Abstract
Tamoxifen is a central component of the treatment of estrogen receptor (ER)-positive breast cancer as a partial agonist of ER. It has been clinically used for the last 30 years and is currently available as a chemopreventive agent in women with high risk for breast cancer. The most challenging issue with tamoxifen use is the development of resistance in an initially responsive breast tumor. This review summarizes the roles of ER as the therapeutic target of tamoxifen in cancer treatment, clinical values and issues of tamoxifen use, and molecular mechanisms of tamoxifen resistance. Emerging knowledge on the molecular mechanisms of tamoxifen resistance will provide insight into the design of regimens to overcome tamoxifen resistance and discovery of novel therapeutic agents with a decreased chance of developing resistance as well as establishing more efficient treatment strategies.
Keywords: Breast cancer, Estrogen receptor, Tamoxifen, Antiestrogen resistance
INTRODUCTION
Breast cancer is one of the most common cancers and the second leading cause of cancer death in American women according to the estimation by the American Cancer Society(ACS). Over 200,000 new cases of breast cancer are diagnosed each year and more than 40,000 deaths have been reported in women in the USA in 2010 alone (Jemal et al., 2010). Despite advances in early detection, therapeutic regimens, and understanding the molecular basis of breast cancer biology, nearly 30% of all patients with early stage breast cancer have recurring disease, which is metastatic in most cases. Breast cancer is a hormone-dependent tumor and estrogen is known to play a major role in the initiation and progression of the disease. Biological actions of estrogen are mediated with the estrogen receptor (ER) and nearly 70% of breast tumors express the ER, progesterone receptor (PR), and/or estrogen-responsive and ER-dependent gene products. Therefore, targeting the ER using ER antagonists or antiestrogens has been a reliable therapeutic measure for all stages of the disease in both pre- and postmenopausal women.
Tamoxifen is a triphenylethylene derivative pharmacologically classifi ed as a selective ER modulator (SERM) that acts as an agonist in the uterus but as an antagonist in the breast. Tamoxifen is the most commonly used chemotherapeutic agent for patients with ER–positive breast cancer, which represents almost 70% of all cases. In the hormone-sensitive breast, tamoxifen acts as a partial antagonist, impairing ER function by competing with estrogen for binding to the receptor (Banerjee et al., 2003). Tamoxifen bound ER complex prevents the genes from being switched on by estrogen, leading to inhibition of estrogenic effects, which are responsible for cancer cell growth or proliferation (Chang et al., 2007). Many ER-positive patients regardless of the high level of ER demonstrate intrinsic resistance to hormonal therapies. A substantial proportion of patients with localized disease and all nearly with advanced disease who initially respond to tamoxifen develop de novo or acquired resistance (EBCTCG, 2005). Interestingly, many patients who relapse on tamoxifen therapy will respond to different types of hormonal manipulation including either ER downregulators/ER angatonists or aromatase inhibitors (AIs), implying that ER continues to play a major role in breast cancer progression (Pike et al., 1993; Forbes et al., 2008; Mouridsen et al., 2009).
Although the molecular mechanisms underlying resistance to tamoxifen remains unclear, various mechanisms have been proposed; for example, differential metabolic activation of tamoxifen, loss of ER function/expression, alterations in crosstalk between ER and growth factor-mediated signaling pathways, the presence of ER-negative cancer stem cells,and dynamic responses to oxidative stress.
This review will focus the molecular basis of the ER-mediated mammary carcinogenesis, clinical use of tamoxifen in breast cancer treatment targeting the ER, and various molecular mechanisms of tamoxifen resistance. A better understanding of the mechanisms for tamoxifen resistance may provide novel strategies to overcome or bypass tamoxifen resistance and make further improvements in cancer therapeutics. Finally, this review will suggest future directions on tamoxifen-associated endocrine therapies.
THE BIOLOGY OF ESTROGEN RECEPTOR-MEDIATED SIGNALING IN MAMMARY CARCINOGENESIS
Mechanisms of action of the estrogen receptors
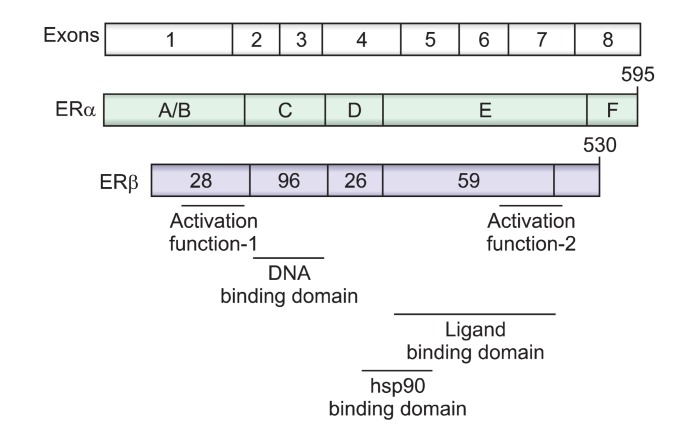
Two ERs, ERα and ERβ, belong to the nuclear receptor (NR) family of transcription factors (Greene et al., 1986). Both receptors are encoded by unique genes composed of eight exons, which are located on different chromosomes with ERα found on the long arm of chromosome 6 and ERβ on chromosome 14 (Fig. 1) (Chang et al., 2002). They contain the evolutionarily conserved modular structure of functional domains characteristic of many other NR families (Mangelsdorf et al., 1995). The central and most conserved domain, the DNA-binding domain (DBD), is involved in DNA recogni-tion and binding, and exhibits a high degree of homology between both ERα and ERβ (Fig. 1). Another functional domain includes the ligand-binding domain (LBD), which occurs near the C-terminal with 60% homology between two ERs. The N-terminal domain represents the most variable domain where homology between two ERs was found to be only 18%. In addition, hinge (E region) and F regions are also not well conserved (Mosselman et al., 1996). Transcriptional activation is facilitated by two distinct activation functions, AF-1 and AF-2. From the structural and functional points of view, it is reasonable to conclude that the two ERs interact with the same DNA response elements and have similar binding affinities for 17β-estradiol (E2) (Kuiper et al., 1997).
Fig. 1. Schematic representation of the common structural andfunctional domains of ERα and ERβ. The cDNA coding for ER iscomposed of 8 exons. The functional domains of the receptors areindicated (A-F), as are the regions responsible for transcriptional activation, DNA binding, ligand binding, and heat shock protein (hsp) 90 binding. The percentage of amino acid homology betweenregions A through F in ERα and ERβ is indicated by the numberswritten on the structure of ERβ. Two transcriptional activation domains, activation function (AF) domains are present in the A/B and E region of the ERs.
Binding of E2 to its receptor, ERα/β, amplifies signals in either the genomic, nongenomic, or mitochondrial ER-mediated signaling pathways that lead to increased cell proliferation and inhibition of apoptosis, uncontrolled cell division or growth and
tumor promotion (Björnström and Sjöberg, 2005). Molecular pathways for ER-mediated signaling transduction are found elsewhere (Heldring et al., 2007) and therefore, they will not be discussed in this review.
Tamoxifen binds to the ER and undergoes conformational change in a similar fashion in which E2 interacts with the ER, except for the repositioning of helix 12 (Shiau et al., 1998). Analysis of the E2-ER LBD complex showed that helix 12 is packed against helix 3, 5/6, and helix 11, sealing the ligand binding pocket like a lid (Brzozowski et al., 1997). This conformation allows the specific amino acids in the AF-2 region to interact with coactivators for transcriptional activation of the target genes. In the 4-OHT [4-hydroxytamoxifen is the active metabolite of tamoxifen in vivo (Jordan, 1977; Jordan et al., 1977)]-ER LBD complex, helix 12 binds to and occludes the coactivator recognition site. The binding mode of the side chain of 4-OHT results in repositioning of helix 12 that prevents the binding of coactivators and thus AF-2 transcription is blocked. Therefore, tamoxifen acts as an ER antagonist on genes which depend on AF-2 activation for ER-mediated transcription. On the other hand, tamoxifen may function as an agonist in the transcription of genes where the AF-1 domain, instead of AF-2 plays a critical role in transcription (McGuire et al., 1977; Tzukerman et al., 1994). The presence of cell or tissue-specific AF-1 or AF-2-activated genes may explain the differential agonistic or antagonistic activity of tissue-specific tamoxifen. The potential for the agonist activity of tamoxifen to outweigh its antagonist effects may serve as one of the molecular ER-mediated mechanisms of tamoxifen resistance. Since the therapeutic target for breast cancer intervention is the ERα, ER refers to ERβ throughout this review unless specified otherwise.
Estrogen and ER-mediated carcinogenesis
Estrogen is implicated in the development of breast cancer, based on the data from the relationship between life-time estrogen exposure and cancer incidence (Henderson and Feigelson, 2000). The greater increase in the risk of developing breast cancer, the longer women are exposed to estrogen either through early onset of menstruation, nulliparity, delayed first childbirth, short duration of breast feeding, late menoause, and estrogen replacement therapy. Estrogens are considered to play a critical role in promoting the proliferation of both the normal and the neoplastic breast epithelium. The most biologically active estrogen, E2, acts locally in the mammary gland, stimulating DNA synthesis and promoting bud formation through an ER-mediated mechanism (Russo et al., 2000). The fact that the normal epithelium contains receptors for estrogen lends support to the receptor-mediated mechanism as a major player in the hormonal regulation of breast development. The role of estrogen on the proliferative activity of the breast, which is indispensable for its normal growth and development, has been for a long time the subject of controversy (Russo and Russo, 2006). Experimental animal systems and clinical observations, however, support the carcinogenicity of estrogens. There are three major mechanisms that have been considered as the molecular mechanisms underlying the estrogen-induced carcinogenesis; 1) ER-mediated hormonal activity, which has been related to stimulation of cell proliferation and rates of cell division, resulting in more opportunities for accumulation of genetic damages (Russo et al., 1988; Habel and Stanford, 1993; Russo et al., 1999), 2) direct genotoxic effects through metabolic activation of estrogens (Liehr, 1998; Cavalieri et al., 2006; Bolton and Thatcher, 2008), and 3) the induction of aneuploidy by estrogen (Russo and Russo, 2006; Quick et al., 2008). These events may disrupt normal and proper cellular processes such as apoptosis, cellular proliferation, or DNA repair (Yue et al., 2005; Russo and Russo, 2006; Yager and Davidson, 2006).
