal of Applied Microbiology. 2007;102(4):1026–1032. doi: 10.1111/j.1365-2672.2006.03154.x. [DOI] [PubMed] [Google Scholar]
- 61.Mirlohi M, Soleimanian-Zad S, Dokhani S, Sheikh-Zeinodin M, Abghary A. Investigation of acid and bile tolerance of native Lactobacilli isolated from fecal samples and commercial probiotics by growth and survival studies. Iranian Journal of Biotechnology. 2009;7(4):233–240. [Google Scholar]
- 62.Both E, Gyorgy E, Kibedi-szabo CZ, et al. Acid and bile tolerance, adhesion to epithelial cells of probiotic microorganisms. UPB Scientific Bulletin B. 2010;72(2):1454–2331. [Google Scholar]
- 63.Singhal K, Joshi H, Chaudhary BL. Bile and acid tolerance ability of probiotic Lactobacillus strains. Journal of Global Pharma Technology. 2010;2(12):17–25. [Google Scholar]
- 64.Sahadeva RPK, Leong SF, Chua KH, et al. Survival of commercial probiotic strains to pH and bile. International Food Research Journal. 2011;18(4):1515–1522. [Google Scholar]
- 65.Saxelin M, Pessi T, Salminen S. Fecal recovery following oral administration of Lactobacillus strain GG (ATCC 53103) in gelatine capsules to healthy volunteers. International Journal of Food Microbiology. 1995;25(2):199–203. doi: 10.1016/0168-1605(94)00091-j. [DOI] [PubMed] [Google Scholar]
- 66.Goldin BR, Gorbach SL, Saxelin M, Barakat S, Gualtieri L, Salminen S. Survival of Lactobacillus species (strain GG) in human gastrointestinal tract. Digestive Diseases and Sciences. 1992;37(1):121–128. doi: 10.1007/BF01308354. [DOI] [PubMed] [Google Scholar]
- 67.Satokari RM, Vaughan EE, Akkermans ADL, Saarela M, de Vos WM. Polymerase chain reaction and denaturing gradient gel electrophoresis monitoring of fecal Bifidobacterium populations in a prebiotic and probiotic feeding trial. Systematic and Applied Microbiology. 2001;24(2):227–231. doi: 10.1078/0723-2020-00035. [DOI] [PubMed] [Google Scholar]
- 68.Önning G, Berggren A, Drevelius M, Jeppsson B, Lindberg AM, Johansson Hagslätt ML. Influence of a drink containing different antioxidants and Lactobacillus plantarum 299v on plasma total antioxidant capacity, selenium status and faecal microbial flora. International Journal of Food Sciences and Nutrition. 2003;54(4):281–289. doi: 10.1080/0963748031000091964. [DOI] [PubMed] [Google Scholar]
- 69.Haschke F, Wang W, Ping G, et al. Clinical trials prove the safety and efficacy of the probiotic strain Bifidobacterium Bb12 in follow-up formula and growing-up milks. Monatsschrift fur Kinderheilkunde. 1998;146(1, supplement):S26–S30. [Google Scholar]
- 70.Mathys S, von Ah U, Lacroix C, et al. Detection of the pediocin gene pedA in strains from human faeces by real-time PCR and characterization of Pediococcus acidilactici UVA1. BMC Biotechnology. 2007;7(article article 55) doi: 10.1186/1472-6750-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choi SS, Kang BY, Chung MJ, et al. Safety assessment of potential lactic acid bacteria Bifidobacterium longum SPM1205 isolated from healthy Koreans. Journal of Microbiology. 2005;43(6):493–498. [PubMed] [Google Scholar]
- 72.Hill MJ. Intestinal flora and endogenous vitamin synthesis. European Journal of Cancer Prevention. 1997;6(1):S43–S45. doi: 10.1097/00008469-199703001-00009. [DOI] [PubMed] [Google Scholar]
- 73.Scholz-Ahrens KE, Ade P, Marten B, et al. Prebiotics, probiotics, and synbiotics affect mineral absorption, bone mineral content, and bone structure. Journal of Nutrition. 2007;137(3):838S–846S. doi: 10.1093/jn/137.3.838S. [DOI] [PubMed] [Google Scholar]


