Abstract
Hesperidin, a flavanone present in citrus fruits, has been studied as potential therapeutic agents that have anti-tumor activity and apoptotic effects in several cancers, but there is no report about the apoptotic effect of hesperidin in human malignant pleural mesothelioma through the specificity protein 1 (Sp1) protein. We investigated whether hesperidin inhibited cell growth and regulated Sp1 target proteins by suppressing the levels of Sp1 protein in MSTO-211H cells. The IC50 value of hesperidin was determined to be 152.3 μM in MSTO-211H cells for 48 h. Our results suggested that hesperidin (0-160 μM) decreased cell viability, and induced apoptotic cell death. Hesperidin increased Sub-G1 population in MSTO-211H cells. Hesperidin significantly suppressed mRNA/protein level of Sp1 and modulated the expression level of the Sp1 regulatory protein such as p27, p21, cyclin D1, Mcl-1, and survivin in mesothelioma cells. Also, hesperidin induced apoptotic signaling including: cleavages of Bid, caspase-3, and PARP, upregulation of Bax, and down-regulation of Bcl-xl in mesothelioma cells. These results show that hesperidin suppressed mesothelioma cell growth through inhibition of Sp1. In this study, we demonstrated that Sp1 acts as a novel molecular target of hesperidin in human malignant pleural mesothelioma.
Keywords: Rotavirus, Hepatitis A virus, Recombinant chimera protein
INTRODUCTION
Malignant pleural mesothelioma (MPM) occurs from the mesothelial cells and is the most common primary tumor of the pleura. MPM is difficult to detect at an early stage and is a highly aggressive cancer (Robinson and Lake, 2005). Approximately 80% of MPM is caused from exposure to asbestos fiber and other factors include simian virus 40, radiation, and erionite (Carbone et al., 2002). Survival time of most patients after their first symptoms is very short (median < 12 months) despite treatments, and conventional treatments such as, chemotherapy and radiotherapy have shown to be quite ineffective (Robinson and Lake, 2005). For more than 20 years, studies for MPM have continued, but no biomarker was used clinically. Diagnostic, therapeutic, and prevention methods for MPM is understood by a variety of molecular pathways. Based on molecular pathways of MPM, diverse clinical trials using targeted agents have been conducted (Zucali et al., 2011). Development of several targeted treatments is necessary for understanding MPM carcinogenesis.
Clinical therapies for cancer treatments are limited to radiation, chemotherapy, immunosuppression and surgery (Neergheen et al., 2009). It is expected that potential chemo-preventive and therapeutic activities of polyphenols have an influence on intracellular signaling of cancers (Ramos, 2008). Flavonoids have various biological functions, such as anti-oxidant, anti-tumor, anti-inflammatory and anti-allergenic effects, at nontoxic concentrations in organisms (Dimmock et al., 1999; Horváthová et al., 2001; Ren et al., 2003). It is generally known that flavonoids have anti-cancer properties. Then, the study for the effects of flavonoids for cancer chemoprevention and chemotherapy is very significant (Ren et al., 2003; Li et al., 2007). Phytochemicals are non-toxic as a natural product in origin and have been known as inhibitors of various transcription factors within in vitro and animal models of cancer (Tsuda et al., 2004; Fresco et al., 2006).
Hesperidin (30,5,9-dihydroxy-40-methoxy-7-orutinosyl flavanone) is a flavanone present in citrus fruits like oranges and lemons (Justesen et al., 1998; Nielsen et al., 2002) (Fig. 1A). Hesperidin was first discovered in 1827, but had been studied as a combination product complex until 1986 (Garg et al., 2001). In nature, hesperidin exist as glycoside form, a beta-7-rutinoside of hesperitin, but dietary hesperidin is hydrolyzed to hesperitin (Preston et al., 1953; Ameer et al., 1996). Hesperidin
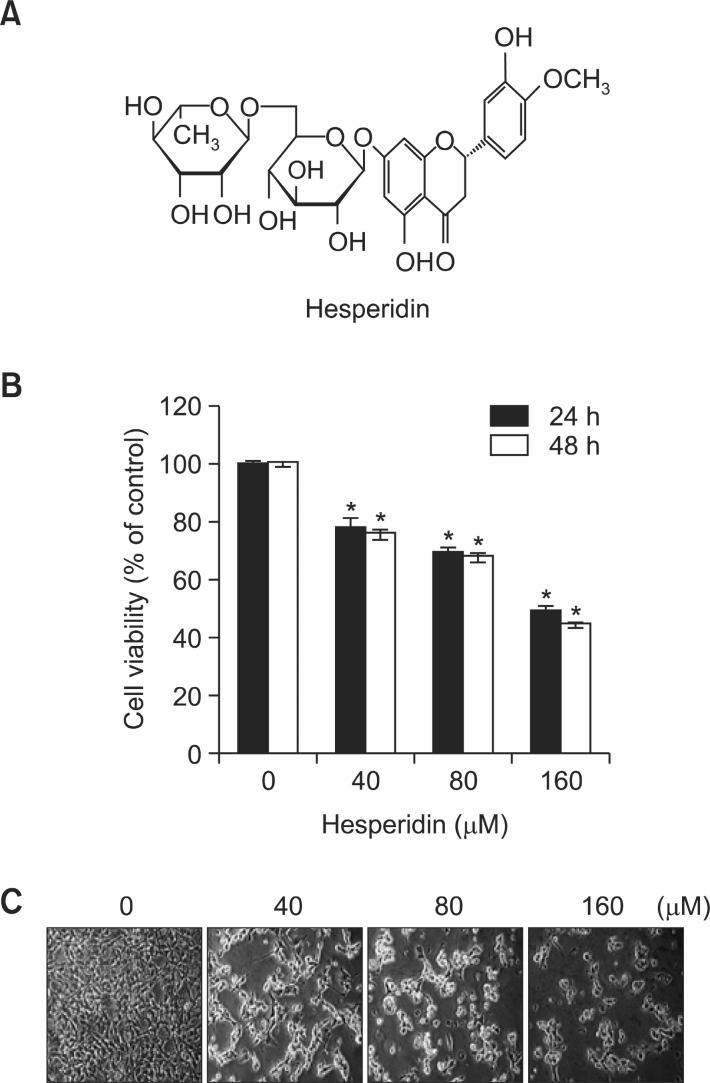
Fig. 1. The effect of hesperidin on cell viability in MSTO-211H cells. (A) Chemical structure of hesperidin. (B) Cell viability effectsof hesperidin on MSTO-211H cells. MSTO-211H cells (3×103 cells/200 μl) were treated with hesperidin (40-160 μM) in 5% FBS-RPMI1640 for 24 h and 48 h. Cell viabilities were measured with MTS assay, as described under “Material and Methods”. Results are indicated as cell viability relative to untreated with hesperidin, as determined from three independent experiments. Data representedas mean ± SD. The asterisk indicated a significant difference compared with the negative control (*p<0.05). (C) Cell morphological changes in MSTO-211H cells treated or untreated with hesperidin for 48 h.