ANTIESTROGEN THERAPY IN ER-POSITIVE BREAST CANCER
Antiestrogens targeting ER
ER signaling is a key regulator of breast tumor proliferation and targeting the ER has been a reliable therapeutic modality for all stages of breast cancer. ER-targeted therapy for breast cancer was first used over a century ago in the form of oophorectomy and it has evolved to become the most effective and least toxic systemic therapy for ER-positive breast cancer (Love and Philips, 2002). Strategies using this type of endocrine manipulation for the treatment of breast cancer include;1) targeting the ER itself with the selective estrogen receptor modulators (SERMs) such as tamoxifen, 2) suppressing the amount of available ER ligand, E2 either with gonadal suppression in premenopausal women by means of an ovariectomy or lutenizing hormone-releasing hormone agonists, or with aromatase inhibitors in postmenopausal women, or 3) downregulating the ER expression for limited ER signaling responses. Considering their proven efficacy and generally tolerable side effects, ER-targeting endocrine therapies are widely used in the treatment of both early stage and recurrent and/or metastatic breast cancer (EBCTCG, 1998).
In patients with early stage breast cancer, adjuvant anti-estrogen therapy given for 5 years has been proven to delay local and distant relapses and prolong overall survival (EBCTCG, 1998). More benefit of this adjuvant tamoxifen therapy has been proposed by the substantial reduction in the incidence of contra-lateral breast cancer in patients with primary breast cancer by half. The results of 5 years of treatment in women aged less than 40 years with ER-positive breast cancer demonstrated the benefits of adjuvant tamoxifen including significant reduction in annual recurrence rate and mortality rate (EBCTCG, 2005). Current clinical practice acknowledged the added benefit from aromatase inhibitor (AI) therapy in terms of disease-free survival and favorable tolerability profiles to long-term tamoxifen therapy (Pike et al., 1993; Forbes et al., 2008; Mouridsen et al., 2009). Although there is a key challenge in predicting the degree of hormone sensitivity and responsiveness to a drug within an individual’s tumor and in determining endocrine and/or chemotherapy regimens, tamoxifen remains the gold standard of treatment option in premenopausal women with early stage breast cancer.
In both pre- and postmenopusal patients with ER-positive metastatic breast cancer, tamoxifen has represented the standard therapy of choice in first-line treatment. For example, in neoadjuvant settings with metastatic breast cancer, more than half of patients with ER-positive tumors achieve an objective response or tumor stabilization as a result of tamoxifen treatment (Jaiyesimi et al., 1995; Anderson et al., 2002). However, use of either the ER antagonist, fulvestrant, or AI provides promising results in these patients in terms of prolonged median time to progression (Paridaens et al., 2008) and in particular, AIs become an integral component of adjuvant therapy.
Tamoxifen plays a central role in the regimen of treatment of ER-positive breast cancer in the adjuvant, advanced, and increasingly, neoadjuvant settings. Clinical trials are ongoing to define the clinical values of tamoxifen in the context of optimal use, duration of treatment, and as sequential treatment option with AIs (Regan et al., 2011; Ring et al., 2011)
Development of antiestrogen resistance
Despite obvious benefits of tamoxifen in the treatment and/or chemoprevention of breast cancer, most initially responsive breast tumors experience a recurrence, indicating that patients eventually develop acquired antiestrogen resistance.
Antiestrogen resistance is generally characterized into two categories; 1) de novo resistance and 2) acquired resistance. de novo resistance is found in ER-positive breast cancers which are nonresponsive to antiestrogen therapy from the beginning of treatment. This type of resistance has been demonstrated in MCF-7, an ER-positive human breast cancer cell line, transfected with the HER2/neu gene which induced tumor growth in xenograft mice even during tamoxifen treatment (Benz et al., 1992). It is speculated that growth factor receptor signaling pathways are involved in tamoxifen resistant cell growth in these cells. On the other hand, acquired resistance is developed after long term therapy in ER-positive tumors that has initially responded to antiestrogen therapy (Jordan, 2004). ER-positive breast tumors with acquired resistance may exhibit either tamoxifen-nonresponsive or tamoxifen-dependent/stimulated growth while continuing to express ER, which renders the tamoxifen-resistant cells responsive to second line therapies such as AIs or fulvestrant. Acquired resistance is well demonstrated when MCF-7 cells are inoculated into ovariectomized athymic mice treated with tamoxifen. Most tumors in these mice initially respond to tamoxifen and do not grow but some tumors begin to grow even in the presence of antiestrogen after about a year. Interestingly enough, the growing tumors are able to continue to grow in other athymic mice in response to either E2 or tamoxifen (Gottardis and Jordan, 1988; O'Regan et al., 2006).
MECHANISMS OF ENDOCRINE RESISTANCE
The precise biological mechanisms underlying acquired resistance to tamoxifen remain unclear. This is partly due to an incomplete understanding of the signaling transduction pathways and components affecting cell proliferation, survival, and death in addition to their estrogen-mediated regulation in breast cancer and complexity of such signaling pathways that are interconnected or converged each other. It is unlikely that any single determinant such as the specific gene or molecular mechanistic pathway is attributed to tamoxifen resistance. It is generally accepted that that several mechanisms exist that encompass various cellular events leading to antiestrogen resistance. Moreover, mechanisms may vary within tumors considering heterogeneity of breast tumor tissues in terms of antiestrogen responsiveness (Ricketts and Coombes, 1989; Clarke et al., 1990; Kurosumi, 2003). The intratumor variability in antiestrogen responsiveness also changes over time and thus several and complex mechanisms are involved in developing resistance to antiestrogen.
In this review, mechanisms of antiestrogen resistance will be introduced in the context of the presence of ER protein and function, genomic and nongenomic ER-mediated signaling network, pharmacologic and immunologic aspects, cell fates, cancer stem cells, and transcription factor-ER interactions. As mentioned earlier, it should be noted that there is not a single mechanism alone responsible for development of antiestrogen resistance and it is very likely that resistance comes from various cellular events. Considering the clinical values of tamoxifen in treatment of breast cancer, it is clear that preventing and overcoming tamoxifen resistance remains important clinical goals. A better understanding of resistance mechanisms would aid in the development of novel strategies to overcome tamoxifen resistance and provide the basis for breast cancer treatment options.
Loss of ER expression and function
Since therapeutic effects of tamoxifen are derived from its ability to mediate ER signaling as an ER antagonist, loss of its cellar target, ER, is attributed to resistance to therapy. Lack of ER expression is clearly the dominant mechanism of de novo resistance to tamoxifen (Ingle et al., 1991; Jaiyesimi et al., 1995). It has been reported that more than 90% of ER/PR-negative tumors do not respond to antiestrogens. Most studies suggest that the majority of patients who develop resistance still express ER at progression, although loss of ER expression is the primary mechanism of tamoxifen resistance in some patients (Encarnación et al., 1993; Dowsett and Haynes, 2003). This is evidenced by the successful use of the AI such as letrozole after 5 years of tamoxifen use in patients with ER-positive tumors but resistance to tamoxifen (Goss et al., 2005). This result could be interpreted as the slow development of acquired resistance by breast cancer micrometastases during the 5 years of tamoxifen treatment so that these patients respond to a non-cross resistant therapy such as AIs or ER-downregulators leading to blocking estrogen synthesis or ER-signaling activity (Howell et al., 2002; EBCTCG, 2005; Goss et al., 2007).
Loss of ER expression involves a switch from an initially ER-positive to ER-negative phenotype. Two potential mechanisms responsible for loss of ER expression are suggested:transcriptional repression of the ER gene and population remodeling leading to overpopulation of ER-negative cells from seemingly heterogeneous ER-positive tumors. These are primarily demonstrated in cell-culture models using T47D or ZR-75-1 cell lines (van den Berg et al., 1989; Graham et al., 1992). Epigenetic modification such as hypermethylation of CpG islands or histone deacetylation is considered as one of major molecular mechanisms for inactivation of ER gene expression (Sharma et al., 2005; Zhou et al., 2007). These studies demonstrate that inhibitors of DNA methyltransferases(DNMT) and histone deacetylases (HDAC) could reactivate the ERα expression in initially ER-negative breast cancer cells, implying that epigenetic modulation can be a valuable to resensitize ER-negative cells to antiestrogen therapies.Studies using epigenetic modulators that inhibit either histone deacetylation or DNA methylation are ongoing in some solid cancers, including breast cancer, to investigate the roles for epigenetic therapies in cancer treatment (Lyko and Brown, 2005; Hurtubise and Momparler, 2006).
Mutations in the ER gene, negative feedback regulation of ER protein expression, and abnormal splicing also confers loss of ER function (Jiang et al., 1992; Fuqua, 1994). Although naturally occurring mutations or artifi cially generated mutations in the ER which affects the antagonistic property of tamoxifen to the agonist-like property have been demonstrated in cultured cell lines, such mutations are detected infrequently in patient samples. In addition, antiestrogen resistance can be certainly seen in the absence of any apparent mutations (Brünner et al., 1997). Therefore, it remains unclear if ER mutations are clinically relevant to antiestrogen resistance (Levenson et al., 1997). Altered ER splicing variants have been yet to be determined for their biological relevance in tamoxifen resistance. Taken together, ER mutations or abnormal ER transcriptional outcomes are not likely a required or dominant mechanism of resistance.
Altered expression patterns of coregulatory proteins
The transcriptional activity of the ligand bound ER is dependent on the proteins called coregulatory proteins that consist of the transcription complex at the promoter region of estrogen target genes (Girault et al., 2006). Coregulators may either activate (coactivators) or inhibit (corepressors) ER-driven transcription. The type, availability, and/or cellular levels of various coregulatory proteins determine the ER’s transcriptional activities. The conformational change in the ER induced by the binding of either agonist or antagonist also determines the differential ability of the ER to recruit coregulatory proteins (Bocchinfuso and Korach, 1997; Zajchowski et al., 2000). For example, tamoxifen may exert the agonistic activity via interaction of the coactivator SRC-1 with the ER AF-1 domain, implying that the agonist or antagonist activity of SERMs such as tamoxifen depend on the cellular and promoter context (Webb et al., 1998). Thus, high expression of some coactivators may enhance the ER agonist activity of tamoxifen and contribute to tamoxifen resistance or low levels of coregulator may be attributed to switch tamoxifen from an antagonist to agonist, leading to tamoxifen resistance and tamoxifen-dependent cell proliferation.
The most studied coactivator, amplified in breast 1 (AIB1), also called steroid receptor coactivator-3 (SRC-3) or thyroid hormone receptor activator molecule 1 (TRAM-1), has been shown to be overexpressed in more than half of breast tumors and also highly expressed in MCF7 cells being essential for the cell growth in vitro or in a xenograft mouse model (Anzick et al., 1997; List et al., 2001). In addition, high AIB1 expression has been associated with a better outcome in the tamoxifen-untreated group, whereas it has been associated with worse prognosis in terms of free survival in the tamoxifen-treated patients when the AIB1 levels were compared between tamoxifen untreated- and treated patients (Osborne et al., 2003). Since AIB1 may interact with HER2 and enhance cyclin D1 expression, and thus affect growth factor-mediated signaling pathways and cell cycle progression, high levels of AIB1 are associated with agonistic activity of tamoxifen and resistance.
