Abstract
All-trans retinoic acid (ATRA) is currently used in adjuvant differentiation-based treatment of residual or relapsed neuroblastoma (NB). It has been reported that short-term ATRA treatment induces migration and invasion of SH-SY5Y via transglutaminase-2 (Tgase-2). However, the detailed mechanism of Tgase-2's involvement in NB cell invasion remains unclear. Therefore we investigated the role of Tgase-2 in invasion of NB cells using SH-SY5Y cells. ATRA dose-dependently induced the invasion of SH-SY5Y cells. Cystamine (CTM), a well known tgase inhibitor suppressed the ATRA-induced invasion of SH-SY5Y cells in a dose-dependent manner. Matrix metalloproteinase-9 (MMP-9) and MMP-2, well known genes involved in invasion of cancer cells were induced in the ATRA-induced invasion of the SH-SH5Y cells. Treatment of CTM suppressed the MMP-9 and MMP-2 enzyme activities in the ATRA-induced invasion of the SH-SY5Y cells. To confirm the involvement of Tgase-2, gene silencing of Tgase-2 was performed in the ATRA-induced invasion of the SH-SH5Y cells. The siRNA of Tgase-2 suppressed the MMP-9 and MMP-2 activity of the SH-SY5Y cells. MMP-2 and MMP-9 are well known target genes of NF-κB. Therefore the relationship of Tgase-2 and NF-κB in the ATRA-induced invasion of the SH-SY5Y cells was examined using siRNA and CTM. ATRA induced the activation of NF-κB in the SH-SY5Y cells and CTM suppressed the activation of NF-κB. Gene silencing of Tgase-2 suppressed the MMP expression by ATRA. These results suggested that Tgase-2 might be a new target for controlling the ATRA-induced invasion of NBs.
Keywords: All-trans retinoic acid, Transglutaminase-2, Invasion, Neuroblastoma, NF-κB, Matrix metalloproteinase
INTRODUCTION
Neuroblastoma (NB) is the most common extracranial solid tumor in the pediatric population. NB accounts for 7-10% of all childhood cancers and 15% of all cancer-related mortalities in children. It is the most common cancer diagnosed during infancy (Gurney et al., 1997; Mueller and Matthay, 2009). The prevalence is about 1 case per 7,000 live births, and there are 700 new cases in the United States annually (Brodeur, 2002). NB is a malignant tumor composed of neural-crest-derived un-differentiated neuro-ectodermal cells of the adrenal medulla or sympathetic neurons (Cianfarani and Rossi, 1997). Typically, NB tumors occur in the adrenal medulla or paraspinal sympathetic ganglia of the abdomen, chest, or neck. The causes of NB and the factors implicating the anabasis of the disease have yet to be fully elucidated. NB is metastatic in 70% of patients at the time of diagnosis (Ara and DeClerck, 2006). Metastasis in NB occurs in 70% of the cases in the bone marrow and 56% of the cases in the bone (DuBois et al., 1999). The mechanisms by which NB cells invade the bone marrow and bone have only begun to be elucidated recently. Despite current therapeutic advances, ongoing clinical trials and basic science investigations, neuroblastoma remains a complex medical challenge with an unpredictable clinical course and dismal overall outcome for advanced-stage disease (Modak and Cheung, 2010; Lee, 2012). Therefore, many approaches are still undergoing (Roy Choudhury et al., 2012).
Although the proper care for NB is not established, there are alternative therapeutic approaches. All-trans retinoic acid (ATRA), a vitamin A derivative, is a potent agent of cellular differentiation and growth inhibition in normal and cancer cell types (Freemantle et al., 2003; Garattini et al., 2007). In vitro studies with ATRA in solid tumor cell lines, including NB, have shown great potential for ATRA as a new adjuvant therapeutic agent (Redfern et al., 1995; Freemantle et al., 2003). However, despite encouraging laboratory findings, retinoids have instruchad disappointing results in patients with solid tumors due to acquired resistance mechanisms (Freemantle et al., 2003).
The relationships of retinoidresistance with invasion and metastasis of neuroblastomas have not been fully investigat-ed. It has been reported that ATRA induces NF-κB activation and matrix metalloproteinases-9 expression and enhances the basement membrane invasiveness of all-trans retinoic acid-resistant human SK-N-BE 9N neuroblastoma cells (Farina et al., 2002). However, it is not known how all-trans-retinoic acid induces NF-κB activation and matrix metalloproteinases-9 expression. Also a recent report showed that short term ATRA treatment promotes the migration and invasion of neuroblastoma SH-SY5Y cells via retinoic acid receptors and Tgase-2 (Joshi et al., 2006). Tgase-2 is one of the most ATRA-induced proteins in NB (Murtaugh et al., 1986). It is well known that ATRA-induced Tgase-2 is involved in promoting the neurite outgrowth and differentiation of NB. It is interesting and surprising that Tgase-2 has a role in invasion of ATRA-induced NBs. However, the detailed role and mechanism of Tgase-2's involvement in ATRA-induced invasion of NB remains unclear.In this report, we showed that Tgase-2 is involved in ATRA-induced invasion and MMP expression by NF-κB activation.
MATERIALS AND METHODS
Materials
The All-trans retinoic acid and other common chemicals were purchased from Sigma (St Louis, MO, USA). Tissue culture media and serum were acquired from WelGENE (Seoul, Korea). The matrigel matrix was obtained from BD Biosciences (Franklin Lakes, NJ, USA). Lipofectamine 2000 Reagent was from Invitrogen (Carlsbad, CA, USA). Tgase-2 antibody was purchased from Neomarkers (Newington, NH, USA), and antibodies against β-actin were acquired from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit polyclonal antibodies against phospho-65, IκBα were from Cell Signaling Technology Inc. (Beverly, MA, USA). shRNA-lentiviral particles against human transglutaminase 2 and control lentiviral particles were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
Cell culture and ATRA treatment
SH-SY5Y cells were grown in a medium containing 10% serum, and 1% antibiotics (10,000 μg/ml streptomycin and 10,000 units/ml penicillin) at 37℃ in a humidified incubator with 5% CO2. The cells were incubated in a medium containing 3% serum for 12 hr and then treated with 5 μM RA or vehicle (DMSO) for 2 days. Treatment of cystamine was performed also in a medium containing 3% serum.