has non-toxic activity in normal cells, but it is reported that hesperidin suppresses cell proliferation in several cancer types. Dietary hesperidin acts as an anticarcinogenic agent in some tumors. Hesperidin, such as flavonoid is known to have anti-inflammatory, anti-viral, UV protecting, anti-oxidant, proapoptotic, anti-proliferative and anti-tumor properties and protective effects against cerebrovascular disease and diabetes mellitus (Garg et al., 2001; Knekt et al., 2002; Choi, 2007; Akiyama et al., 2010; Ghorbani et al., 2012; Kamaraj et al., 2011; Lee et al., 2011). In addition, hesperidin as a radioprotective and chemoprotective therapeutic agent is expected to prevent invasion or metastasis of human cancers (Lee et al., 2010). Anti-cancer effects of hesperidin was studied in tumorimplanted animal models or culture cell lines of several cancer types, including colon cancer, bladder cancer, hepatocarcinoma cancer, and breast cancer, but the mechanism of this compound was not understood (Tanaka et al., 1997; Yang et al., 1997; Choi, 2007; Park et al., 2008; Yeh et al., 2009; Lee et al., 2010).
Normally, it is known that several transcription factors are upregulated in cancer. Transcription factors may function as targets for the development of new anti-cancer drugs,because these can regulate gene expression by binding to specific DNA sequences within cancer-related gene promoter regions (Safe and Abdelrahim, 2005). The level of Sp1 was elevated in cancer cells compared to normal cells. It was reported that Sp1 was overexpressed in several cancers including prostate cancer, breast cancer, gastric cancer, pancreatic cancer, thyroid cancer, hepatocellular carcinomas, colorectal cancer and lung cancer (Davie et al., 2008; Chuang et al., 2009; Kong et al., 2010; Sankpal et al., 2011). So highly expressed Sp1 protein upregulate genes concerned in tumor development, growth and metastasis by binding to promoter sequences. Thus, Sp1 protein was expected to be a negative prognostic factor and potential therapeutic target for cancer chemotherapy (Safe and Abdelrahim, 2005). Thus, based on the cancer-related functions of Sp1, it has been expected that Sp1 is a significant target for researches of cancer therapy.
It was not reported whether MPM was influenced by the chemoprevention effects of hesperidin. Also, t the association of hesperidin and Sp1 signaling has not been discovered. In the study, we investigated whether hesperidin inhibited cell growth, and regulated Sp1 target proteins, resulting in apoptosis by suppressing the levels of Sp1 protein in MSTO-211H cells.
MATERIALS AND METHODS
Antibodies
The following antibodies were purchased: anti-p21 (F-5), anti-p27 (C-19), anti-Cyclin D1 (M-20), anti-Sp1 (1C6), anti-caspase-3 (H-277), horseradish-peroxidase-conjugated anti-mouse IgG and horseradish-peroxidase-conjugated anti-rabbit IgG (all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-Poly ADP-ribose polymerase (PARP) (BD Biosciences, San Diego, California), anti-Mcl-1, anti-survivin, anti-Bid, anti-Bax, anti-Bclxl (Cell Signaling, Danvers, Massachusetts), anti-β-actin (AC-74) (Sigma-Aldrich, Inc. St. Louis, Missouri).
Cell culture
MSTO-211H cells were purchased from the American Tissue Culture Collection (Manassas, Virginia). The MSTO-211H cells were maintained in Hyclone RPMI-1640 supplemented with 5% fetal bovine serum (FBS) and 100 U/ml each of penicillin and streptomycin (Thermo Scientific, Logan, Utah) at 37°C in a humidified chamber with 5% CO2 and 95% air, and the medium was changed every 3 days.
MTS assay
MTS (3-(4,5-dimethylthiazol–2-yl)-5-(3–carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H–tetrazolium) Assay Kit (Pro-mega, Madison, Wisconsin) was used to measure the viability of MSTO-211H cell, according to the manufacturer’s protocol. The MSTO-211H cells were plated at a density of 3×103 cells/100 ul/well on a 96-well microtiter plate for 24 h. Hesperidin was treated with a final concentration of 0, 40, 80 and 160μM in cells for 24 h and 48 h, and then a MTS cell proliferation assay reagent was added to the cells and incubated at 37℃ in 5% CO2 for 2 h. The absorbance was measured at 490 nm using GloMax-Multi Microplate Multimode Reader (Promega, Madison, Wisconsin) and calculated as the difference of test and reference wavelength. Percentage of viability was calculated as an equation (optical density ratio of hesperidin-treated sample/non-treated sample) ×100.
DAPI staining
Apoptosis of hesperidin-treated cells were determined by 4’-6-diamidino-2-phenylindole (DAPI) staining. Nuclear condensation and fragmentation was observed by nucleic acid staining with DAPI (Sigma-Aldrich, Inc. St. Louis, Missouri). The MSTO-211H cells treated with 0-160 μM hesperidin for 48 h were harvested by trypsinization and fixed in 100% methanol at room temperature for 20 min. The cells were spread on slides, then stained with DAPI solution (2 μg/ml) and observed through a FluoView confocal laser microscope (Fluoview FV10i, Olympus Corporation, Tokyo, Japan).