The nuclear receptor corepressor 1, NcoR1, has been shown to associate with ER in the presence of a metabolite of tamoxifen, trans-hydroxytamoxifen, and knockdown of NcoR1 mediated agonistic action of this antiestrogen metabolite instead of antagonistic activity (Lavinsky et al., 1998).
It should be noted that the coregulatory proteins associated with ER may interact with other nuclear receptors or with other transcription factors unrelated to the nuclear receptors (Klinge, 2000; Myers et al., 2005). Thus, altered expression of coregulatory proteins may play a limited role in the mechanisms for tamoxifen resistance.
Growth factor receptors/kinase signal transduction pathways in antiestrogen resistance
Signaling cascades through nongenomic epidermal growth factor (EGFR) 2, also known as HER2, are known to influence the genomic actions mediated by ER. HER2, a receptor tyrosine kinase, is a member of the EGFR family and its overexpression is frequently associated with an aggressive phenotype of cancers (Dawson et al., 2009). The ER can be phosphorylated at the Ser 118 or 167 within the AF-1 domain by MAPK and Akt, respectively, which are downstream components of the HER2 signaling pathway. This interaction leads to ligand-independent activation of ER. Preclinical data demonstrated that ER function is augmented by crosstalk between ER and HER2 signaling and tamoxifen resistance is also associated with this crosstalk. Use of a growth factor receptor kinase inhibitor (RKI) in combination with tamoxifen is suggested as the therapeutic opportunity to either prevent or circumvent tamoxifen resistance (Creighton et al., 2008). Some preclinical studies have shown that the antitumor activity of tamoxifen can be restored or delayed when a growth factor RKI is combined in either HER2 overexpressing breast cancer cell lines or xenograft models (Gee et al., 2003; Arpino et al., 2007). Several clinical studies are ongoing to investigate combination strategies using growth factor RKIs with tamoxifen. Inhibition of a variety of key signal transduction mediators of growth factor signaling such as farnesyl transferase, mTOR, or Raf are also investigated as a measure to delay resistance or resensitize the cellular responsiveness to tamoxifen (Johnston et al., 2008).
Increased crosstalk between ER and HER2 coupled with high expression of coactivator SRC3 is suggested as one of mechanisms by which cells do not respond to tamoxifen by switching tamoxifen bound ER from an antagonistic configuration to that of an agonist (Shou et al., 2004). In this case, tamoxifen-bound ER do not recruit corepressors but rather co-activator such as SRC3 and silencing the SRC3 or inhibiting the activity of HER2 was able to resensitize cells to tamoxifen treatment (Shou et al., 2004; Mc Ilroy et al., 2006). In addition, Akt, which is rapidly activated by E2, is also activated by 4-OHT in breast cancer cells overexpressing HER2 in a HER2-dependent manner, implying conversion of 4-OHT to an agonist. In support of this, high levels of SRC3 along with HER2 overexpression are associated with worse outcomes following tamoxifen therapy, indicating roles for growth factor receptor signaling in tamoxifen-stimulated growth and resistance (Osborne et al., 2003).
One of potential mechanisms for HER2-mediated tamoxifen resistance is that overactivity or overexpression of HER2 and its downstream MAPK may contribute to the loss of ER, which is directly attributed to endocrine resistance. It has been shown that ER levels are negatively correlated with those of EGFR and HER2 (Dawson et al., 2009). Preclinical data suggest that increased growth factor signaling induced by receptor-specific ligands such as EGF, IGF-1, transforming growth factor (TGF)-β, and heregulin can downregulate ER protein expression and thus lead to a more hormone-independent phenotype (Stoica et al., 2003). Overactivity of kinases like p42/44 MAPK has been associated with acquired loss of ER via a nuclear factor-kappa B (NF-κB)-mediated mechanism (Holloway et al., 2004). On the other hand, other clinical data suggest that expression of ER can be reverted from ER-negative to ER-positive after treatment with a HER2 inhibitor, trastuzumab, implying that endocrine therapy becomes benefi cial in this case (Munzone et al., 2006). If the ability of inhibition of growth factor kinase receptors to restore ER expression is clinically confi rmed, it will provide an additional therapeutic opportunity to endocrine therapies.
Pharmacological and metabolic aspects of antiestrogen resistance
Decrease in intracellular concentrations of a certain drug is a general mechanism of drug resistance as a result of increased efflux or decreased influx (Gonzalez-Angulo et al., 2007). Indeed, significantly lower intratumoral tamoxifen concentrations were found in breast tumor samples that had been known to develop acquired resistance to tamoxifen compared to those with de novo resistance (Johnston et al., 1993). It is likely that reduced uptake of tamoxifen from extracellular sources and lower availability of intracellular tamoxifen could confer resistance, resulting in a lack of the intracellular tamoxifen to effectively compete with E2 for binding to ER. Although the precise mechanisms for lower intracellular tamoxifen levels remain unclear, potential mechanisms include the presence of microsomal antiestrogen binding site proteins (AEBSs) (Katzenellenbogen et al., 1985) or increased tamoxifen efflux via multi-drug resistance (MDR) P-glycoprotein drug pump. Accumulating data suggest that MDR is not responsible for tamoxifen accumulation (Clarke et al., 1992). On the other hand, overexpression of AEBSs and high affinity of tamoxifen to AEBS are speculated to be involved in acquired tamoxifen resistance in the presence of continued ER expression .AEBSs do not bind either to endogenous estrogens or the steroidal antiestrogens, but binds to tamoxifen, which belongs to the non-steroidal antiestrogens (Pavlik et al., 1992). It has also been reported that the affinity of tamoxifen for AEBS is particularly high at the 1 nM level, which is significantly greater than its affinity for the ER (Denton et al., 2002).
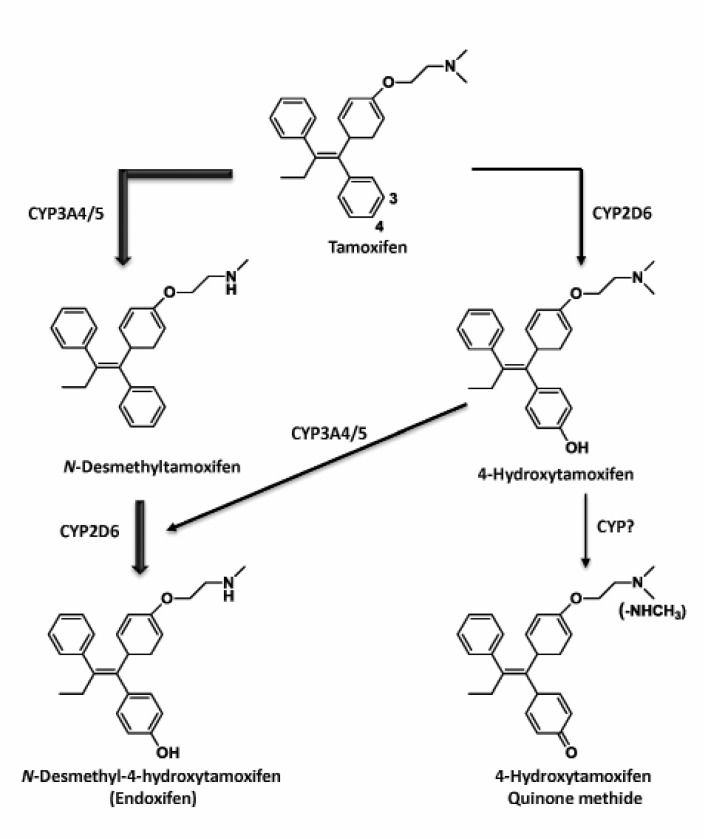
Tamoxifen is a prodrug that requires metabolism to form the pharmacologically active metabolites such as 4-OHT or 4-hydroxy-N-desmethyltamoxifen (endoxifen) by cytochrome P450 (CYP)-mediated catalysis (Fig. 2) (Massarweh and Schiff, 2006; Johnston, 2010). These hydroxylated metabolites of tamoxifen have a high binding affinity for the ER and exert antiestrogenic and antitumor activities (Jordan et al., 1977; Johnson et al., 2004). CYP3A4/5 and 2D6 are major CYP isozymes involved in tamoxifen metabolism and are known to display several genotypes that may lead to different enzyme activities and personal variation in therapeutic effects. There is little evidence for a relevant contribution of CYP3A4 gene expression and enzyme function, whereas genetic poly-morphisms in a CYP3A5*3 allele, define much of the variation in CYP3A5 (Kuehl et al., 2001). It was hypothesized that pharmacogenomics of CYP3A5*3, would affect the clinical outcomes of tamoxifen therapy; however, the current available studies suggests that there is no clinically meaningful association between CYP3A5 genotype and benefits from tamoxifen therapy (Tucker et al., 2005; Wegman et al., 2007).
Fig. 2. Metabolic activation of tamoxifen. Phase I metabolism of tamoxifen produces phenolic metabolites that have a high bindingaffinity for the ER and thus exerts potent antiestrogenic activity. Metabolic pathways mainly include cytochrome P450 3A4/5 and 2D6. Further oxidative metabolism may contribute to formation of reactive intermediates such as quinone methides. The thickness of each arrow indicates the relative contribution of the respective pathway to the formation of a specific metabolite. The principal metabolizingenzymes responsible for biotransformation are indicated next to the arrow.
Endoxifen is the ultimate metabolite formed by CYP2D6-catalyzed phase I metabolism of tamoxifen. The dominant role of CYP2D6 in the formation of endoxifen renders poly-morphisms of CYP2D6 genotypes and phenotypes at the center of tamoxifen pharmacogenetics. Interestingly, the serum
concentrations of endoxifen showed large variations among patients receiving adjuvant tamoxifen therapy, implying that individual differences in metabolic profiles of tamoxifen exist and may affect the therapeutic outcomes (Stearns et al., 2003; Jin et al., 2005). For example, it has been reported that the plasma concentration of endoxifen in breast cancer patients is 6-fold higher than that of 4-OHT (Stearns et al., 2003).
Based on the therapeutic effects coming from endoxifen and biotransformation of tamoxifen, it can be rationalized that the catalytic activity of CYP2D6 is an important parameter in devising a tamoxifen treatment plan. CYP2D6 has been shown to have over 80 different single nucleotide polymorphisms that lead to four metabolic classes; poor, intermediate, extensive, and ultrarapid metabolizers. Therefore, CYP2D6 polymorphisms affect the metabolism of tamoxifen and, thus, its therapeutic effects in vivo (Jin et al., 2005; Schroth et al., 2007; Mürdter et al., 2011). For example, it can be expected that the poor metabolizers would not respond properly to tamoxifen treatment despite the presence of ER. Therefore, CYP2D6 polymorphisms may represent one of the molecular mechanisms for tamoxifen resistance.