Invasion assay using transwell plates
Cell invasion was studied using matrigel-coated (0.7 μg/ml) transwell inserts, as described previously (Mehta et al., 2004; Cha et al., 2011). Trypsinized cells were suspended in serum-free medium without RA, and 5×105 cells were added to the upper chamber of the transwell inserts. Medium with 3% serum was added to the lower chamber. After 24 hr incubation of SH-SY5Y cells, the nonmigrated cells on the upper surface of the membrane were scraped off, and the cells on the lower surface were stained using the Hema 3 staining system (Fisher Scientific, Houston, TX, USA), photographed (40× magnification) and counted from 10 randomly selected fields. All of the experiments were repeated at least three times with two replicates each.
Zymography
Samples were subjected to regular non-reducing SDS-PAGE in gel copolymerized with 1 mg/ml gelatin. After electrophoresis, gels were washed in 2.5% Triton X-100, rinsed in water, and incubated in a buffer containing 50 mM Tris, 0.2 M NaCl, and 5 mM CaCl2 overnight at 37℃. Enzyme/inhibitor activity was visualized by staining with Coomassie Blue.
Cell culture and gene silencing of tgase-2
Human neuroblastoma SH-SY5Y cells were obtained from the American Type Culture Collection. SH-SY5Y cells were maintained in MEM media supplemented with 10% fetal bovine serum (FBS) (Welgene, Korea). All cells were grown at 37℃ in a humidified 5% CO2 atmosphere.
A small interfering RNA (siRNA) duplex targeting human Tgase-2, 5'-AAG AGC GAG AUG AUC UGG AAC-3' (Invitrogen) was introduced into the cells using Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA), according to the manufacturer's instruction. Universal negative siRNA (Invitrogen, Carlsbad, CA) was employed as the negative controls.
For stable expression of shRNA against Tgase-2 through lentiviral infection, SH-SY5Y cells were grown in MEM containing 10% FBS for 24 h and incubated with polybrene (5 μg/ml) for 1 h before the addition of the lentiviral vector (approximately 1 molar ratio of infection). After 24 h, the medium was replaced and cells were grown for 1 day. SH-SY5Y sh control (SH5YShCon) and SH-SY5Y sh Tgase-2 (SH5YShTG2) stable cell lines were selected in 5 μg/ml puromycin dihydrochloride and maintained in growth medium containing 2 μg/ml puromycin dihydrochloride. To avoid clonal variations, we pooled individual infectants for each stable cell line produced by infection.
Western blot
After incubation, the cells were collected and washed twice with cold PBS. The cells were lysed in a lysis buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% triton X-100, 2 mM EDTA, 1% DOC (Deoxycholic acid), 0.1% SDS, 1 mM NaVO3, 10 mM NaF, 1 mM DTT] and centrifuged to yield whole-cell lysates. Protein concentration was measured using the Bradford method. Aliquots of the lysates (10-20 μg of protein) were separated on a 4-12% SDS-polyacrylamide gel and transferred onto a polyvinylidene fluoride (PVDF) membrane (Invitrogen, Carlsbad, CA, USA) with a glycine transfer buffer [192 mM glycine, 25 mM Tris-HCl (pH 8.8), 10% MeOH (v/v)]. After blocking the nonspecific site with 3% non-fat dry milk, the membrane was then incubated with specific primary antibody in 3% BSA at 4℃ for overnight. The membrane was further incubated for 60 min with a peroxidase-conjugated secondary antibody (1:5,000, Santa Cruz, CA, USA) at room temperature. Immunoactive proteins were detected using the PowerOpti-ECL western blotting detection reagent (Animal Genetics Inc., Gyeonggi, Korea).
Preparation of nuclear and cytosolic fractions
Nuclear extract was prepared using CelLytic Nuclear Extraction Kit (Sigma, USA) following the manufacturer's instructions
(Guidez et al., 1998). Cell pellets were resuspended in isotonic buffer (10 mM HEPES, pH 7.5, 2 mM MgCl2, 3 mM CaCl2, 300 mM Sucrose, 0.2 mM PMSF, 0.5 mM DTT, 10 μg/ml aprotinin) and incubated on ice for 15 min. Cells were then lysed by adding 0.6% IGEPAL A-630 solution and vortexed vigorously for 10 s. Centrifuge immediately for 30 seconds at 10,000g. Transfer the supernatant to a fresh tube. This fraction is the cytoplasmic fraction. Resuspend the crude nuclei pellet in high salt buffer (20 mM HEPES, pH 7.9, 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 1 mM NaF, 1 mM sodium orthovanadate) and centrifuge at 12,000g for 5 min at 4℃. This fraction is the nucleus fraction.
Statistical analysis
The statistical significance of the data was evaluated using a one-way analysis of variance, the 0.05 probability level indicating significant differences.