Propidium iodide staining
After 48 h of the treatment of hesperidin, the detached cells(floaters) were collected by centrifugation and combined with the adherent cells. The cells were fixed in 70% ice-cold ethanol overnight at -20℃, and treated with 150 μg/ml RNase A and 20 μg/ml propidium iodide (PI; Sigma-Aldrich, Inc. St.Louis, Missouri). DNA content was analyzed by flow cytometry using a MACSQuant Analyzer (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany).
Western blotting
The hesperidin treated cells were washed twice with Phosphate Buffered Saline (PBS), then the cells were homogenized with a PRO-PREPTM Protein Extraction Solution (iNtRON Biotechnology, KOREA) containing 1 μg/ml aprotinin, 1 μg/ml leupeptin and 1 mM PMSF. The extracted protein was measured using the DC Protein Assay Reagent (BIO-RAD Laboratories Inc., Hercules, California). Fifty micrograms of protein per lane were electrophoresed to SDS-polyacrylamide gel and transferred to PVDF membranes. The membrane was blocked for 2 h at room temperature with 5% non-fat dried milk in PBS containing 0.1% tween-20, then incubated with specific antibodies overnight at 4℃ followed by incubation with respective secondary antibodies conjugated with horseradish peroxidase and development by Pierce ECL Western Blotting Substrate (Thermo scientific, Rockford, Illinois).
Reverse transcription-polymerase chain reaction
Total RNA was isolated from the cells using the TRIzol® Reagent (Life Technologies, Carlsbad, California), and 2 μg of RNA was used to synthesize cDNA using the HelixCriptTM 1st-strand cDNA synthesis kit (NanoHelix, Korea). RT-PCR was performed using HelixAmpTM Ready-2x-Go Series kit (Nano-Helix, Korea). PCR amplification was performed under the following conditions (30 cycles: 1 min at 95℃, 1 min at 60℃,and 1 min at 72℃). The primers used for β-actin as an internal standard were 5'-GTG-GGG-CGC-CCC-AGG-CAC-CA-3'(sense) and 5'-CTC-CTT-AAT-GTC-ACG-CAC-GAT-TTC-3'(antisense). The primers used for Sp1 were 5’-ATG-CCT-AAT ATT-CAG-TAT-CAA-GTA-3’ (sense) and 5’-CCC-TGA-GGTGAC-AGG-CTG-TGA-3’ (antisense).
Sp1 small interfering RNA (siRNA)
TARGETplus SMARTpool siRNAs sequences targeting Sp1 and non targeting controls were purchased from Thermo Scientific Dharmacon (Lafayette, CO). MSTO-211H cells were seeded in 96 well plates and 100mm dishes, and transfected transiently with 50 nM siRNA using DharmaFECT2 transfection reagent. After transfection, the cells were analyzed by MTS assay and immunoblotting.
Statistical analysis
Results were presented as mean ± SD. Differences between the means were assessed by one way analysis of variance. Data was analyzed for statistical significance using a Student’s t-test. The minimum level of significance was set at
p<0.05 compared with the vehicle control.
RESULTS
Hesperidin suppresses the cell viability of MSTO-211H cells
In order to evaluate the effects of hesperidin on the viability of MSTO-211H cells using the MTS assay kit, Hesperidin was treated with a dose-dependent manner of 0, 40, 80, and 160 μM on MSTO-211H cells. The IC50 value of hesperidin was determined to be about 152.3 μM in MSTO-211H cells for 48 h (Fig. 1B). Then, Cell viabilities were 82.0 ± 1.6%, 77.7 ± 1.4%, and 53.9 ± 1.5% of the control at 40, 80, and 160 μM hesperidin, respectively (Fig. 1B). Hesperidin treatment resulted in a significant concentration- and time dependent inhibition of cell growth with IC50 values of about 154 μM in other malignant mesothelioma cells, HT28 (Data not shown). To investigate the cell morphological changes of hesperidin in malignant mesothelioma cells, MSTO-211H cells were treated with hesperidin at various concentrations (0-160 μM). The results showed that the size of cells decreased and MSTO-211H cells changed to a round cell shape when treated or untreated with hesperidin for 48 h (Fig. 1C).