Another factor to modulate the activity of a drug-metabolizing enzyme is drug-drug interaction in which co-administered drug inhibits the primary metabolizing enzyme responsible for a specific drug’s metabolism (Caraci et al., 2011). There is an increased usage of selective serotonin uptake inhibitors (SSRIs) with long-term tamoxifen treatment, especially in pre-menopausal patients, to alleviate hot flashes. Unfortunately, SSRIs such as fluoxetine and paroxetine are known to inhibit the CYP2D6 enzyme activity (Caraci et al., 2011). Therefore, the efficacy of tamoxifen, potentially derived from endoxifen, can be undermined by the use of co-administered SSRIs (Stearns et al., 2003; Jin et al., 2005). This speculation has led to development of SSRIs, such as venlafaxine with little inhibitory action on the CYP2D6 activity (Borges et al., 2006).
It remains unambiguous whether genetic variations in the tamoxifen metabolizing enzymes or drug-drug interactions would affect therapeutic outcomes (Goetz et al., 2005; Goetz et al., 2007). Clearly, reasons for the different conclusions derived from various studies need to be elucidated and large scale clinical studies are warranted to derive the correlation between CYP polymorphism and tamoxifen treatment outcomes.
Cancer stem cells in the context of antiestrogen resistance
Overgrowth of ER-negative cell populations in tamoxifen-resistant tumors has been considered as one of the mechanisms for ER loss or tamoxifen-resistance (Jordan, 2004). In this view, a little portion of ER-negative cells present in a tumor region grow over the growth-arrested ER-positive cells followed by antiestrogen treatment, leading to transition of ER status from positive to negative. The presence of ER-negative cells in seemingly ER-positive tumors is now reckoned as the ER-negative cancer stem cells (CSC) originally present in breast tissue during normal development or carcinogenesis (Sheridan et al., 2006; Ouhtit et al., 2007; Croker et al., 2009).
In the context of E2 responsiveness, ER status, and development of normal breast, there is developmental plasticity at the tissue level, implying that a stem cell population renews and differentiates to form a cellular hierarchy according to highly regulated functional cues (Stingl, 2011). For example, the rudimentary mammary development begins from week 12, whereas expression of ER does not initiate until 30 weeks of gestation (Clarke et al., 1997; Russo et al., 1999; Keeling et al., 2000). Approximately 10-15% of luminal epithelial cells within the normal breast express immunodetectable ER and PR. Both ER and PR-positive cells in mature normal mammary glands are associated with a differentiated cell phenotype in close proximity to mitotic cells and thus with limited replicative capacity. In contrast to the normal mammary gland, actively dividing ER and PR-positive cells are dominant in hyperplasia or cancers of the breast. Thus, stem or progenitor cells are mainly ER-negative and differentiation renders the expression of the ER. Cell proliferation and division of the differentiated ER-negative cells are regulated by ER-independent pathways such as growth factor signaling.
Based on the cellular hierarchy of normal and malignant breast in terms of the ER levels, it was proposed that ER-negative CSCs are derived from the mutated ER-negative normal stem cells (Dontu et al., 2004). This ER-negative CSC population has the potential to differentiate to luminal cancer cells and thus to seed relapses and metastasize despite endocrine therapy (Sheridan et al., 2006; Ouhtit et al., 2007; Croker et al., 2009).
The possibility that the ER status of the tumor is associated with the cellular origin emphasizes that profi ling of stem and progenitor cells in normal breast tissues as well as in carcinomas has profound implications for the further development of existing therapies and new strategies for bypassing antiestrogen resistance.
Cell fate regulation and antiestrogen resistance
Therapeutic strategies to manage breast cancer generally include regulation of cell fate-associated pathways. Endocrine therapies consistently induce a notable growth arrest in hormone-sensitive tumors in addition to cell death. The relative importance or roles of growth arrest, cell death, and cell survival in cancers generally remain unambitious.
Cell death pathways include apoptosis, autophagy, mitotic catastrophe, necrosis, and senescence (Tan and White, 2008). The regulatory signaling upstream of these events and how these various signaling cascades are integrated and converged is now incomplete; however, emerging study results suggest that regulation of one or more of these cell death signaling pathways is a measure to modulate cellular responsiveness to chemotherapeutic agents. In this review, roles for autophagy among pathways for cell fate regulation will be specifically introduced which is expected to provide a new therapeutic option for tamoxifen-resistant breast tumors based on the very recent preclinical and clinical data.
Autophagy is a mechanism of self-eating by which a cell digests cystoplasmic contents such as subcellular organelles or unfolded/misfolded/aggregated proteins by double/multi-membrane vacuoles or autophagosomes (Jin, 2006). It provides opportunities for cells to remove damaged organelles as well as proteins and to respond to stresses leading to recovering energy and maintaining metabolic homeostasis. Autophagy plays duel facets in regulating cell fates; it can act as a cell survival mechanism when extracellular nutrients or growth factors are limited, or as an alternative cell death pathway to apoptosis. Also, in cancer, autophagy can serve as either a‘tumor suppressor’ or as a ‘tumor promoter’. Clinical studies have demonstrated that disruption of the autophagic process via inhibition or allelic loss of vital autophagy components is a key event in tumorigenesis. Various preclinical studies using small molecule inhibitors of autophagy or siRNA to knockdown vital components of autophagy demonstrate the critical role of autophagy in chemotherapeutic sensitization of cancer cells.Regulation of cell fate, cell death or cell survival by autophagy is dependent on the number of autophagosomes in each cell. Thus, the outcome of autophagy in antiestrogen therapy and development of resistance is somewhat confounding. For example, antiestrogens exert cytotoxic effects through stimulation of autophagy (Bursch et al., 1996). On the other hand, a prosurvival role of autophagy in antiestrogen therapy was demonstrated where inhibition of the autophagic process via 3-methyladenine (a known autophagy inhibitor) or beclin-1(a key component of autophagy signaling) siRNA induced antiestrogen-dependent cell death (Samaddar et al., 2008). It has been shown in various ER-positive breast cancer cell lines that concurrent inhibition of autophagy signaling molecules such as beclin-1 or Atg 5 and treatment with tamoxifen resulted in increased mitochondria-mediated apoptosis and reduced cell viability (Qadir et al., 2008). In addition, resensitization of the cell’s responsiveness to tamoxifen through inhibition of autophagy was achieved in a fulvestrant and tamoxifen cross-resistant MCF-7 subcell line. This study also emphasized that dual inhibition through chemical inhibitor and siRNA technology to knockdown the autophagy-associated gene is effective in restoring the cellular responsiveness to antiestrogens (Crawford et al., 2010).
Overexpression of beclin-1 is often associated with a lack of estrogen-regulated growth in conjunction with a decrease in estrogen-responsive genes including c-myc and c-fos (John et al., 2008). Thus, high expression of beclin-1 seems associated with its antitumor activity and autophagy but also leads to a loss of sensitivity to tamoxifen, implying that autophagy plays a role in promoting antiestrogen resistance (John et al., 2008). Numerous preclinical data collectively indicated that autophagy inhibition may resensitize breast tumors to antiestrogens or other therapeutic agents and clinical trials targeting autophagy have already begun. Of particular interest is a study in ductal carcinoma in situ where either tamoxifen, an autophagy inhibitor chloroquinone, or a combination of both prior to surgical removal of the tumor (USA government identifier number: NCT01023477). This study will investigate whether inhibition of autophagy in combination with tamoxifen treatment will reduce the growth and invasiveness of this type of breast tumor. The results of this trial and other ongoing clinical trials targeting autophagy are expected to answer many questions pertaining to the role of autophagy in cancer and clinical values of autophagy modulators.
Regulation of redox status and antiestrogen resistance
Tamoxifen undergoes oxidative metabolism by molecular oxygen and CYP450 system giving rise to reactive oxygen species (ROS) formation in the reduction/oxidation cycling process (Fan and Bolton, 2001). The ROS formed, in particular, hydroxyl radicals, lead to oxidative DNA damage which is refl ected by 8-hydroxydeoxyguanosine (8-OHdG) formation. Numerous in vitro and in vivo studies have shown a strong correlation between 8-OHdG production and tumor promotion or carcinogenesis (Kim and Wells, 1996; Kryston et al., 2011). Moreover, tamoxifen was found to induce the tamoxifen-activating enzymes that turn out to cause excessive formation of harmful metabolites and persistence of toxic effects (Pathak et al., 1996). Metabolic activation has been linked to the genotoxicity and carcinogenic potentials of tamoxifen (Fan and Bolton, 2001; Crewe et al., 2002). In addition, cells are continuously exposed to ROS which are produced during normal metabolic reactions, specifically in the mitochondria (Fridovich, 1999; Murphy, 2009). There are diverse mechanisms that exist to protect cells from continuous exposure to ROS.
Peroxiredoxins (Prxs), a family of thiol peroxidases, are one of the antioxidant proteins that modulate intracellular redox cycling and play a critical role in apoptosis and protection of cells from oxidative stimulus (Neumann and Fang, 2007). In particular, Prx5 was shown to protect cells from oxidative stress by modulating apoptosis in various types of cells (Mikhaǐlov et al., 2002; Yuan et al., 2004; Chang et al., 2007). It has been reported that expression of Prx5 in concert with Prx1, 3, and 4 is higher in breast cancer tissues and is significantly greater if tumors are larger or have lymph-node metastases (Karihtala et al., 2003). This implies that Prx5 expression is associated with mammary carcinogenesis. It has recently demonstrated that GATA1 acts as a transcription repressor on Prx5 gene expression and this activity is associated with agonist-bound ER (Seo et al., 2012). Moreover, it has been suggested that tamoxifen-bound ER acts like an agonist-activated receptor in tamoxifen-resistant cells, implying that one of molecular mechanisms of tamoxifen resistance involves GATA1-ER
interaction and their activities on the Prx5 gene expression leads to decreased apoptosis in an ER ligand-dependent manner (Fig. 3 ).
Fig. 3. A scheme showing the different mechanisms of tamoxifen resistance: (1) Loss of ERα expression and function lead to disappearance of the molecular target for tamoxifen (2) altered expression of coactivators or coregulators that play a critical role in ER-mediated gene transcription, (3) ligand-independent growth factor signaling cascades that activate kinases and ER-phosphorylation, (4) altered availability of active tamoxifen metabolites regulated by drug-metabolizing enzymes such as CYP2D6, (5) regulation of autophagy and/or apoptosis,(6) ER-negative cancer stem cells that differentiate over growth inhibition of ER-positive cancer cells upon antiestrogen treatment, and(7) antioxidant protein-mediated cell survival in which tamoxifen prevents repression of antioxidant proteins, such as Prx5 leading to cell survival and resistance to tamoxifen treatment.