RESULTS
One day ATRA treatment promotes invasion and cysta-mine inhibits ATRA-induced invasion of SH-SY5Y
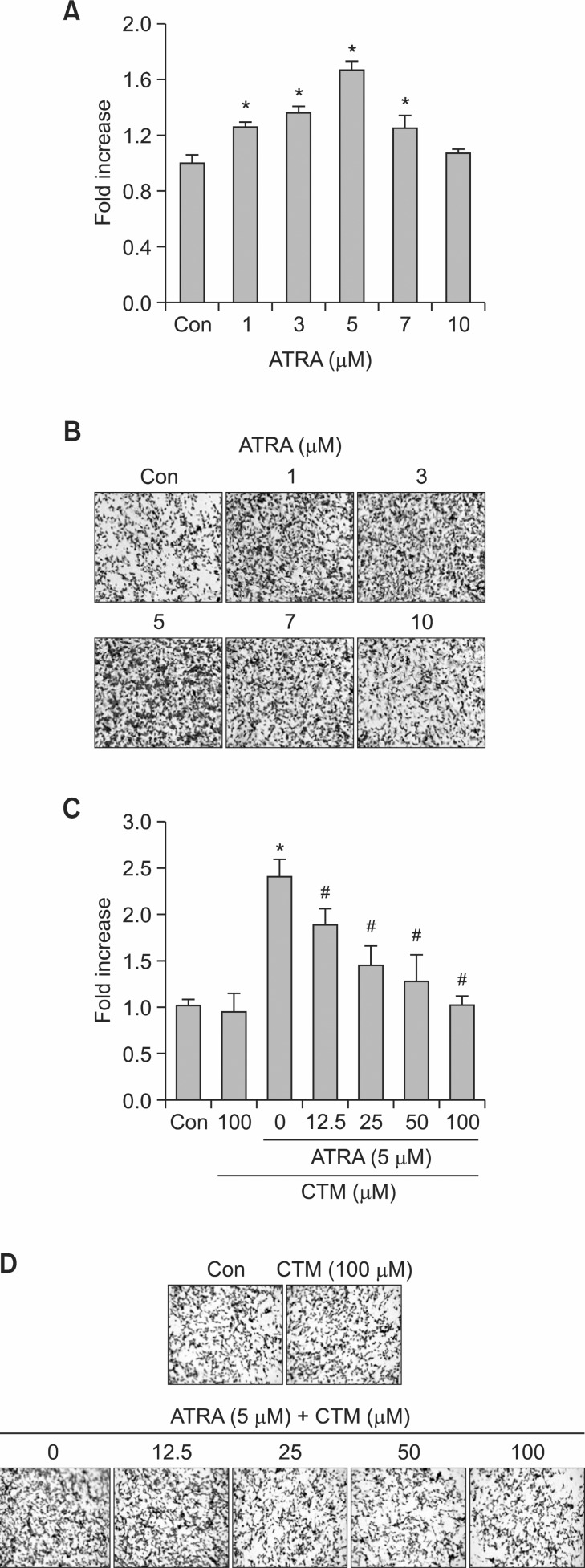
To examine the role of Tgase-2 in 1 day treatment effect of ATRA on invasion of SH-SY5Y cells, ATRA is added to SH-SY5Y cells and invasion was examined by in vitro invasion assay, using matrigel matrix-coated transwell inserts. The effect of ATRA on the capacity of the cells to invade tissue barriers was studied in an ATRA treatment for 1-2 days. ATRA dose-dependently increased the invasion capacity (Fig. 1A, 1B). Cystamine (CTM), well-known Tgase inhibitor, dose-dependently suppressed ATRA-induced invasion of SH-SY5Y(Fig. 1C, 1D).
Fig. 1. Invasion of ATRA-treated SH-SY5Y cells and effects of cystamine on invasion of ATRA-treated SH-SY5Y cells. (A) Effects of ATRA on invasion of SH-SY5Y cells. The SH-SY5Y cells were treated with various amount of ATRA (1-10 μM). Average number of invaded cell from (B). (B) Photographs of invaded SH-SY5Y cells. Cells invaded through the membrane after 48 h of incubation were stained and photographed. (C) Effects of cystamine on invasion of SH-SY5Y cells. The SH-SY5Y cells were treated with (5 μM) or without ATRA for 24 h or treated with ATRA and various amounts of cystamine for 1 h. Average number of invaded cells from (D). (D) Photographs of invaded SH-SY5Y cells. Cells invaded through the membrane after 48 h of incubation were stained and photographed. *p<0.05 versus control (Con) or #p<0.05 versus ATRA (5 μM).
ATRA induces MMP-9 and MMP-2 activation and cystamine inhibits MMP-9 and MMP-2 in SH-SY5Y cells
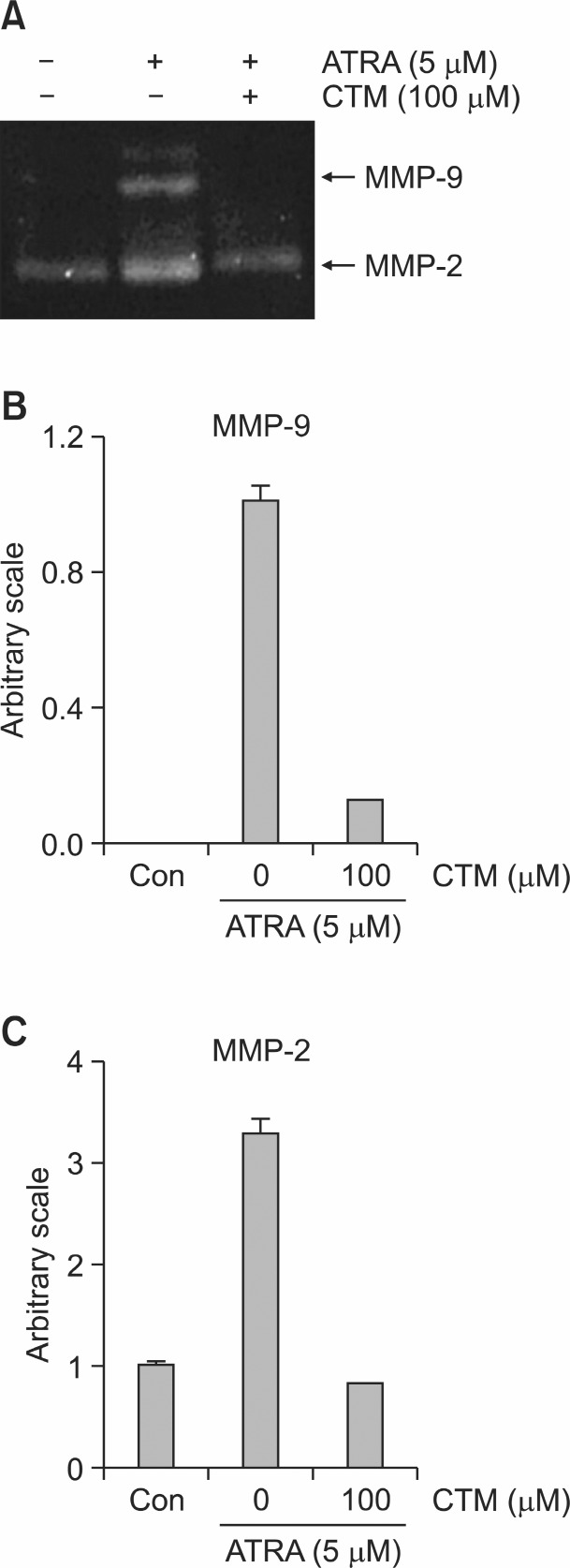
