Abstract
The purpose of this study was to investigate the modification of expression and functionality of the drug transporter P-glycoprotein (P-gp) by tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ) at the blood-brain barrier (BBB). We used immortalized human brain microvessel endothelial cells (iHBMEC) and primary human brain microvessel endothelial cells (pHBMEC) as in vitro BBB model. To investigate the change of p-gp expression, we carried out real time PCR analysis and Western blotting. To test the change of p-gp activity, we performed rhodamin123 (Rh123) accumulation study in the cells. In results of real time PCR analysis, the P-gp mRNA expression was increased by TNF-α or IFN-γ treatment for 24 hr in both cell types. However, 48 hr treatment of TNF-α or IFN-γ did not affect P-gp mRNA expression. In addition, co-treatment of TNF-α and IFN-γ markedly increased the P-gp mRNA expression in both cells. TNF-α or IFN-γ did not influence P-gp protein expression whatever the concentration of cytokines or duration of treatment in both cells. However, P-gp expression was increased after treatments of both cytokines together in iHBMEC cells only compared with untreated control. Furthermore, in both cell lines, TNF-α or IFN-γ induced significant decrease of P-gp activity for 24 hr treatment. And, both cytokines combination treatment also decreased significantly P-gp activity. These results suggest that P-gp expression and function at the BBB is modulated by TNF-α or/and IFN-γ. Therefore, the distribution of P-gp depending drugs in the central nervous system can be modulated by neurological inflammatory diseases.
Keywords: TNF-α, IFN-γ, P-glycoprotein; Human brain microvascular endothelial cell, Blood-brain barrier, Efflux transport
INTRODUCTION
The blood-brain barrier (BBB), which is comprised by tight junctions of the brain micorvascular endothelial cells, is highly restrictive of the passage of endogenous and exogenous compounds between the blood and the brain parenchyma cells (Cornford, 1985; Pardridge, 1988). This highly effective barrier contributes to central nervous system (CNS) homeostasis and protects against xenobiotics but also greatly limits entry of drugs used in the treatment of many CNS disorders (Konsman et al., 2004). Although the BBB exerts this physical barrier by limiting paracellular uptake of compounds, it has several transporters like glucose transporter (GLUT1), and these transporters carry out the brain distribution of specific endogenous compounds as well as efficacy of many pharmacological agents (Spector, 1989). P-glycoprotein (P-gp), the major efflux transporter contributes to protection against neurotoxins by pumping its substances from the brain into the blood at the BBB (Schinkel et al., 1994). Also, P-gp plays a role in the drug transport through the BBB (Tamai and Tsuji, 2000). Changes in functional activity of P-gp at the BBB may result in alteration of drug disposition in the brain and, therefore, might induce changes of its clinical efficiency. Several reports demonstrated that CNS inflammatory diseases, like multiple sclerosis (MS), bacterial meningitis or stroke modify the integrity of the BBB by transmigration of numerous activated neutrophils, lymphocytes or monocytes across the BBB (Perry et al., 1997). Several proinflammatory cytokines including tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ) and interleukin-1β (IL-1β) were shown to control molecules relevant for leukocyte-endothelial interaction in rodent and human microvascular endothelial cell in vitro (McCarron et al., 1993; Kallmann et al., 2000). In experimental allergic encephalomyelitis (EAE), inflammatory cytokines such as TNF-α and IL-1β are elevated and these changes match alterations in BBB permeability (Gijbels et al., 1990). Also, the alteration of P-gp by proinflammatory cytokines has been demonstrated at the BBB in various in vitro and in vivo studies (Théron et al., 2003; Hartz et al., 2004; Hartz et al., 2006; Bauer et al., 2007; Yu et al., 2007). Nevertheless, the results are controversial on the regulation of P-gp by cytokines according to species and cell type. Some reports demonstrated P-gp mRNA and/or protein up-regulation, whereas in other studies a decrease in P-gp function was observed. Also, most data are about the regulation of P-gp by TNF-α in animal models so information regarding effects of other proinflammatory cytokines on P-gp is more limited in humans. Therefore, the aim of the present study was the evaluation of specific effects on P-gp expression and functionality by representative proinflammatory cytokines, IFN-γ as well as TNF-α in human brain microvascular endothelial cells. Especially, we studied the regulation of P-gp by IFN-γ and TNF-α using both immortalized and primary brain microvascular endothelial cell lines as in vitro BBB model.