It has been reported that AP-1, oxidative stress-responsive transcription factor composed of the Jun and Fos families (Angel and Karin, 1991), is activated in tamoxifen-resistant MCF-7 xenograft tumors in concert with increases in antioxidant biomarkers such as antioxidant enzymes, glutathione, and lipid peroxidation (Schiff et al., 2000). This study suggested that tamoxifen-induced oxidative stress may contribute to increases in intracellular redox status followed by AP-1 activity that leads to several mitogenic signaling pathways and, therefore, tumor growth.
Protective mechanisms in responsive to oxidative stress involve regulation of cell death/survival signaling pathways. Abnormal redox status induced during tamoxifen treatment cues cell survival signaling activation, implying that modulation of cell survival or apoptosis may be an ancillary molecular target in addition to tamoxifen therapy and warrants further investigation.
CONCLUSIONS AND FUTURE DIRECTIONS
The endocrine therapy in women with ER-positive breast tumor has become more complex than previously envisioned as data are generated on molecular signaling transduction between estrogen/antiestrogen and ER or ER and growth factor receptors. Tamoxifen therapy is a well-established and valuable ER-targeted approach to manipulate breast cancer in both the neo- and adjuvant settings in either pre- or post-menopausal women. It has been a critical limit to tamoxifen use for initially responding tumors to develop resistance or for ER-positive tumors not to respond at all. Mechanisms for de novo or acquired resistance to tamoxifen appear very complex and are dominated by crosstalk between ER and growth factor signaling pathways, the presence of ER-negative undifferentiated cells, cell fate regulation through autophagy or apoptosis, antioxidant protein-gene regulation, and genetic polymorphisms of a specific tamoxifen-metabolizing enzyme(Fig. 3 ). Mechanistic understanding of tamoxifen resistance will expand our knowledge on devising new therapy regimens and benefit the breast cancer patients. For example, endocrine treatments such as fulvestrant expand the choice for postmenopausal women with resistance to tamoxifen therapy. Femara® (letrozole) is evaluated as the extended adjuvant therapy in ER-positive breast cancer with tamoxifen resistance. Based on the preclinical and clinical trials translated from molecular studies, new options are being developed for sequencing and combination treatment using many types of endocrine modulators such as AIs or ER downregulators. As growth factor/kinase signaling pathways are involved in ER-mediated or independent estrogen signaling pathways, co-targeting these molecules and other estrogen signaling components are expected to more effectively modulate ER-mediated actions during progression of breast cancer. This approach may provide novel and efficient therapeutic measures for endocrine-resistant states by preventing or delaying the onset of endocrine resistance.
Although much information about ER and cancer has been provided in the past three decades since the arrival of tamoxifen in the clinic, a lot more needs to be elucidated for favorable therapeutic outcomes. More detailed molecular mechanisms relevant to tamoxifen resistance and the interaction between tamoxifen and ER are still actively being studied. In addition, more concrete research outcomes will warrant the translational research that may lead to more efficient and safer treatment options for patients as well as women at high risk of breast cancer.
Acknowledgments
This work was supported by the National Research Foundation of Korea grant funded by the Korea government (2011-0030074) to M. Chang (2011-B02).
References
- 1.Anderson W. F. Chatterjee N. Ershler W. B. Brawley O. W. Estrogen receptor breast cancer phenotypes in the Surveillance, Epidemiology, and End Results database. Breast Cancer Res. Treat. (2002);76:27–36. doi: 10.1023/a:1020299707510. [DOI] [PubMed] [Google Scholar]
- 2.Angel P. Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta. (1991);1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 3.Anzick S. L. Kononen J. Walker R. L. Azorsa D. O. Tanner M. M. Guan X. Y. Sauter G. Kallioniemi O. P. Trent J. M. Meltzer P. S. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. (1997);277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 4.Arpino G. Gutierrez C. Weiss H. Rimawi M. Massarweh S. Bharwani L. De Placido S. Osborne C. K. Schiff R. Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J. Natl. Cancer Inst. (2007);99:694–705. doi: 10.1093/jnci/djk151. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee S. Saxena N. Sengupta K. Banerjee S. K. 17alpha-estradiol-induced VEGF-A expression in rat pituitary tumor cells is mediated through ER independent but PI3K-Akt dependent signaling pathway. Biochem. Biophys. Res. Commun. (2003);300:209–215. doi: 10.1016/s0006-291x(02)02830-9. [DOI] [PubMed] [Google Scholar]
- 6.Benz C. C. Scott G. K. Sarup J. C. Johnson R. M. Tripathy D. Coronado E. Shepard H. M. Osborne C. K. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res. Treat. (1992);24:85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 7.Björnström L. Sjöberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol. Endocrinol. (2005);19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 8.Bocchinfuso W. P. Korach K. S. Estrogen receptor residues required for stereospecific ligand recognition and activation. Mol. Endocrinol. (1997);11:587–594. doi: 10.1210/mend.11.5.9931. [DOI] [PubMed] [Google Scholar]
- 9.Bolton J. L. Thatcher G. R. Potential mechanisms of estrogen quinone carcinogenesis. Chem. Res. Toxicol. (2008);21:93–101. doi: 10.1021/tx700191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borges S. Desta Z. Li L. Skaar T. C. Ward B. A. Nguyen A. Jin Y. Storniolo A. M. Nikoloff D. M. Wu L. Hillman G. Hayes D. F. Stearns V. Flockhart D. A. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin. Pharmacol.Ther. (2006);80:61–74. doi: 10.1016/j.clpt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Brünner N. Boysen B. Jirus S. Skaar T. C. Holst-Hansen C. Lippman J. Frandsen T. Spang-Thomsen M. Fuqua S. A. Clarke R. MCF7/LCC9: an antiestrogen-resistant MCF-7 variant in which acquired resistance to the steroidal antiestrogen ICI 182780 confers an early cross-resistance to the nonsteroidal antiestrogen tamoxifen. Cancer Res. (1997);57:3486–3493. [PubMed] [Google Scholar]
- 12.Brzozowski A. M. Pike A. C. Dauter Z. Hubbard R. E. Bonn T. Engström O. Ohman L. Greene G. L. Gustafsson J. A. Carlquist M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. (1997);389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 13.Bursch W. Ellinger A. Kienzl H. Török L. Pandey S. Sikorska M. Walker R. Hermann R. S. Active cell death induced by the anti-estrogens tamoxifen and ICI 164 384 in human mammary carcinoma cells (MCF-7) in culture: the role of autophagy. Carcinogenesis. (1996);17:1595–1607. doi: 10.1093/carcin/17.8.1595. [DOI] [PubMed] [Google Scholar]
- 14.Caraci F. Crupi R. Drago F. Spina. E. Metabolic drug interactions between antidepressants and anticancer drugs: focus on selective serotonin reuptake inhibitors and hypericum extract. Curr. Drug Metab. (2011);12:570–577. doi: 10.2174/138920011795713706. [DOI] [PubMed] [Google Scholar]
- 15.Cavalieri E. Chakravarti D. Guttenplan J. Hart E. Ingle J. Jankowiak R. Muti P. Rogan E. Russo J. Santen R. Sutter T. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim. Biophys. Acta. (2006);1766:63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Dansette P. M, editor; Snyder R, editor; Delaforge M, editor; Gibson G. G, editor; Greim H, editor; Jollow D. J, editor; Monks T. J, editor; Sipes I.G, editor. Hormonal and growth factors. In Cancer of the Breast. Saunders; Philadelphia, USA.: (2002). pp. 417–441. [Google Scholar]
- 17.Chang X. Z. Li D. Q. Hou Y. F. Wu J. Lu J.S. Di G. H. Jin W. Ou Z. L. Shen Z. Z. Shao Z. M. Identifi cation of the functional role of peroxiredoxin 6 in the progression of breast cancer. Breast Cancer Res. (2007);9:R76. doi: 10.1186/bcr1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke R. Currier S. Kaplan O. Lovelace E. Boulay V. Gottesman M. M. Dickson R. B. Effect of P-glycoprotein expression on sensitivity to hormones in MCF-7 human breast cancer cells. J. Natl. Cancer Inst. (1992);84:1506–1512. doi: 10.1093/jnci/84.19.1506. [DOI] [PubMed] [Google Scholar]
- 19.Clarke R. Dickson R. B. Brünner N. The process of malignant progression in human breast cancer. Ann. Oncol. (1990);1:401–407. doi: 10.1093/oxfordjournals.annonc.a057790. [DOI] [PubMed] [Google Scholar]
- 20.Clarke R. B. Howell A. Potten C. S. Anderson E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. (1997);57:4987–4991. [PubMed] [Google Scholar]
- 21.Crawford A. C. Riggins R. B. Shajahan A. N. Zwart A. Clarke R. Co-inhibition of BCL-W and BCL2 restores antiestrogen sensitivity through BECN1 and promotes an autophagy-associated necrosis. PLoS One. (2010);5:e8604. doi: 10.1371/journal.pone.0008604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Creighton C. J. Massarweh S. Huang S. Tsimelzon A. Hilsenbeck S. G. Osborne C. K. Shou J. Malorni L. Schiff R. Development of resistance to targeted therapies transforms the clinically associated molecular profile subtype of breast tumor xenografts. Cancer Res. . (2008);68:7493–7501. doi: 10.1158/0008-5472.CAN-08-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crewe H. K. Notley L. M. Wunsch R. M. Lennard M. S. Gillam E. M. Metabolism of tamoxifen by recombinant human cytochrome P450 enzymes: formation of the 4-hydroxy, 4'-hydroxy and N-desmethyl metabolites and isomerization of trans-4-hydroxy-tamoxifen. Drug Metab. Dispos. (2002);30:869–874. doi: 10.1124/dmd.30.8.869. [DOI] [PubMed] [Google Scholar]