To elucidate how CTM inhibits the ATRA-induced invasion of the SH-SY5Y cells, we examined whether CTM affects the MMPs activity of SH-SY5Y cells. We tested the activities of MMPs in ATRA-induced invasion of SH-SY5Y cells by zymography. ATRA (5 μM) induced MMP-9 and MMP-2 in SH-SY5Y cells after 1 day of treatment (Fig. 2A). CTM reduced the activity
Fig. 2. Expression of MMP-2 and MMP-9 in ATRA-induced invasion in SH-SY5Y. (A) Representative gelatin zymograms demonstrating MMP-9 and MMP-2 activity of SH-SY5Y cells treated with 5 μM ATRA (w/wo CTM at 100 μM) in 24 h serum-free conditioned medium. (B) Densitometric analysis of MMP-9 from zymogram of (A). (C) Densitometric analysis of MMP-2 from zymogram of (A).
of MMP-9 and MMP-2 in the SH-SY5Y cells (Fig. 2A-C).
Gene silencing of Tgase-2 inhibits the expression of MMP-2 and MMP-9 in ATRA induced invasion of SH-SY5Y cell
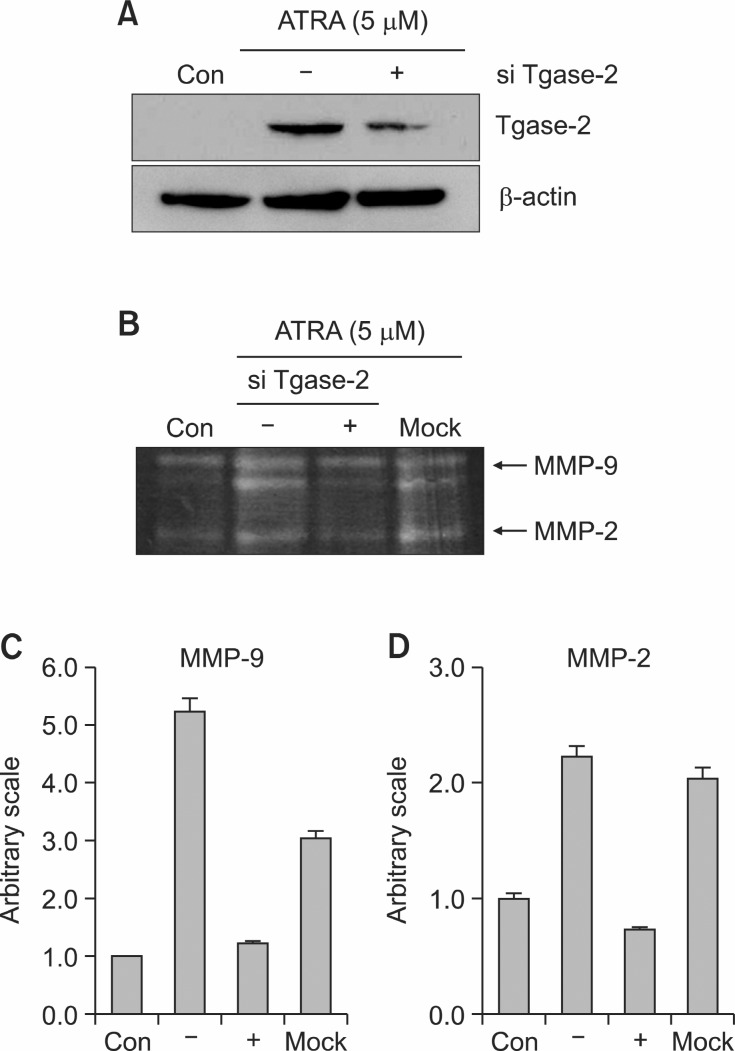
CTM is non-specific Tgase inhibitor. To clarify the involvement of Tgase-2 in the mechanism of CTM which suppressed the MMP-2 and MMP-9 activation of ATRA-induced invasion of SH-SY5Y cells, gene silencing of Tgase-2 was done. The siRNA of tgase-2 decreased the expression of Tgase-2 protein (Fig. 3A). A zymogram showed that ATRA increased the activities of MMP-2 and MMP-9 and the gene silencing of Tgase-2 reduced the MMP-9 and MMP-2 activity compared with mock or negative control (Fig. 3B-D).
Fig. 3. Effects of gene silencing of Tgase-2 on expression of MMP-2 and MMP-9 in ATRA-induced invasion of SH-SY5Y. (A) Western blot of Tgase-2 in ATRA and with or without Tgase-2 siRNA treated SH-SY5Y cells. (B) Representative gelatin zymograms demonstrating MMP-9 and MMP-2 activity of SH-SY5Y cells treated with 5 μM ATRA with or without Tgase-2 siRNA in 24-h serum-free conditioned medium. (C) Densitometric analysis of MMP-9 from zymogram of (B). (D) Densitometric analysis of MMP-2 from zymogram of (B).