Hesperidin induces apoptotic cell death in MSTO-211H cells
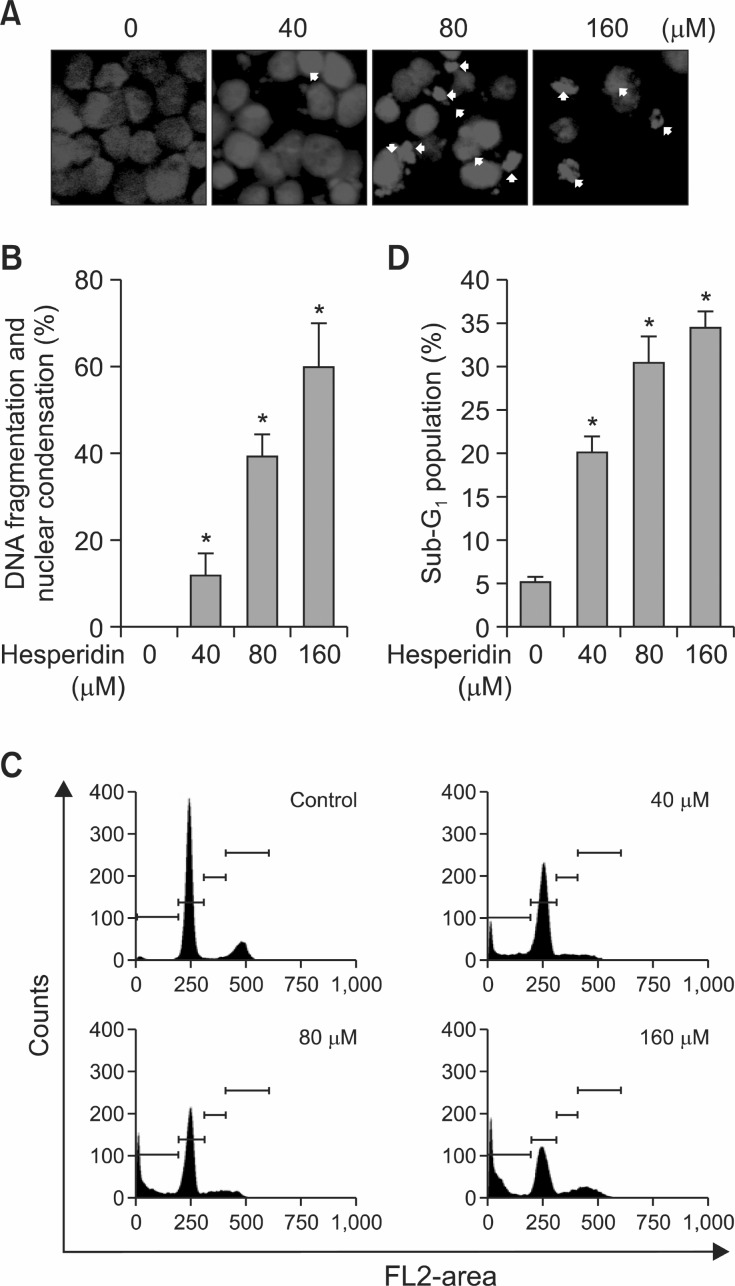
The effects of hesperidin on apoptosis of MSTO-211H cells were determined with DAPI staining and PI staining. It was performed to investigate the number of cells showing nuclear
condensation and fragmentation by DAPI. As shown in Fig. 2A, hesperidin treatment of mesothelioma cells led to an increase in nuclear condensation and fragmentation compared with the control. We determined the effect of hesperidin on Sub-G1 population in mesothelioma cells by PI staining (Fig. 2C and D). It showed that the Sub-G1 phase increased from
Fig. 2. The apoptotic effect induced by hesperidin in MSTO-211H cells. Cells were incubated with hesperidin (40 80 and 160 μM) treated and untreated (DMSO) of MSTO-211H cells for 48 h. The cells were harvested and prepared for DAPI staining and PI staining as described under Materials and Methods. (A) Analysis of DNA fragmentation and nuclear condensation (white arrows) by fluorescence microscopy (magnification ×600) after treatment of hesperidin in MSTO-211H cells (B) DNA fragmentation and nuclear condensation were quantified and the results in triplicates are expressed as the mean ± SD. (C) Analysis of cell cycle by flow cytometry after hesperidin treatment of cells for 48 h. (D) Representative histograms of Sub-G1 population. The hesperidin-treated cells were compared with untreated cells, and the graphs are shown as the average of triplicated data from three independent experiments (*p<0.05).
20-35% in 40-160 μM hesperidin-treated MSTO-211H cells (Fig. 2D).
Hesperidin regulates specifi city protein 1 in MSTO-211H cells
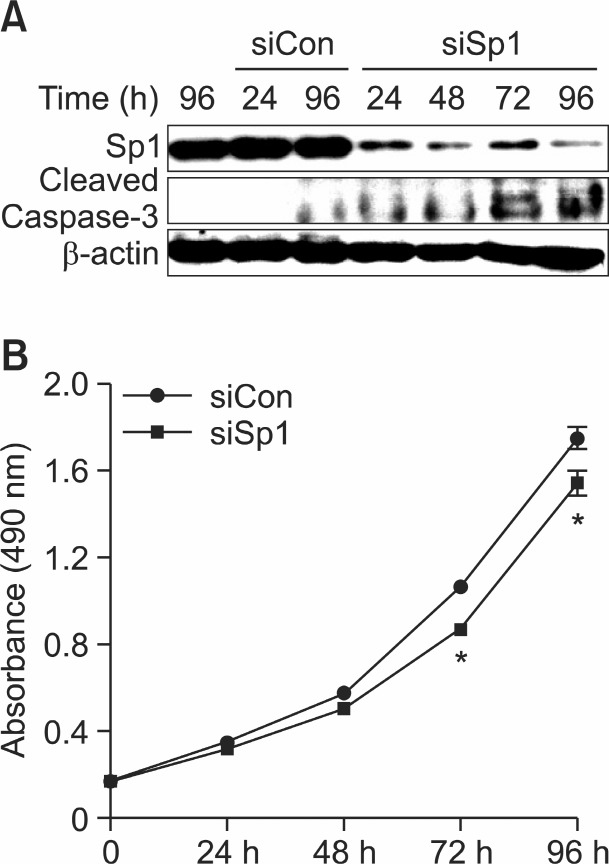
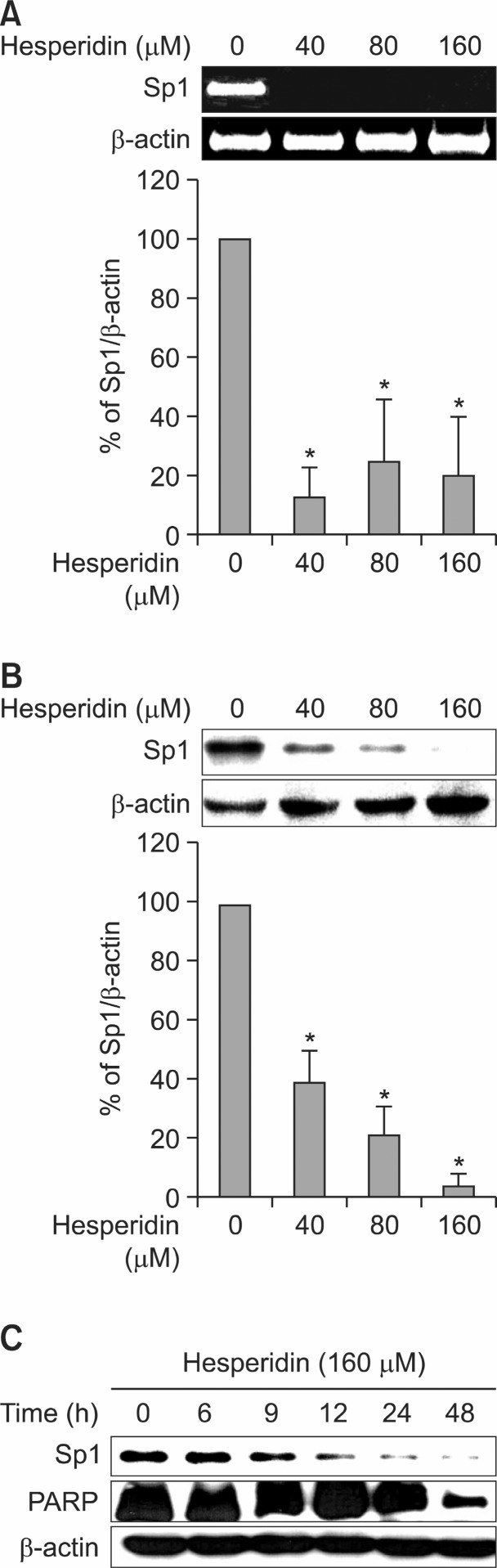
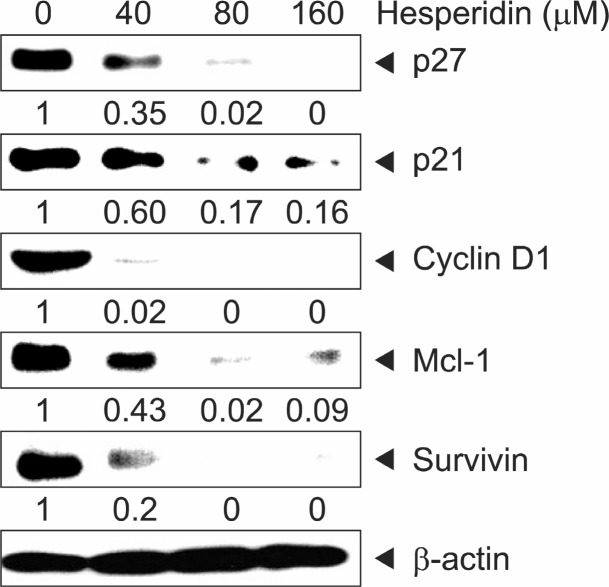
It is reported that Sp1 induced or suppressed the activity of promoters for genes involved in cell progression and apoptotic cell death (Li and Davie, 2010). In order to confirm whether Sp1 influenced MSTO-211H cell proliferation, we transfected with RNA interference with small interfering Sp1 (siSp1) in MSTO-211H cell. Cell transfection of siSp1 decreased cell viability of MSTO-211H cells by interfering Sp1 signaling (Fig.3B). Additionally, it was analyzed that Sp1 expression was significantly down-regulated while Cleaved-Caspase-3 was upregulated by siSp1 (Fig. 3A). In order to determine if the suppression of cell growth by hesperidin was due to the inhibition of Sp1 protein, we investigated the level of Sp1 protein and Sp1 mRNA on MSTO-211H cells treated with hesperidin, using