- 24.Croker A. K. Goodale D. Chu J. Postenka C. Hedley B. D. Hess D. A. Allan A. L. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J. Cell Mol. Med. (2009);13:2236–2252. doi: 10.1111/j.1582-4934.2008.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawson S. J. Provenzano E. Caldas C. Triple negative breast cancers: clinical and prognostic implications. Eur. J. Cancer. (2009);45(Suppl 1):27–40. doi: 10.1016/S0959-8049(09)70013-9. [DOI] [PubMed] [Google Scholar]
- 26.Denton K. M. Shweta A. Anderson W. P. Preglomerular and postglomerular resistance responses to different levels of sympathetic activation by hypoxia. J. Am. Soc. Nephrol. (2002);13:27–34. doi: 10.1681/ASN.V13127. [DOI] [PubMed] [Google Scholar]
- 27.Dontu G. El-Ashry D. Wicha M. S. Breast cancer stem/progenitor cells and the estrogen receptor. Trends Endocrinol. Metab. (2004);15:193–197. doi: 10.1016/j.tem.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Dowsett M. Haynes B. P. Hormonal effects of aromatase inhibitors: focus on premenopausal effects and interaction with tamoxifen. J. Steroid Biochem. Mol. Biol. (2003);86:255–263. doi: 10.1016/s0960-0760(03)00365-0. [DOI] [PubMed] [Google Scholar]
- 29.EBCTCG (Early Breast Cancer Trialist’ Collaborative Group). Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. (1998);351:1451–1467. [PubMed] [Google Scholar]
- 30.EBCTCG (Early Breast Cancer Trialist’ Collaborative Group). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. (2005);365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 31.Encarnación C. A. Ciocca D. R. McGuire W. L. Clark G. M. Fuqua S. A. Osborne C. K. Measurement of steroid hormone receptors in breast cancer patients on tamoxifen. Breast Cancer Res. Treat. (1993);26:237–246. doi: 10.1007/BF00665801. [DOI] [PubMed] [Google Scholar]
- 32.Fan P. W. Boltonm J L. Bioactivation of tamoxifen to metabolite E quinone methide: reaction with glutathione and DNA. Drug Metab. Dispos. (2001);29:891–896. [PubMed] [Google Scholar]
- 33.Forbes J. F. Cuzick J. Buzdar A. Howell A. Tobias J. S. Baum M Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists' Group. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. (2008);9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 34.Fridovich I. Fundamental aspects of reactive oxygen species, or what's the matter with oxygen? Ann. N. Y. Acad. Sci. (1999);893:13–18. doi: 10.1111/j.1749-6632.1999.tb07814.x. [DOI] [PubMed] [Google Scholar]
- 35.Fuqua S. A. Estrogen receptor mutagenesis and hormone resistance. Cancer. (1994);74(3 Suppl):1026–1029. doi: 10.1002/1097-0142(19940801)74:3+<1026::aid-cncr2820741509>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 36.Gee J. M. Harper M. E. Hutcheson I. R. Madden T. A. Barrow D. Knowlden J. M. McClelland R. A. Jordan N. Wakeling A. E. Nicholson R. I. The antiepidermal growth factor receptor agent gefitinib (ZD1839/Iressa) improves antihormone response and prevents development of resistance in breast cancer in vitro. Endocrinology. (2003);144:5105–5117. doi: 10.1210/en.2003-0705. [DOI] [PubMed] [Google Scholar]
- 37.Girault I. Bièche I. Lidereau R. Role of estrogen receptor alpha transcriptional coregulators in tamoxifen resistance in breast cancer. Maturitas. (2006);54:342–351. doi: 10.1016/j.maturitas.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Goetz M. P. Knox S. K. Suman V. J. Rae J. M. Safgren S. L. Ames M. M. Visscher D. W. Reynolds C. Couch F. J. Lingle W.L. Weinshilboum R. M. Fritcher E. G. Nibbe A. M. Desta Z. Nguyen A. Flockhart D. A. Perez E. A. Ingle J. N. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res. Treat. (2007);101:113–121. doi: 10.1007/s10549-006-9428-0. [DOI] [PubMed] [Google Scholar]
- 39.Goetz M. P. Rae J. M. Suman V. J. Safgren S. L. Ames M. M. Visscher D. W. Reynolds C. Couch F. J. Lingle W. L. Flockhart D. A. Desta Z. Perez E. A. Ingle J. N. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J. Clin. Oncol. (2005);23:9312–9318. doi: 10.1200/JCO.2005.03.3266. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Angulo A. M. Morales-Vasquez F. Hortobagyi G. N. Overview of resistance to systemic therapy in patients with breast cancer. Adv. Exp. Med. Biol. (2007);608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 41.Goss P. E. Ingle J. N. Martino S. Robert N. J. Muss H. B. Piccart M. J. Castiglione M. Tu D. Shepherd L. E. Pritchard K. I. Livingston R. B. Davidson N. E. Norton L. Perez E. A. Abrams J.S. Cameron D. A. Palmer M. J. Pater J. L National Cancer Institute of Canada Clinical Trials Group MA.17. Effi cacy of letrozole extended adjuvant therapy according to estrogen receptor and progesterone receptor status of the primary tumor: National Cancer Institute of Canada Clinical Trials Group MA.17. J. Clin. Oncol. (2007);25:2006–2011. doi: 10.1200/JCO.2006.09.4482. [DOI] [PubMed] [Google Scholar]
- 42.Goss P. E. Ingle J. N. Martino S. Robert N. J. Muss H. B. Piccart M. J. Castiglione M. Tu D. Shepherd L. E. Pritchard K. I. Livingston R. B. Davidson N. E. Norton L. Perez E. A. Abrams J. S. Cameron D. A. Palmer M. J. Pater J. L. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J. Natl. Cancer Inst. (2005);97:1262–1271. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 43.Gottardis M. M. Jordan V. C. Development of tamoxifen-stimulated growth of MCF-7 tumors in athymic mice after long-term antiestrogen administration. Cancer Res. (1988);48:5183–5187. [PubMed] [Google Scholar]
- 44.Graham M. L. 2nd. Smith J. A. Jewett P. B. Horwitz K. B. Heterogeneity of progesterone receptor content and remodeling by tamoxifen characterize subpopulations of cultured human breast cancer cells: analysis by quantitative dual parameter flow cytometry. Cancer Res. (1992);52:593–602. [PubMed] [Google Scholar]
- 45.Greene G. L. Gilna P. Waterfi eld M. Baker A. Hort Y. Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. (1986);231:1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- 46.Habel L. A. Stanford J. L. Hormone receptors and breast cancer. Epidemiol. Rev. (1993);15:209–219. doi: 10.1093/oxfordjournals.epirev.a036107. [DOI] [PubMed] [Google Scholar]
- 47.Heldring N. Pike A. Andersson S. Matthews J. Cheng G. Hartman J. Tujague M. Ström A. Treuter E. Warner M. Gustafsson J. A. Estrogen receptors: how do they signal and what are their targets. Physiol. Rev. (2007);87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 48.Henderson B. E. Feigelson H. S. Hormonal carcinogenesis. Carcinogenesis. (2000);21:427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 49.Holloway J. N. Murthy S. El-Ashry D. A cytoplasmic substrate of mitogen-activated protein kinase is responsible for estrogen receptor-alpha down-regulation in breast cancer cells: the role of nuclear factor-kappaB. Mol. Endocrinol. (2004);18:1396–1410. doi: 10.1210/me.2004-0048. [DOI] [PubMed] [Google Scholar]
- 50.Howell A. Robertson J. F. Quaresma Albano J. Aschermannova A. Mauriac L. Kleeberg U. R. Vergote I. Erikstein B. Webster A. Morris C. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J. Clin. Oncol. (2002);20:3396–3403. doi: 10.1200/JCO.2002.10.057. [DOI] [PubMed] [Google Scholar]
- 51.Hurtubise A. Momparler R. L. Effect of histone deacetylase inhibitor LAQ824 on antineoplastic action of 5-Aza-2'-deoxycytidine(decitabine) on human breast carcinoma cells. Cancer Chemother. Pharmacol. (2006);58:618–625. doi: 10.1007/s00280-006-0225-6. [DOI] [PubMed] [Google Scholar]
- 52.Ingle J. N. Mailliard J. A. Schaid D. J. Krook J. E. Gesme D. H. Jr. Windschitl H. E. Pfeifle D.M. Etzell P. S. Gerstner J. G. Long H. J et al. A double-blind trial of tamoxifen plus prednisolone versus tamoxifen plus placebo in postmenopausal women with metastatic breast cancer. A collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic. Cancer. (1991);68:34–39. doi: 10.1002/1097-0142(19910701)68:1<34::aid-cncr2820680107>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 53.Jaiyesimi I. A. Buzdar A. U. Decker D. A. Hortobagyi G. N. Use of tamoxifen for breast cancer: twenty-eight years later. J. Clin. Oncol. (1995);13:513–529. doi: 10.1200/JCO.1995.13.2.513. [DOI] [PubMed] [Google Scholar]
- 54.Jemal A. Siegel R. Xu J. Ward E. Cancer statistics, 2010. CA Cancer J. Clin. (2010);60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 55.Jiang S. Y. Langan-Fahey S. M. Stella A. L. McCague R. Jordan V. C. Point mutation of estrogen receptor (ER) in the ligand-binding domain changes the pharmacology of antiestrogens in ER-negative breast cancer cells stably expressing complementary DNAs for ER. Mol. Endocrinol. (1992);6:2167–2174. doi: 10.1210/mend.6.12.1491696. [DOI] [PubMed] [Google Scholar]
- 56.Jin S. Autophagy, mitochondrial quality control, and oncogenesis. Autophagy. (2006);2:80–84. doi: 10.4161/auto.2.2.2460. [DOI] [PubMed] [Google Scholar]
- 57.Jin Y. Desta Z. Stearns V. Ward B. Ho H. Lee K. H. Skaar T. Storniolo A. M. Li L. Araba A. Blanchard R. Nguyen A. Ullmer L. Hayden J. Lemler S. Weinshilboum R. M. Rae J. M. Hayes D. F. Flockhart D. A. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J. Natl. Cancer Inst. (2005);97:30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]
- 58.John S. Nayvelt I. Hsu H. C. Yang P. Liu W. Das G. M. Thomas T. Thomas T. J. Regulation of estrogenic effects by beclin 1 in breast cancer cells. Cancer Res. (2008);68:7855–7863. doi: 10.1158/0008-5472.CAN-07-5875. [DOI] [PubMed] [Google Scholar]
- 59.Johnson M. D. Zuo H. Lee K. H. Trebley J. P. Rae J. M. Weatherman R. V. Desta Z. Flockhart D. A. Skaar T. C. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res. Treat. (2004);85:151–159. doi: 10.1023/B:BREA.0000025406.31193.e8. [DOI] [PubMed] [Google Scholar]
- 60.Johnston S. R. New strategies in estrogen receptor-positive breast cancer. Clin. Cancer Res. (2010);16:1979–1987. doi: 10.1158/1078-0432.CCR-09-1823. [DOI] [PubMed] [Google Scholar]