ATRA induces activation of NF-κB, and CTM inhibits it
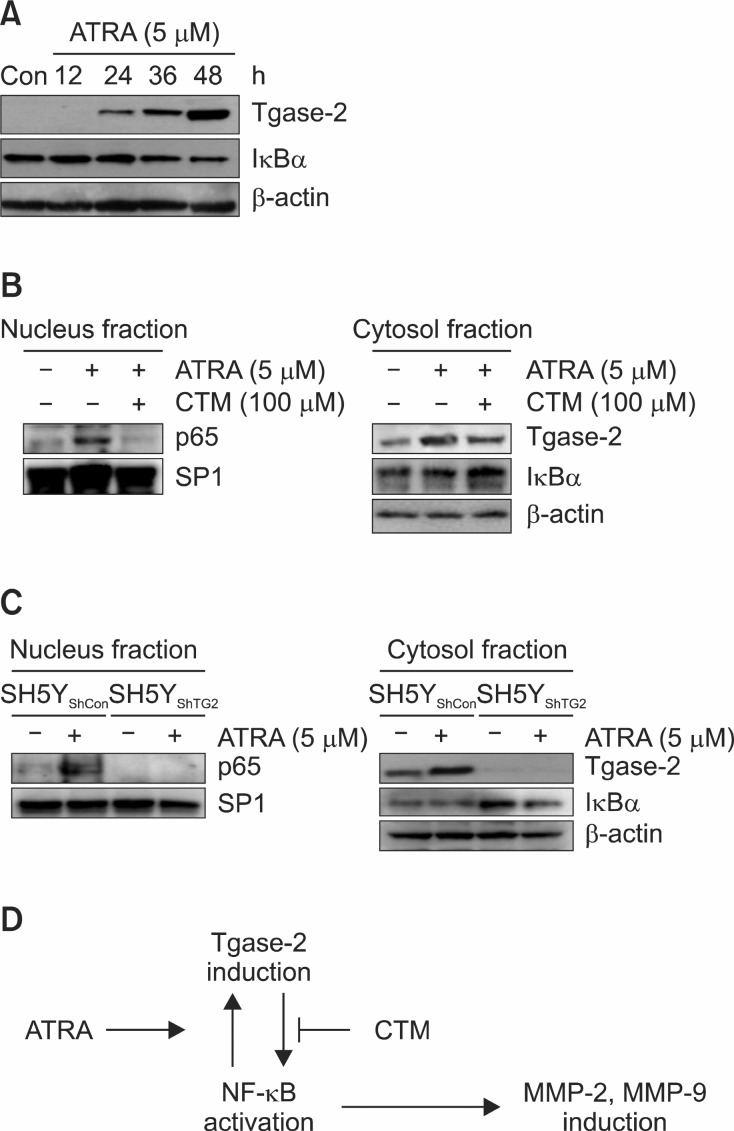
MMP-2 and MMP-9 are related with NF-κB activation. The involvement of Tgase-2 in NF-κB in SH-SY5Y cells with 1 day treatment of ATRA was examined using CTM and gene silencing. ATRA dose-dependently increased Tgase-2 and decreased the expression of IκBα in the cell and increased the
expression of Tgase-2 (Fig. 4A).
Fig. 4. Activation of ATRA-induced NF-κB and suppression of ATRA-induced NF-κB activation by CTM or gene silencing. (A) Western blot of Tgase-2 and IκBα in ATRA-induced invasion of SH-SY5Y cells. (B) Western blot of NF-κB p65 in nucleus fraction and IκBα in cytosol fraction of ATRA-treated SH-SY5Y cells (w/wo CTM at 100 μM). (C) Western blot of NF-κB p65 and Tgase-2 in nucleus fraction and IκBα and Tgase-2 in cytosol fraction in SH-SY5Y and SH-SY5YShTG2 with or without ATRA. (D) Proposed scheme of involvement of Tgase-2 in ATRA-induced invasion of SH-SY5Y cells.
Moreover, ATRA treatment increased the nucleus p65 and Tgase inhibitor CTM suppressed the p65 in nucleus (Fig. 4B). CTM treatment also decreased the Tgase-2 expression and increased the expression of IκBα. We established Tgase-2 deficient SH-SY5Y cells (SH5YShTG2) with lentivirus containing Tgase-2 (Fig. 4C). ATRA did not induce the translocation of p65 to nucleus in SH5YShTG2 cells (Fig. 4C). In contrast, ATRA did not degrade the IκBα in cytosol of SH5YShTG2 cells compared with SH-5YShcon cells (Fig 4C).
DISCUSSION
Joshi et al. clearly showed the involvement of Tgase-2 in short-term treatment of ATRA-induced SH-SY5Y migration (Joshi et al., 2006). In here, we showed that 1 day treatment of ATRA promotes invasion and cystamine inhibits ATRA-in-duced invasion of SHSY5Y (Fig. 1).
Tgase-2 is an enzyme which mediates the crosslinking of lysine and glutamine residues leading to form isopeptide bond (Lee and Kim, 2009). This enzyme is involved in survival and resistance of anticancer agents such as doxorubicin via activation of NF-κB (Kim et al., 2006; Lee and Kim, 2009). Tgase-2 is also involved in migration of cancer cells. Tgase-2 is a strong binding partner of fibronectin, and interactions between surface Tgase-2 and integrins and proteins in ECM such as fibronectin are known to play an important role in adhesion and migration (Akimov et al., 2000; Priglinger et al., 2004; Zemskov et al., 2006). Recently we showed that cytosolic Tgase-2 mediates the sphingosylphosphorylcholine-induced migration (Park et al., 2011).
However, the specifics of how cytosolic Tgase-2 is involved in invasion are unknown. Especially the fact that cystamine suppressed the ATRA-induced invasion of SH-SY5Y cells suggested that Tgase-2 plays an important role in neuroblastoma invasion.
Fig. 2's zymography confirms that ATRA-induced invasion of SH-SY5Y cells is implicated in activation of MMP-2 and MMP-9. These results are similar to those for ATRA-resistant SK-N-BE(9) (Farina et al., 2002).