immunoblot and RT-PCR. In the results, Sp1 protein and Sp1 mRNA expression levels were down-regulated by 40, 80, and 160 μM hesperidin (Fig. 4A, B). Also, Sp1 protein levels on MSTO-211H cells by 160 μM hesperidin treatment were suppressed sequentially through time-course incubation of 6, 9, 12, 24, and 48h (Fig. 4C). In addition, downstream target proteins of Sp1 were regulated by hesperidin in MSTO-211H cells. We suggested that hesperidin decreased the level of p27, p21, cyclin D1, Mcl-1, and survivin (Fig. 5).
Fig. 3. Function of specificity protein 1 (Sp1) in the proliferation of MSTO-211H cells. (A) RNA interference with small interfering Sp1 (siSp1). MSTO-211H cells were transfected with siCon (nonspecific) or siSp1 for 24 h, 48 h, 72 h, and 96 h. Transfected cell lysates were determined by western blotting. β-actin was probed to determine the equal amounts of the loading protein extract from each treatment. (B) The cell proliferation effect of siCon-transfected or siSp1-transfected MSTO-211H cells were determined by MTS assay.Results were indicated from three independent experiments. Data was represented as mean ± SD. The asterisk indicated a significant difference in siSp1 transfected cells compared with siCon transfected cells (*p<0.05).
Fig. 4. Down-regulation of specificity protein 1 (Sp1) by hesperidinon MSTO-211H. (A) The effect of hesperidin for 48 h on Sp1mRNA expression was determined by RT-PCR. The graphs indicated percentages of Sp1/β-actin. (B) The effect of hesperidin for 48 h on Sp1 protein expression was determined by western blotting. Actin was probed to determine the equal of the loading protein extract from each treatment. Data are shown as the mean ± SD of three independent experiments. The asterisks (*p<0.05) indicated a significant decrease in Sp1 protein in hesperidin-treated cells compared with untreated cells. (C) The time-dependent effect of hesperidin on Sp1 and apoptotic protein of PARP protein expression was performed in MSTO-211H cells were treated with 160 μM of hesperidin for 6 h, 9 h, 12 h, 24 h, and 48 h.
Fig. 5. The effect of hesperidin on downstream target proteins by specificity protein 1 (Sp1). MSTO-211H cells were treated with hesperidin (0, 40, 80, and 160 μM) for 48 h. The protein expression of p27, p21, Cyclin D1, Mcl-1, and Survivin were analyzed by Western blot analysis. Fifty micrograms of cellular extract per lane was separated on SDS-PAGE gel as described in Materials and methods. Equal loading of proteins was confirmed by incubating the same membrane with anti-β-actin antibody.