- 61.Johnston S. R. Haynes B. P. Smith I. E. Jarman M. Sacks N. P. Ebbs S. R. Dowsett M. Acquired tamoxifen resistance in human breast cancer and reduced intra-tumoral drug concentration. Lancet. (1993);342:1521–1522. doi: 10.1016/s0140-6736(05)80088-1. [DOI] [PubMed] [Google Scholar]
- 62.Johnston S. R. Semiglazov V. F. Manikhas G. M. Spaeth D. Romieu G. Dodwell D. J. Wardley A. M. Neven P. Bessems A. Park Y. C. De Porre P. M. Perez Ruixo J. J. Howes A. J. A phase II randomized blinded study of the farnesyltransferase inhibitor tipifarnib combined with letrozole in the treatment of advanced breast cancer after antiestrogen therapy. Breast Cancer Res. Treat. (2008);110:327–335. doi: 10.1007/s10549-007-9726-1. [DOI] [PubMed] [Google Scholar]
- 63.Jordan V. C. Effects of tamoxifen in relation to breast cancer. Br. Med. J. (1977);1:1534–1535. doi: 10.1136/bmj.1.6075.1534-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jordan V. C. Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell. (2004);5:207–213. doi: 10.1016/s1535-6108(04)00059-5. [DOI] [PubMed] [Google Scholar]
- 65.Jordan V. C. Collins M. M. Rowsby L. Prestwich G. A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J. Endocrinol. (1997);75:305–316. doi: 10.1677/joe.0.0750305. [DOI] [PubMed] [Google Scholar]
- 66.Karihtala P. Mäntyniemi A. Kang S. W. Kinnula V. L. Soini Y. Peroxiredoxins in breast carcinoma. Clin. Cancer Res. (2003);15:3418–3424. [PubMed] [Google Scholar]
- 67.Katzenellenbogen B. S. Miller M. A. Mullick A. Sheen Y. Y. Antiestrogen action in breast cancer cells: modulation of proliferation and protein synthesis, and interaction with estrogen receptors and additional antiestrogen binding sites. Breast Cancer Res. Treat. (1985);5:231–243. doi: 10.1007/BF01806018. [DOI] [PubMed] [Google Scholar]
- 68.Keeling J. W. Ozer E. King G. Walker F. Oestrogen receptor alpha in female fetal, infant, and child mammary tissue. J. Pathol. (2000);191:449–451. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH661>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 69.Kim P. M. Wells P. G. Genoprotection by UDP-glucuronosyltransferases in peroxidase-dependent reactive oxygen species-mediated micronucleus initiation by the carcinogens 4-(methylnitrosamino)-1-(3-py-ridyl)-1-butanone and benzo[a]pyrene. Cancer Res. (1996);56:1526–1532. [PubMed] [Google Scholar]
- 70.Klinge C. M. Estrogen receptor interaction with co-activators and co-repressors. Steroids. (2000);65:227–251. doi: 10.1016/s0039-128x(99)00107-5. [DOI] [PubMed] [Google Scholar]
- 71.Kryston T. B. Georgiev A. B. Pissis P. Georgakilas A. G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. (2011);711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 72.Kuehl P. Zhang J. Lin Y. Lamba J. Assem M. Schuetz J. Watkins P. B. Daly A. Wrighton S. A. Hall S. D. Maurel P. Relling M. Brimer C. Yasuda K. Venkataramanan R. Strom S. Thummel K. Boguski M. S. Schuetz E. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat. Genet. (2001);27:383–391. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 73.Kuiper G. G. Carlsson B. Grandien K. Enmark E. Häggblad J. Nilsson S. Gustafsson J. A. Comparison of the ligand binding specifi city and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. (1997);138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 74.Kurosumi M. Significance of immunohistochemical assessment of steroid hormone receptor status for breast cancer patients. Breast Cancer. (2003);10:97–104. doi: 10.1007/BF02967633. [DOI] [PubMed] [Google Scholar]
- 75.Lavinsky R. M. Jepsen K. Heinzel T. Torchia J. Mullen T. M. Schiff R. Del-Rio A. L. Ricote M. Ngo S. Gemsch J. Hilsenbeck S. G. Osborne C. K. Glass C. K. Rosenfeld M. G. Rose D. W. Diverse signaling pathways modulate nuclear receptor recruitment of N-CoR and SMRT complexes. Proc. Natl. Acad. Sci. USA. (1998);95:2920–2925. doi: 10.1073/pnas.95.6.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levenson A. S. Catherino W. H. Jordan V. C. Estrogenic activity is increased for an antiestrogen by a natural mutation of the estrogen receptor. J. Steroid. Biochem. Mol. Biol. (1997);60:261–268. doi: 10.1016/s0960-0760(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 77.Liehr J. G. Catecholestrogens in the induction of tumors in the kidney of the Syrian hamster. Adv. Pharmacol. (1998);42:824–828. doi: 10.1016/s1054-3589(08)60873-x. [DOI] [PubMed] [Google Scholar]
- 78.List H. J. Lauritsen K. J. Reiter R. Powers C. Wellstein A. Riegel A. T. Ribozyme targeting demonstrates that the nuclear receptor coactivator AIB1 is a rate-limiting factor for estrogen-dependent growth of human MCF-7 breast cancer cells. J. Biol. Chem. (2001);276:23763–23768. doi: 10.1074/jbc.M102397200. [DOI] [PubMed] [Google Scholar]
- 79.Love R. R. Philips J. Oophorectomy for breast cancer: history revisited. J. Natl. Cancer Inst. (2002);94:1433–1434. doi: 10.1093/jnci/94.19.1433. [DOI] [PubMed] [Google Scholar]
- 80.Lyko F. Brown R. DNA methyltransferase inhibitors and the development of epigenetic cancer therapies. J. Natl. Cancer Inst. (2005);97:1498–1506. doi: 10.1093/jnci/dji311. [DOI] [PubMed] [Google Scholar]
- 81.Mangelsdorf D. J. Thummel C. Beato M. Herrlich P. Schütz G. Umesono K. Blumberg B. Kastner P. Mark M. Chambon P. Evans R. M. The nuclear receptor superfamily: the second decade. Cell. (1995);83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Massarweh S. Schiff R. Resistance to endocrine therapy in breast cancer: exploiting estrogen receptor/growth factor signaling crosstalk. Endocr. Relat. Cancer. (2006);13(Suppl 1):S15–24. doi: 10.1677/erc.1.01273. [DOI] [PubMed] [Google Scholar]
- 83.Mc Ilroy M. Fleming F. J. Buggy Y. Hill A. D. Young L. S. Tamoxifen-induced ER-alpha-SRC-3 interaction in HER2 positive human breast cancer; a possible mechanism for ER isoform specific recurrence. Endocr. Relat. Cancer. (2006);13:1135–1145. doi: 10.1677/erc.1.01222. [DOI] [PubMed] [Google Scholar]
- 84.McGuire W. L. Horwitz K. B. Pearson O. H. Segaloff A. Current status of estrogen and progesterone receptors in breast cancer. Cancer. (1977);39(6 Suppl):2934–2947. doi: 10.1002/1097-0142(197706)39:6<2934::aid-cncr2820390680>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 85.Mikhaĭlov V. M. Kropotov A. V. Zelenin A. V. Krutilina R. I. Kolesnikov V. A. Zelenina I. A. Baranov A. N. Shteĭn G. I. Ostapenko O. V. Tomilin N. V. Baranov V. S. The BCL-xL and ACR-1 genes promote differentiation and reduce apoptosis in muscle fibers of mdx mice. Genetika. (2002);38:1445–1450. [PubMed] [Google Scholar]
- 86.Mosselman S. Polman J. Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. (1996);392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 87.Mouridsen H. Giobbie-Hurder A. Goldhirsch A. Thürlimann B. Paridaens R. Smith I. Mauriac L. Forbes J. F. Price K. N. Regan M. M. Gelber R. D. Coates A. S. BIG 1-98 Collaborative Group. (2009) Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N. Engl. J. Med. (2009);361:766–776. doi: 10.1056/NEJMoa0810818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Munzone E. Curigliano G. Rocca A. Bonizzi G. Renne G. Goldhirsch A. Nolè F. Reverting estrogen-receptor-negative phenotype in HER-2-overexpressing advanced breast cancer patients exposed to trastuzumab plus chemotherapy. Breast Cancer Res. (2006);8:R4. doi: 10.1186/bcr1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mürdter T. E. Schroth W. Bacchus-Gerybadze L. Winter S. Heinkele G. Simon W. Fasching P. A. Fehm T German Tamoxifen and AI Clinicians Group. Eichelbaum M. Schwab M. Brauch H. Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin. Pharmacol.Ther. (2011);89:708–717. doi: 10.1038/clpt.2011.27. [DOI] [PubMed] [Google Scholar]
- 90.Murphy M. P. How mitochondria produce reactive oxygen species. Biochem. J. (2009);417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Myers E. Hill A. D. Kelly G. McDermott E. W. O'Higgins N. J. Buggy Y. Young L. S. ssociations and interactions between Ets-1 and Ets-2 and coregulatory proteins, SRC-1, AIB1, and NCoR in breast cancer. Clin. Cancer Res. (2005);11:2111–2122. doi: 10.1158/1078-0432.CCR-04-1192. [DOI] [PubMed] [Google Scholar]
- 92.Neumann C. A. Fang Q. Are peroxiredoxins tumor suppressors? Curr. Opin. Pharmacol. (2007);7:375–380. doi: 10.1016/j.coph.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 93.O'Regan R. M. Osipo C. Ariazi E. Lee E. S. Meeke K. Morris C. Bertucci A. Sarker M. A. Grigg R. Jordan V. C. Development and therapeutic options for the treatment of raloxifene-stimulated breast cancer in athymic mice. Clin. Cancer Res. (2006);12:2255–2263. doi: 10.1158/1078-0432.CCR-05-2584. [DOI] [PubMed] [Google Scholar]
- 94.Osborne C. K. Bardou V. Hopp T. A. Chamness G. C. Hilsenbeck S. G. Fuqua S. A. Wong J. Allred D. C. Clark G. M. Schiff R. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J. Natl. Cancer Inst. (2003);95:353–361. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 95.Ouhtit A. Abd Elmageed Z. Y. Abdraboh M. E. Lioe T. F. Raj M. H. In vivo evidence for the role of CD44s in promoting breast cancer metastasis to the liver. Am. J. Pathol. (2007);171:2033–2039. doi: 10.2353/ajpath.2007.070535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paridaens R. J. Dirix L. Y. Beex L. V. Nooij M. Cameron D. A. Cufer T. Piccart M. J. Bogaerts J. Therasse P. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: the European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J. Clin. Oncol. (2008);26:4883–4890. doi: 10.1200/JCO.2007.14.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pathak D. N. Pongracz K. Bodell W. J. Activation of 4-hydroxytamoxifen and the tamoxifen derivative metabolite E byuterine peroxidase to form DNA adducts: comparison with DNAadducts formed in the uterus of Sprague-Dawley rats treated withtamoxifen. Carcinogenesis. (1996);17:1785–1790. doi: 10.1093/carcin/17.9.1785. [DOI] [PubMed] [Google Scholar]