Initially, the involvement of Tgase-2 in activation of MMPs induced by ATRA was confirmed by cystamine (Fig. 2). Metalloproteinases such as MMP-9 are well-known to be involved in invasion in many tumor cells (Woessner, 2002). Indeed, a positive correlation has been observed between the expression of metalloproteinases and the aggressiveness of NBs and other human malignancies (Mackay et al., 1992; Sato et al., 1995; Festuccia et al., 1996; Sugiura et al., 1998).
Cystamine inhibited the Tgase-2 via SH-group of cystein in active site and is not quite specific for Tgase-2. To confirm whether Tgase-2 is involved in MMP-2 and MMP-9 activity, gene silencing of Tgase-2 experiment was done. As a result, gene silencing of Tgase-2 suppressed the MMP-2 and MMP-9 in ATRA-induced invasion of SH-SY5Y cells (Fig. 3). These results suggested that Tgase-2 is involved in MMP-9 and MMP-2 activation leading to invasion of neuroblastoma.
To investigate how Tgase-2 is involved in the activation of MMP-2 and MMP-9 in ATRA-induced invasion of neuroblastoma, we tested effect of the Tgase inhibitor on the activation of NF-κB in neuroblastoma. In classical model, activation of the transcriptional response is mediated by translocation of the nuclear factor-κB such as p65, which is held by inhibitors of NF-κB proteins such as IκBα (Natoli and Chiocca, 2008). At first, we tested whether ATRA induced activation of NF-κB in SH-SY5Y cells. ATRA-induced NF-κB activation was proved by disappearance of IκBα band and increase of p65 in nuclear fraction by western blot (Fig. 4). Therefore decrease of IκBα and translocation of p65 result in NF-κB activation (Fig. 4). ATRA-induced NF-κB activation also was observed in ATRA-resistant SK-N-BE(9) cells and neuro-2 cells (Farina et al., 2002; Condello et al., 2008). Therefore, these similarities suggested that NF-κB is important in ATRA-resistance of neuroblastoma and Tgase-2 might play a role in ATRA resistance via NF-κB activation.
Cystamine (Tgase inhibitor) and gene silencing of Tgase-2 suppressed the activation of NF-κB of neuroblastoma by ATRA (Fig. 4). It is proved by p65 and IκBα western blot. Cystamine suppressed the translocation of p65 to nucleus. It is not available the exact explanation how ATRA induced activation of NF-κB activation. One possible explanation is that RAR or RXR might form complex with p65 or p50 (subunit of NF-κB)to activate NF-κB. Examples of interaction of RAR and NF-κB were reported (De Bosscher et al., 2006). Other explanation is that proteins induced by ATRA stimulate the activation of NF-κB. The latter one is more plausible since it is reported that Tgase-2 activates the NF-κB by IκB polymerization in microglia cells and SH-SY5Y/TG2 cells expressing full-length human Tgase-2 (Lee et al., 2004).
In Fig. 4B, IκBα level in the cytosolic fraction was not decreased by ATRA, while ATRA clearly decreased IκBα in the whole cell lysates (Fig. 4A). This discrepancy suggested that the decreased amount of IκBα in the whole lysates might be contributed by the IκBα in the nuclear fraction. Several reports demonstrated that IκBα enter the nucleus and dissociates NF-κB from DNA and shuttle it back to the cytoplasm (Arenzana-Seisdedos et al., 1995; Karin and Ben-Neriah, 2000). But our explanations require further studies.
The role and precise mechanism of activation of NF-κB in neuroblastoma is not clear, it is expected that Tgase-2 induced by ATRA might mediate the activation of NF-κB in neuroblastoma. These expectation suggested that Tgase-2 might be involved in the activation of NF-κB in ATRA-resistant neuroblastoma such as SK-N-BE(9) (Farina et al., 2002). Well-known role of Tgase-2 in neuroblastoma is related with out-growth of neurite in differentiated NB cells. Therefore it is very interesting involvement of Tgase-2 in activation of NF-κB. Recent reports emphasize the role of Tgase-2 in survival: Doxorubicin induces the persistent activation of intracellular transglutaminase-2 that protects from cell death (Cho et al., 2012); Tgase-2 is associated with a protective role in TNF-α-induced cell death as well as in 1-methyl-4-phenylpyridinum-induced cytotoxicity (Kweon et al., 2004; Beck et al., 2006); moreover, overexpression of Tgase-2 in SH-SY5Y cells induces inflammation–related genes (Lee et al., 2006). In fact it is now commonly accepted that the signaling pathways and the genetic programs that orchestrate embryonic development and tissue morphogenesis including differentiation are reactivated in cancer cells and can lead to the neoplastic dissemination of tumours (Schmidt et al., 1995; Boccaccio and Comoglio, 2006). Therefore Tgase-2 in neuroblastoma must be reconsidered to be an important target for activation of MMPs and NF-κB in NB which are important players in invasion of NB cells (Fig. 4D).
In conclusion, our results showed that Tgase-2 is involved in ATRA-induced activation of MMP-2 and MMP-9 and invasion of SH-SY5Y cells via activation of NF-κB. The results also suggested that the suppression of ATRA-induced Tgase-2 might suggest a new way of blocking the invasion and metastasis of neuroblastoma.
Acknowledgments
This work was partly supported by a Research Grant (NCC1110011-2) from the National Cancer Center in Korea and a Research Program for New Drug Target Discovery grant (2011-0030173) from the Ministry of Education, Science & Technology, Korea, the Ministry of Education, Science & Technology, Korea (2011-0015839) and GRRC program of Gyeonggi Province (GRRC Dongguk2011-B01).