Hesperidin regulates the expression of anti-apoptotic and apoptotic molecule in MSTO-211H cells
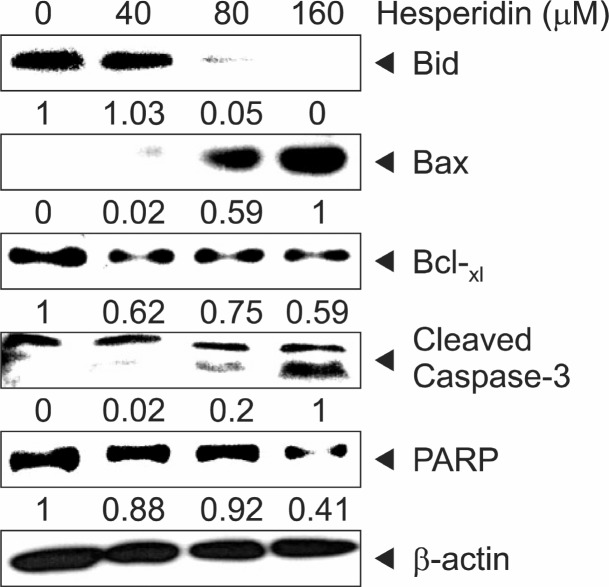
In order to investigate how hesperidin regulated the expression of apoptotic marker proteins in MSTO-211H cells, we determined the level of several pro- and anti-apoptotic proteins in hesperidin-treated cells by immunoblotting after treatment with hesperidin for 48 h, the activation of PARP and Bid, the cleavage of caspase3, and the induction of Bax, which are the hallmarks of apoptotic activity as well as the down-regulation of Bcl-xl in MSTO-211H cells (Fig. 6).
Fig. 6. The effect of hesperidin on apoptosis of hesperidin-treated MSTO-211H. Immunoblot detection of the Bid, Bcl-xL, Cleaved-Caspase-3, PARP, and Bax in whole cell lysates from various concentration of hesperidin-treated MSTO-211H cells for 48 h. β-actin was used to normalize the protein loading from each treatment.
DISCUSSION
MPM is rarely detected with only 2000-3000 new cases occurring each year (Raja et al., 2011). Asbestos caused most MPM (Carbone et al., 2002). MPM is difficult to detect early on (Robinson and Lake, 2005). For investigations of MPM treatments, several studies have executed chemotherapy in combination with radiation and surgery for resectable MPM and chemotherapy only in unresectable MPM. At present, several clinical studies including molecular targeting agents, immunotherapy and gene therapy have been continuously tried for cancer therapy (Takigawa et al., 2011). Also, it is known that many clinical trials based on diverse molecular pathways of MPM were examined. But, several target agents for molecular pathways were still ineffective in clinical trials (Zucali et al., 2011).
Flavonoids are studied as anti-cancer agents for chemo-therapy of cancer, for its advantage like nontoxic characters from natural products. Representative flavonoids like resveratrol, quercetin, curcumin and EGCG are studied for clinical trials (Shanafelt et al., 2009; Howells et al., 2010; la Porte et al., 2010; Kanai et al., 2011). However, it has not yet been developed for clinical treatments regarding cancer therapy. Hesperidin and metabolites have significant potential as therapeutic agents for a wide range of diseases and disorders. So far, it is not yet very widely used therapeutically (Garg et al., 2001). The pharmacological effects of hesperidin involved in the inhibition of transcriptional factors and induction of apoptosis are continuously studied for cancer therapy (Garg et al., 2001; Park et al., 2008; Yeh et al., 2009), however, the precise mechanism of cancer prevention has not yet been discovered in human mesothelioma cells.
Sp1 is upregulated in different types of cancer including breast carcinomas, thyroid cancer, gastric cancer, and lung cancer (Davie et al., 2008; Chuang et al., 2009; Kong et al., 2010). The ubiquitous transcription factors of Sp1 are known to regulate molecular target genes in several biological processes such as differentiation, metabolism, cell growth, angiogenesis and apoptosis regulation (Li and Davie, 2010). Inhibition of key proteins in signal transduction pathways, inhibition of transcription factor, modulation of cell-cycle regulation, and induction of apoptosis have been studied as target strategies for understanding carcinogenesis-related mechanisms and cancer prevention (Fresco et al., 2006).
In this paper, we investigated whether expression of transcription factor, Sp1, is inhibited and apoptotic cell death is induced by hesperidin in MSTO-211H cells. Preferentially,we previously confi rmed the roles of Sp1 in cell proliferation and apoptosis of MSTO-211H cells by interference of Sp1 signaling using siSp1 (Fig. 3). Our results showed nuclear condensation and fragmentation and sub-G1 population on MSTO-211H cells, resulting in the induction of apoptosis by hesperidin treatments, as determined by DAPI staining and PI staining (Fig. 2). Our studies strongly suggested that hesperidin suppressed expression of Sp1 mRNA and Protein in a dose dependent manner. Sp1 could contribute to cell growth via the regulation of numerous gene expressions such as p21, p27, cyclin D1, Mcl-1 and survivin, etc. in prostate, breast, pancreatic, cervical, and oral cancer cells (Li and Davie, 2010). Our results demonstrated that hesperidine strongly affected Sp1 and regulated Sp1-downstream target proteins including p27, p21, cyclin D1, Mcl-1 and survivin in MSTO-211H cells (Fig. 5). Moreover, these results showed that hesperidin had a direct influence on the apoptosis-related proteins by regulating expression of Bcl-Xl, Bax, caspase-3 and PARP in MSTO-211H cells (Fig. 6). These results proved that Sp1 is an efficient therapeutic target for cancer, as reported (Neergheen et al., 2009). Hesperidin indicated cancer therapeutic effects similar to those of several flavonoids, but the inhibitory mechanisms of hesperidin at the molecular pathway of carcinogenesis need to be confirmed. Also, an in vivo study for clinical application is necessary in MPM.
Finally, due to the conventional approach of chemotherapy and chemoprevention of cancer, hesperidin needs to be studied so as to improve non-toxicity, water-solubility, absorption rate, optimal dose, interference and metabolic conversion for its safe and efficient clinical application as a dietary treatment (Neergheen et al., 2009). Since metabolites can function differently with hesperidin in regards to chemoprevention, structure-property-activity studies are necessary (Fresco et al., 2006; 2010). In conclusion, we demonstrated that Sp1 can be used as an effective therapeutic target in cancer research, and that hesperidin may be essential as a potential cancer drug or supplement regarding chemotherapeutic and chemo preventative agents for MPM.