- 98.Pavlik E. J. Nelson K. Srinivasan S. Powell D. E. Kenady D. E. DePriest P. D. Gallion H. H. van Nagell J. R. Jr. Resistance to tamoxifen with persisting sensitivity to estrogen: possible mediation by excessive antiestrogen binding site activity. Cancer Res. (1992);52:4106–4112. [PubMed] [Google Scholar]
- 99.Pike M. C. Spicer D. V. Dahmoush L. Press M. F. Estrogensprogestogens normal breast cell proliferation and breast cancer risk. Epidemiol. Rev. (1993);15:17–35. doi: 10.1093/oxfordjournals.epirev.a036102. [DOI] [PubMed] [Google Scholar]
- 100.Qadir M. A. Kwok B. Dragowska W. H. To K. H. Le D. Bally M. B. Gorski S. M. Macroautophagy inhibition sensitizes tamoxifen-resistant breast cancer cells and enhances mitochondrial depolarization. Breast Cancer Res. Treat. (2008);112:389–403. doi: 10.1007/s10549-007-9873-4. [DOI] [PubMed] [Google Scholar]
- 101.Quick E. L. Parry E. M. Parry J. M. Do oestrogens induce chromosome specific aneuploidy in vitro, similar to the pattern of aneuploidy seen in breast cancer? Mutat. Res. (2008);651:46–55. doi: 10.1016/j.mrgentox.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 102.Regan M. M. Neven P. Giobbie-Hurder A. Goldhirsch A. Ejlertsen B. Mauriac L. Forbes J. F. Smith I. Láng I. Wardley A. Rabaglio M. Price K. N. Gelber R. D. Coates A. S. Thürlimann B BIG 1-98 Collaborative Group; International Breast Cancer Study Group (IBCSG). Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1-98 randomised clinical trial at 8·1 years median follow-up. Lancet Oncol. (2011);12:1101–1108. doi: 10.1016/S1470-2045(11)70270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ricketts D. Coombes R. C. What's new in steroid receptor immunocytochemistry in clinical oncology? Pathol. Res. Pract. (1989);185:935–941. doi: 10.1016/S0344-0338(89)80301-2. [DOI] [PubMed] [Google Scholar]
- 104.Ring A. Sestak I. Baum M. Howell A. Buzdar A. Dowsett M. Forbes J. F. Cuzick J. Influence of comorbidities and age on risk of death without recurrence: a retrospective analysis of the Arimidex, Tamoxifen Alone or in Combination trial. J. Clin. Oncol. (2011);29:4266–4272. doi: 10.1200/JCO.2011.35.5545. [DOI] [PubMed] [Google Scholar]
- 105.Russo J. Russo I. H. The role of estrogen in the initiation of breast cancer. J. Steroid. Biochem. Mol. Biol. (2006);102:89–96. doi: 10.1016/j.jsbmb.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Russo J. Ao X. Grill C. Russo I. H. Pattern of distribution of cells positive for estrogen receptor alpha and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res. Treat. (1999);53:217–227. doi: 10.1023/a:1006186719322. [DOI] [PubMed] [Google Scholar]
- 107.Russo J. Hu Y. F. Yang X. Russo I. H. Developmental, cellular, and molecular basis of human breast cancer. J. Natl. Cancer Inst. Monogr. (2000);27:17–37. doi: 10.1093/oxfordjournals.jncimonographs.a024241. [DOI] [PubMed] [Google Scholar]
- 108.Russo J. Reina D. Frederick J. Russo I. H. Expression of phenotypical changes by human breast epithelial cells treated with carcinogens in vitro. Cancer Res. (1988);48:2837–2857. [PubMed] [Google Scholar]
- 109.Samaddar J. S. Gaddy V. T. Duplantier J. Thandavan S. P. Shah M. Smith M. J. Browning D. Rawson J. Smith S. B. Barrett J. T. Schoenlein P. V. A role for macroautophagy in protection against 4-hydroxytamoxifen-induced cell death and the development of antiestrogen resistance. Mol. Cancer Ther. (2008);7:2977–2987. doi: 10.1158/1535-7163.MCT-08-0447. [DOI] [PubMed] [Google Scholar]
- 110.Schiff R. Reddy P. Ahotupa M. Coronado-Heinsohn E. Grim M. Hilsenbeck S. G. Lawrence R. Deneke S. Herrera R. Chamness G. C. Fuqua S. A. Brown P. H. Osborne C. K. Oxidative stress and AP-1 activity in tamoxifen-resistant breast tumors in vivo. J. Natl. Cancer Inst. (2000);92:1926–1934. doi: 10.1093/jnci/92.23.1926. [DOI] [PubMed] [Google Scholar]
- 111.Schroth W. Antoniadou L. Fritz P. Schwab M. Muerdter T. Zanger U. M. Simon W. Eichelbaum M. Brauch H. Breast cancer treatment outcome with adjuvant tamoxifen relative to patient CYP2D6 and CYP2C19 genotypes. J. Clin. Oncol. (2007);25:5187–5193. doi: 10.1200/JCO.2007.12.2705. [DOI] [PubMed] [Google Scholar]
- 112.Seo M. J. Liu X. Chang M. Park J. H. GATA-binding protein 1 is a novel transcription regulator of peroxiredoxin 5 in human breast cancer cells. Int. J. Oncol. (2012);40:655–664. doi: 10.3892/ijo.2011.1236. [DOI] [PubMed] [Google Scholar]
- 113.Sharma D. Blum J. Yang X. Beaulieu N. Macleod A. R. Davidson N. E. Release of methyl CpG binding proteins and histone deacetylase 1 from the Estrogen receptor alpha (ER) promoter upon reactivation in ER-negative human breast cancer cells. Mol. Endocrinol. (2005);19:1740–1751. doi: 10.1210/me.2004-0011. [DOI] [PubMed] [Google Scholar]
- 114.Sheridan C. Kishimoto H. Fuchs R. K. Mehrotra S. Bhat-Nakshatri P. Turner C. H. Goulet R. Jr. Badve S. Nakshatri H. CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. (2006);8:R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shiau A. K. Barstad D. Loria P. M. Cheng L. Kushner P. J. Agard D. A. Greene G. L. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. (1998);95:927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- 116.Shou J. Massarweh S. Osborne C. K. Wakeling A. E. Ali S. Weiss H. Schiff R. Mechanisms of tamoxifen resistance:increased estrogen receptor-HER2/neu crosstalk in ER/HER2-positive breast cancer. J. Natl. Cancer Inst. (2004);96:926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 117.Stearns V. Johnson M. D. Rae J. M. Morocho A. Novielli A. Bhargava P. Hayes D. F. Desta Z. Flockhart D. A. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J. Natl. Cancer Inst. (2003);95:1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 118.Stingl J. Estrogen and progesterone in normal mammary gland development and in cancer. Horm. Cancer. (2011);2:85–90. doi: 10.1007/s12672-010-0055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stoica G. E. Franke T. F. Moroni M. Mueller S. Morgan E. Iann M. C. Winder A. D. Reiter R. Wellstein A. Martin M. B. Stoica A. Effect of estradiol on estrogen receptor-alpha gene expression and activity can be modulated by the ErbB2/PI 3-K/Akt pathway. Oncogene. (2003);22:7998–8011. doi: 10.1038/sj.onc.1206769. [DOI] [PubMed] [Google Scholar]
- 120.Tan T. T. White E. Therapeutic targeting of death pathways in cancer: mechanisms for activating cell death in cancer cells. Adv. Exp. Med. Biol. (2008);615:81–104. doi: 10.1007/978-1-4020-6554-5_5. [DOI] [PubMed] [Google Scholar]
- 121.Tucker A. N. Tkaczuk K. A. Lewis L. M. Tomic D. Lim C. K. Flaws J. A. Polymorphisms in cytochrome P4503A5 (CY-P3A5)may be associated with race and tumor characteristics, but not metabolism and side effects of tamoxifen in breast cancer patients. Cancer Lett. (2005);21:61–72. doi: 10.1016/j.canlet.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 122.Tzukerman M. T. Esty A. Santiso-Mere D. Danielian P. Parker M.G. Stein R. B. Pike J. W. McDonnell D. P. Human estrogen receptor transactivational capacity is determined by both cellular and promoter context and mediated by two functionally distinct intramolecular regions. Mol. Endocrinol. (1994);8:21–30. doi: 10.1210/mend.8.1.8152428. [DOI] [PubMed] [Google Scholar]
- 123.van den Berg H. W. Lynch M. Martin J. Nelson J. Dickson G.R. Crockard A. D. Characterisation of a tamoxifen-resistant variant of the ZR-75-1 human breast cancer cell line (ZR-75-9a1) and ability of the resistant phenotype. Br. J. Cancer. (1989);59:522–526. doi: 10.1038/bjc.1989.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Webb P. Nguyen P. Shinsako J. Anderson C. Feng W. Nguyen M. P. Chen D. Huang S. M. Subramania n S. McKinerney E. Katzenellenbogen B. S. Stallcup M. R. Kushner P. J. Estrogen receptor activation function 1 works by binding p160 co-activator proteins. Mol. Endocrinol. (1998);12:1605–1618. doi: 10.1210/mend.12.10.0185. [DOI] [PubMed] [Google Scholar]
- 125.Wegman P. Elingarami S. Carstensen J. Stål O. Nordenskjöld B. Wingren S. Genetic variants of CYP3A5, CYP2D6, SULT1A1, UGT2B15 and tamoxifen response in postmenopausal patients with breast cancer. Breast Cancer Res. (2007);9:R7. doi: 10.1186/bcr1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yager J. D. Davidson N. E. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. (2006);354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 127.Yuan J. Murrell G. A. Trickett A. Landtmeters M. Knoops B. Wang M. X. Overexpression of antioxidant enzyme peroxiredoxin 5 protects human tendon cells against apoptosis and loss of cellular function during oxidative stress. Biochim. Biophys. Acta. (2004);1693:37–45. doi: 10.1016/j.bbamcr.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 128.Yue W. Wang J. P. Li Y. Bocchinfuso W. P. Korach K. S. Devanesan P. D. Rogan E. Cavalieri E. Santen R. J. Tamoxifen versus aromatase inhibitors for breast cancer prevention. Clin. Cancer Res. (2005);11:925s–930s. [PubMed] [Google Scholar]
- 129.Zajchowski D. A. Kauser K. Zhu D. Webster L. Aberle S. White F. A. 3rd. Liu H. L. Humm R. MacRobbie J. Ponte P. Hegele-Hartung C. Knauthe R. Fritzemeier K. H. Vergona R. Rubanyi G. M. Identifi cation of selective estrogen receptor modulators by their gene expression fingerprints. J. Biol. Chem. (2000);275:15885–15894. doi: 10.1074/jbc.M909865199. [DOI] [PubMed] [Google Scholar]
- 130.Zhou Q. Atadja P. Davidson N. E. Histone deacetylase inhibitor LBH589 reactivates silenced estrogen receptor alpha (ER)gene expression without loss of DNA hypermethylation. Cancer Biol. Ther. (2007);6:64–69. doi: 10.4161/cbt.6.1.3549. [DOI] [PubMed] [Google Scholar]