References
- 1.Akimov S. S. Krylov D. Fleischman L. F. Belkin A. M. Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J. Cell Biol. (2000);148:825–838. doi: 10.1083/jcb.148.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ara T. DeClerck Y. A. Mechanisms of invasion and metastasis in human neuroblastoma. Cancer Metastasis Rev. (2006);25:645–657. doi: 10.1007/s10555-006-9028-9. [DOI] [PubMed] [Google Scholar]
- 3.Arenzana-Seisdedos F. Thompson J. Rodriguez M. S. Bachelerie F. Thomas D. Hay R. T. Inducible nuclear expression of newly synthesized I kappa B alpha negatively regulates DNA-binding and transcriptional activities of NF-kappa B. Mol. Cell Biol. (1995);15:2689–2696. doi: 10.1128/mcb.15.5.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck K. E. De Girolamo L. A. Griffin M. Billett E. E. The role of tissue transglutaminase in 1-methyl-4-phenylpyridinium(MPP+)-induced toxicity in differentiated human SH-SY5Y neuroblastoma cells. Neurosci. Lett. (2006);405:46–51. doi: 10.1016/j.neulet.2006.06.061. [DOI] [PubMed] [Google Scholar]
- 5.Boccaccio C. Comoglio P. M. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat. Rev. Cancer. (2006);6:637–645. doi: 10.1038/nrc1912. [DOI] [PubMed] [Google Scholar]
- 6.Brodeur G. M. Significance of intratumoral genetic heterogeneity in neuroblastomas. Med. Pediatr. Oncol. (2002);38:112–113. doi: 10.1002/mpo.1282. [DOI] [PubMed] [Google Scholar]
- 7.Cha D. S. Shin T. Y. Eun J. S. Kim D. K. Jeon H. Anti-metastatic properties of the leaves of Eriobotrya japonica. Arch. Pharm. Res. (2011);34:425–436. doi: 10.1007/s12272-011-0310-1. [DOI] [PubMed] [Google Scholar]
- 8.Cho S. Y. Jeong E. M. Lee J. H. Kim H. J. Lim J. Kim C. W. Shin D. M. Jeon J. H. Choi K. Kim I. G. Doxorubicin induces the persistent activation of intracellular transglutaminase 2 that protects from cell death. Mol. Cells. (2012);33:235–241. doi: 10.1007/s10059-012-2201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cianfarani S. Rossi P. Neuroblastoma and insulin-like growth factor system. New insights and clinical perspectives. Eur. J. Pediatr. (1997);156:256–261. doi: 10.1007/s004310050595. [DOI] [PubMed] [Google Scholar]
- 10.Condello S. Caccamo D. Currò M. Ferlazzo N. Parisi G. Ientile R. Transglutaminase 2 and NF-kappaB interplay during NGF-induced differentiation of neuroblastoma cells. Brain Res. (2008);1207:1–8. doi: 10.1016/j.brainres.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 11.De Bosscher K. Vanden Berghe W. Haegeman G. Crosstalk between nuclear receptors and nuclear factor kappaB. Oncogene. (2006);25:6868–6886. doi: 10.1038/sj.onc.1209935. [DOI] [PubMed] [Google Scholar]
- 12.DuBois S. G. Kalika Y. Lukens J. N. Brodeur G. M. Seeger R. C. Atkinson J. B. Haase G. M. Black C. T. Perez C. Shimada H. Gerbing R. Stram D. O. Matthay K. K. Metastatic sites in stage IV and IVS neuroblastoma correlate with age tumor biology and survival. J. Pediatr. Hematol. Oncol. (1999);21:181–189. doi: 10.1097/00043426-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Farina A. R. Masciulli M. P. Tacconelli A. Cappabianca L. De Santis G. Gulino A. Mackay A. R. All-trans-retinoic acid induces nuclear factor kappaB activation and matrix metalloproteinase-9 expression and enhances basement membrane invasivity of differentiation-resistant human SK-N-BE 9N neuroblastoma Cells. Cell Growth Differ. (2002);13:343–354. [PubMed] [Google Scholar]
- 14.Festuccia C. Bologna M. Vicentini C. Tacconelli A. Miano R. Violini S. Mackay A. R. Increased matrix metalloproteinase-9 secretion in short-term tissue cultures of prostatic tumor cells. Int. J. Cancer. (1996);69:386–393. doi: 10.1002/(SICI)1097-0215(19961021)69:5<386::AID-IJC6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Freemantle S. J. Spinella M. J. Dmitrovsky E. Retinoids in cancer therapy and chemoprevention: promise meets resistance. Oncogene. (2003);22:7305–7315. doi: 10.1038/sj.onc.1206936. [DOI] [PubMed] [Google Scholar]
- 16.Garattini E. Gianni M. Terao M. Retinoids as differentiating agents in oncology: a network of interactions with intracellular pathways as the basis for rational therapeutic combinations. Curr. Pharm. Des. (2007);13:1375–1400. doi: 10.2174/138161207780618786. [DOI] [PubMed] [Google Scholar]
- 17.Guidez F. Li A. C. Horvai A. Welch J. S. Glass C. K. Differential utilization of Ras signaling pathways by macrophage colony-stimulating factor (CSF) and granulocyte-macrophage CSF receptors during macrophage differentiation. Mol. Cell Biol. (1998);18:3851–3861. doi: 10.1128/mcb.18.7.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurney J. G. Ross J. A. Wall D. A. Bleyer W. A. Severson R. K. Robison L. L. Infant cancer in the U.S.: histology-specific incidence and trends 1973 to 1992. J. Pediatr. Hematol. Oncol. (1997);19:428–432. doi: 10.1097/00043426-199709000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Joshi S. Guleria R. Pan J. DiPette D. Singh U. S. Retinoic acid receptors and tissue-transglutaminase mediate short-term effect of retinoic acid on migration and invasion of neuroblastoma SH-SY5Y cells. Oncogene. (2006);25:240–247. doi: 10.1038/sj.onc.1209027. [DOI] [PubMed] [Google Scholar]