Acknowledgments
This research was supported by Basic Science Research program through the National Research Foundation Korea (NRF) Funded by the Ministry of Education, Science and Technology (2010-0007845 and 2012-0003226).
References
- 1.Akiyama S. Katsumata S. Suzuki K. Ishimi Y. Wu J. Uehara M. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J. Clin. Biochem. Nutr. (2010);46:87–92. doi: 10.3164/jcbn.09-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ameer B. Weintraub R. A. Johnson J. V. Yost R. A. Rouseff R. L. Flavanone absorption after naringin, hesperidin, and citrus administration. Clin. Pharmacol. Ther. (1996);60:34–40. doi: 10.1016/S0009-9236(96)90164-2. [DOI] [PubMed] [Google Scholar]
- 3.Carbone M. Kratzke R. A. Testa J. R. The pathogenesis of mesothelioma. Semin. Oncol. (2002);29:2–17. doi: 10.1053/sonc.2002.30227. [DOI] [PubMed] [Google Scholar]
- 4.Choi E. J. Hesperetin induced G1-phase cell cycle arrest in human breast cancer MCF-7 cells: involvement of CDK4 and p21. Nutr. Cancer. (2007);59:115–119. doi: 10.1080/01635580701419030. [DOI] [PubMed] [Google Scholar]
- 5.Chuang J. Y. Wu C. H. Lai M. D. Chang W. C. Hung J. J. Overexpression of Sp1 leads to p53-dependent apoptosis in cancer cells. Int. J. Cancer. (2009);125:2066–2076. doi: 10.1002/ijc.24563. [DOI] [PubMed] [Google Scholar]
- 6.Davie J. R. He S. Li L. Sekhavat A. Espino P. Drobic B. Dunn K. L. Sun J. M. Chen H. Y. Yu J. Pritchard S. Wang X. Nuclear organization and chromatin dynamics--Sp1, Sp3 and histone deacetylases. Adv. Enzyme Regul. (2008);48:189–208. doi: 10.1016/j.advenzreg.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Dimmock J. R. Elias D. W. Beazely M. A. Kandepu N. M. Bioactivities of chalcones. Curr. Med. Chem. (1999);6:1125–1149. [PubMed] [Google Scholar]
- 8.Fresco P. Borges F. Diniz C. Marques M. P. New insights on the anticancer properties of dietary polyphenols. Med. Res. Rev. (2006);26:747–766. doi: 10.1002/med.20060. [DOI] [PubMed] [Google Scholar]
- 9.Fresco P. Borges F. Marques M. P. Diniz C. The anti-cancer properties of dietary polyphenols and its relation with apoptosis. Curr. Pharm. Des. (2010);16:114–134. doi: 10.2174/138161210789941856. [DOI] [PubMed] [Google Scholar]
- 10.Garg A. Garg S. Zaneveld L. J. Singla A. K. Chemistry and pharmacology of the Citrus bioflavonoid hesperidin. Phytother. Res. (2001);15:655–669. doi: 10.1002/ptr.1074. [DOI] [PubMed] [Google Scholar]
- 11.Ghorbani A. Nazari M. Jeddi-Tehrani M. Zand H. The citrus flavonoid hesperidin induces p53 and inhibits NF-κB activation in order to trigger apoptosis in NALM-6 cells: involvement of PPARγ-dependent mechanism. Eur. J. Nutr. (2012);51:39–46. doi: 10.1007/s00394-011-0187-2. [DOI] [PubMed] [Google Scholar]
- 12.Horváthová K. Vachálková A. Novotný L. Flavonoids as chemoprotective agents in civilization diseases. Neoplasma. (2001);48:435–441. [PubMed] [Google Scholar]
- 13.Howells L. M. Britton R. G. Mazzoletti M. Greaves P. Broggini M. Brown K. Steward W. P. Gescher A. J. Sale S. Preclinical colorectal cancer chemopreventive efficacy and p53-modulating activity of 3',4',5'-trimethoxyflavonol a quercetin analogue. Cancer Prev. Res (Phila). (2010);3:929–939. doi: 10.1158/1940-6207.CAPR-09-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Justesen U. Knuthsen P. Leth T. Quantitative analysis of flavonols flavones and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photodiode array and mass spectrometric detection. J. Chromatogr. A. (1998);799:101–110. doi: 10.1016/s0021-9673(97)01061-3. [DOI] [PubMed] [Google Scholar]
- 15.Kamaraj S. Anandakumar P. Jagan S. Ramakrishnan G. Devaki T. Hesperidin attenuates mitochondrial dysfunction during benzo(a)pyrene-induced lung carcinogenesis in mice. Fundam. Clin. Pharmacol. (2011);25:91–98. doi: 10.1111/j.1472-8206.2010.00812.x. [DOI] [PubMed] [Google Scholar]
- 16.Kanai M. Imaizumi A. Otsuka Y. Sasaki H. Hashiguchi M. Tsujiko K. Matsumoto S. Ishiguro H. Chiba T. Dose-escalation and pharmacokinetic study of nanoparticle curcumin a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chemother. Pharmacol. (2011);69:65–70. doi: 10.1007/s00280-011-1673-1. [DOI] [PubMed] [Google Scholar]
- 17.Knekt P. Kumpulainen J. Järvinen R. Rissanen H. Heliövaara M. Reunanen A. Hakulinen T. Aromaa A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. (2002);76:560–568. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 18.Kong L. M. Liao C. G. Fei F. Guo X. Xing J. L. Chen Z. N. Transcription factor Sp1 regulates expression of cancer-associated molecule CD147 in human lung cancer. Cancer Sci. (2010);101:1463–1470. doi: 10.1111/j.1349-7006.2010.01554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.la Porte C. Voduc N. Zhang G. Seguin I. Tardiff D. Singhal N. Cameron D. W. Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin. Pharmacokinet. (2010);49:449–454. doi: 10.2165/11531820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 20.Lee K. H. Yeh M. H. Kao S. T. Hung C. M. Liu C. J. Huang Y. Y. Yeh C. C. The inhibitory effect of hesperidin on tumor cell invasiveness occurs via suppression of activator protein 1 and nuclear factor-kappaB in human hepatocellular carcinoma cells. Toxicol. Lett. (2010);194:42–49. doi: 10.1016/j.toxlet.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Lee Y. R. Jung J. H. Kim H. S. Hesperidin partially restores impaired immune and nutritional function in irradiated mice. J. Med. Food. (2011);14:475–482. doi: 10.1089/jmf.2010.1269. [DOI] [PubMed] [Google Scholar]