- 20.Karin M. Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu. Rev. Immunol. (2000);18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 21.Kim D. S. Park S. S. Nam B. H. Kim I. H. Kim S. Y. Reversal of drug resistance in breast cancer cells by transglutaminase 2 inhibition and nuclear factor-kappaB inactivation. Cancer Res. (2006);66:10936–10943. doi: 10.1158/0008-5472.CAN-06-1521. [DOI] [PubMed] [Google Scholar]
- 22.Kweon S. M. Lee Z. W. Yi S. J. Kim Y. M. Han J. A. Paik S. G. Ha S. S. Protective role of tissue transglutaminase in the cell death induced by TNF-alpha in SH-SY5Y neuroblastoma cells. J. Biochem. Mol. Biol. (2004);37:185–191. doi: 10.5483/bmbrep.2004.37.2.185. [DOI] [PubMed] [Google Scholar]
- 23.Lee A. C. Neuroblastoma: the challenge remains. Singapore Med. J. (2012);53:1–2. [PubMed] [Google Scholar]
- 24.Lee C. H. Kim S. Y. NF-kappaB and therapeutic approach. Biomol. Ther. (2009);17:219–240. [Google Scholar]
- 25.Lee J. S. Kim I. H. Kim S. Y. Changes in gene expression with increased transglutaminase 2 in a SH-SY5Y cell line. Front Biosci. (2006);11:2774–2781. doi: 10.2741/2007. [DOI] [PubMed] [Google Scholar]
- 26.Lee J. Kim Y. S. Choi D. H. Bang M. S. Han T. R. Joh T. H. Kim S. Y. Transglutaminase 2 induces nuclear factor-kappaB activation via a novel pathway in BV2 microglia. J. Biol. Chem. . (2004);279:53725–53735. doi: 10.1074/jbc.M407627200. [DOI] [PubMed] [Google Scholar]
- 27.Mackay A. R. Ballin M. Pelina M. D. Farina A. R. Nason A. M. Hartzler J. L. Thorgeirsson U. P. Effect of phorbol ester and cytokines on matrix metalloproteinase and tissue inhibitor of metalloproteinase expression in tumor and normal cell lines. Invasion Metastasis. (1992);12:168–184. [PubMed] [Google Scholar]
- 28.Mehta K. Fok J. Miller F. R. Koul D. Sahin A. A. Prognostic significance of tissue transglutaminase in drug resistant and metastatic breast cancer. Clin. Cancer Res. (2004);10:8068–8076. doi: 10.1158/1078-0432.CCR-04-1107. [DOI] [PubMed] [Google Scholar]
- 29.Modak S. Cheung N. K. Neuroblastoma: Therapeutic strategies for a clinical enigma. Cancer Treat. Rev. (2010);36:307–317. doi: 10.1016/j.ctrv.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Mueller S. Matthay K. K. Neuroblastoma: biology and staging. Curr. Oncol. Rep. (2009);11:431–438. doi: 10.1007/s11912-009-0059-6. [DOI] [PubMed] [Google Scholar]
- 31.Murtaugh M. P. Dennison O. Stein J. P. Davies P. J. Retinoic acid-induced gene expression in normal and leukemic myeloid cells. J. Exp. Med. (1986);163:1325–1330. doi: 10.1084/jem.163.5.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Natoli G. Chiocca S. Nuclear ubiquitin ligases NF-kappaB degradation and the control of inflammation. Sci. Signal. (2008);1:pe1. doi: 10.1126/stke.11pe1. [DOI] [PubMed] [Google Scholar]
- 33.Park M. K. Lee H. J. Shin J. Noh M. Kim S. Y. Lee C. H. Novel participation of transglutaminase-2 through c-Jun N-terminal kinase activation in sphingosylphosphorylcholine-induced keratin reorganization of PANC-1 cells. Biochim. Biophys. Acta. (2011);1811:1021–1029. doi: 10.1016/j.bbalip.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Priglinger S. G. Alge C. S. Neubauer A. S. Kristin N. Hirneiss C. Eibl K. Kampik A. Welge-Lussen U. TGF-beta2-induced cell surface tissue transglutaminase increases adhesion and migration of RPE cells on fibronectin through the gelatin-binding domain. Invest. Ophthalmol. Vis. Sci. (2004);45:955–963. doi: 10.1167/iovs.03-0210. [DOI] [PubMed] [Google Scholar]
- 35.Redfern C. P. Lovat P. E. Malcolm A. J. Pearson A. D. Gene expression and neuroblastoma cell differentiation in response to retinoic acid: differential effects of 9-cis and all-trans retinoic acid. Eur. J. Cancer. (1995);31A:486–494. doi: 10.1016/0959-8049(95)00066-r. [DOI] [PubMed] [Google Scholar]
- 36.Roy Choudhury S. Karmakar S. Banik N. L. Ray S. K. Targeting angiogenesis for controlling neuroblastoma. J. Oncol. (2012);2012:782020. doi: 10.1155/2012/782020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato S. Seki N. Hotta Y. Tabata S. Expression profiles of a human gene identified as a structural homologue of meiosis-specific recA-like genes. DNA Res. (1995);2:183–186. doi: 10.1093/dnares/2.4.183. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt C. Bladt F. Goedecke S. Brinkmann V. Zschiesche W. Sharpe M. Gherardi E. Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. (1995);373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 39.Sugiura Y. Shimada H. Seeger R. C. Laug W. E. DeClerck Y. A. Matrix metalloproteinases-2 and -9 are expressed in human neuroblastoma: contribution of stromal cells to their production and correlation with metastasis. Cancer Res. (1998);58:2209–2216. [PubMed] [Google Scholar]
- 40.Woessner J. F. Jr. MMPs and TIMPs-an historical perspective. Mol. Biotechnol. (2002);22:33–49. doi: 10.1385/MB:22:1:033. [DOI] [PubMed] [Google Scholar]
- 41.Zemskov E. A. Janiak A. Hang J. Waghray A. Belkin A. M. The role of tissue transglutaminase in cell-matrix interactions. Front Biosci. (2006);11:1057–1076. doi: 10.2741/1863. [DOI] [PubMed] [Google Scholar]