- 22.Li L. Davie J. R. The role of Sp1 and Sp3 in normal and cancer cell biology. Ann. Anat. (2010);192:275–283. doi: 10.1016/j.aanat.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Li Y. Fang H. Xu W. Recent advance in the research of flavonoids as anticancer agents. Mini. Rev. Med. Chem. (2007);7:663–678. doi: 10.2174/138955707781024463. [DOI] [PubMed] [Google Scholar]
- 24.Neergheen V. S. Bahorun T. Taylor E. W. Jen L. S. Aruoma O. I. Targeting specific cell signaling transduction pathways by dietary and medicinal phytochemicals in cancer chemoprevention. Toxicology. (2009);278:229–241. doi: 10.1016/j.tox.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen S. E. Freese R. Kleemola P. Mutanen M. Flavonoids in human urine as biomarkers for intake of fruits and vegetables. Cancer Epidemiol. Biomarkers. Prev. (2002);11:459–466. [PubMed] [Google Scholar]
- 26.Park H. J. Kim M. J. Ha E. Chung J. H. Apoptotic effect of hesperidin through caspase3 activation in human colon cancer cells, SNU-C4. Phytomedicine. (2008);15:147–151. doi: 10.1016/j.phymed.2007.07.061. [DOI] [PubMed] [Google Scholar]
- 27.Preston R. K. Avakian S. Beiler J. M. Moss J. N. Martin G. J. In vivo and in vitro inhibition of hyaluronidase by organic phosphates. Exp. Med. Surg. (1953);11:1–8. [PubMed] [Google Scholar]
- 28.Raja S. Murthy S. C. Mason D. P. Malignant pleural mesothelioma. Curr. Oncol. Rep. (2011);13:259–264. doi: 10.1007/s11912-011-0177-9. [DOI] [PubMed] [Google Scholar]
- 29.Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. (2008);52:507–526. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- 30.Ren W. Qiao Z. Wang H. Zhu L. Zhang L. Flavonoids: promising anticancer agents. Med. Res. Rev. (2003);23:519–534. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- 31.Robinson B. W. Lake R. A. Advances in malignant mesothelioma. N. Engl. J. Med. (2005);353:1591–1603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- 32.Safe S. Abdelrahim M. Sp transcription factor family and its role in cancer. Eur. J. Cancer. (2005);41:2438–2448. doi: 10.1016/j.ejca.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Sankpal U. T. Goodison S. Abdelrahim M. Basha R. Targeting Sp1 transcription factors in prostate cancer therapy. Med. Chem. (2011);7:518–525. doi: 10.2174/157340611796799203. [DOI] [PubMed] [Google Scholar]
- 34.Shanafelt T. D. Call T. G. Zent C. S. LaPlant B. Bowen D. A. Roos M. Secreto C. R. Ghosh A. K. Kabat B. F. Lee M. J. Yang C. S. Jelinek D. F. Erlichman C. Kay N. E. Phase I trial of daily oral Polyphenon E in patients with asymptomatic Rai stage 0 to II chronic lymphocytic leukemia. J. Clin. Oncol. (2009);27:3808–3814. doi: 10.1200/JCO.2008.21.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takigawa N. Kiura K. Kishimoto T. Medical treatment of mesothelioma: anything new? Curr. Oncol. Rep. (2011);13:265–271. doi: 10.1007/s11912-011-0172-1. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka T. Makita H. Kawabata K. Mori H. Kakumoto M. Satoh K. Hara A. Sumida T. Tanaka T. Ogawa H. Chemo-prevention of azoxymethane-induced rat colon carcinogenesis by the naturally occurring flavonoids, diosmin and hesperidin. Carcinogenesis. (1997);18:957–965. doi: 10.1093/carcin/18.5.957. [DOI] [PubMed] [Google Scholar]
- 37.Tsuda H. Ohshima Y. Nomoto H. Fujita K. Matsuda E. Iigo M. Takasuka N. Moore M. A. Cancer prevention by natural compounds. Drug Metab. Pharmacokinet. (2004);19:245–263. doi: 10.2133/dmpk.19.245. [DOI] [PubMed] [Google Scholar]
- 38.Yang M. Tanaka T. Hirose Y. Deguchi T. Mori H. Kawada Y. Chemopreventive effects of diosmin and hesperidin on Nbutyl-N-(4-hydroxybutyl)nitrosamine-induced urinary-bladder carcinogenesis in male ICR mice. Int. J. Cancer. (1997);73:719–724. doi: 10.1002/(sici)1097-0215(19971127)73:5<719::aid-ijc18>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 39.Yeh M. H. Kao S. T. Hung C. M. Liu C. J. Lee K. H. Yeh C. C. Hesperidin inhibited acetaldehyde-induced matrix metalloproteinase-9 gene expression in human hepatocellular carcinoma cells. Toxicol. Lett. (2009);184:204–210. doi: 10.1016/j.toxlet.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 40.Zucali P. A. Ceresoli G. L. De Vincenzo F. Simonelli M. Lorenzi E. Gianoncelli L. Santoro A. Advances in the biology of malignant pleural mesothelioma. Cancer Treat. Rev. (2011);37:543–558. doi: 10.1016/j.ctrv.2011.01.001. [DOI] [PubMed] [Google Scholar]