MATERIALS AND METHODS
Cell culture
Immortalized human brain microvascular endothelial cell line (iHBMEC), kindly provided by Kwang S. Kim (Division of Pediatric Infectious Diseases, Johns Hopkins University School of Medicine), were seeded on 2% gelatin (Sigma-Aldrich, St. Louis, MO, USA) coated cell culture dishes (Nunc, Roskilde, Denmark) at 37℃ under 5% CO2 and 95% air in RPMI1640 (Biochrome, Berlin, Germany) containing 10% FCS (Biochrome, Berlin, Germany), endothelial cell growth supplement (3 mg/ml; Sigma-Aldrich), heparin (500 U/ml; Biochrome, Berlin, Germany), L-glutamine (200 mM; Biochrome, Berlin, Germany), sodium pyruvate (100 mM; Biochrome) and multi-vitamins (Biochrome, Berlin, Germany). These cells are positive for factor VIII-Rag, carbonic anhydrase IV, occludin, zonula occludens-1 (ZO-1) and claudin-3, and express γ-glutamyltranspeptidase as well as alkaline phosphatase, demonstrating their human brain endothelial cell characteristics (Stins et al., 1997). iHBMEC were passaged every 4 days by treating adherent cells with 0.1% Trypsin/EDTA (Sigma-Aldrich, St. Louis, MO, USA) and splitting 1:4. Only subconfluent cells in passage 8 to 15 were used for the experiments after cell purity had been confirmed by microscopy inspection.
Primary human brain microvascular endothelial cell line (pHBMEC) was purchased from Cell Systems Corporation (Kirkland, WA, USA). pH-BMEC cells were plated on 2% gelatin-coated cell culture plates at 37℃ under 5% CO2 and 95% air in CSC complete medium (Cell Systems Corporation). pHBMEC were passaged every 6-7 days by treating adherent cells with 0.1% Trysin/EDTA and splitting 1:3. Only subconfluent cells in passage 3 to 6 were used for the experiments after cell purity had been confirmed by microscopy inspection.
Cytokine treatment
iHBMEC and pHBMEC cells were grown to confl uence on 2% gelatin-coated six-well culture dishes (Nunc, Roskilde, Denmark) and then treated for 24 and 48 hr with TNF-α (Strathmann Biotech AG) or IFN-γ (R&D systems, Wiesbaden, Germany) at concentrations of 1 and 10 ng/ml. Also, TNF-α and IFN-γ together were added to the cell medium at each concentration of 10 ng/ml for 24 and 48 hr. For functional study, iHBMEC and pHBMEC cells were grown to confluence on 2% gelatin-coated 24 well culture dishes and then treated for 12, 24 and 48 hr with TNF-α or IFN-γ at concentrations of 1 and 10 ng/ml. TNF-α and IFN-γ together were added to the cell medium at each concentration of 10 ng/ml for 12, 24 and 48 hr.
Real-time reverse transcription polymerase chain reaction
Total RNA was isolated from cultured iHMBEC and pHB-MEC by the RNeasy kit from Quiagen (Quiagen, Hilden, Germany) and according to the manufacturer’s instructions. Reverse transcription (RT) was performed using the GeneAmp RNA PCR kit (Applied Biosystems, Weiterstadt, Germany). Quantification of P-gp mRNA was performed as previously described on an ABI PRISM 7700 Sequence Detector System (Perkin Elmer, Foster City, CA, USA) (Uçeyler et al., 2006). 5 μl of complementary DNA (cDNA) were used for real-time PCR. Gene-specific oligonucleotide primers and probes for human P-gp as well as the endogenous control 18s ribosomal RNA (rRNA) were obtained from Applied Biosystems. The reaction contained 25 μl TaqMan Master Mix (Applied Biosystems, Weiterstadt, Germany) and 2.5 μl of the specific primer in a final volume of 50 μl. Reaction condition were 30 min at 48℃, 10 min at 95℃, 40 cycles for 15 sec at 95℃ and 1 min at 60℃, and a final 5 min incubation at 25℃.
Western blotting
The culture medium was removed and cytokine treated iHBMEC and pHBMEC cells were washed twice with PBS. The cells were scraped into radio immunoprecipitation assay (RIPA) lysis buffer (50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1% NP-40; 0.25% sodium deoxycholate; 1x Boehringer COMPLETE® protease inhibitor mix [Boehringer Mannheim]). Samples were shaken vigorously for 30 min at 4℃, and centrifuged at 14,000 rpm for 20 min at 4℃. After being boiled for 3 min in SDS sample buffer with dithiothreitol, 25 (pHBMEC) or 50μg (iHBMEC) of protein per lane was subjected 8% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and then proteins were electro-transferred onto a 0.45 ㎛ nitrocellulose membrane (for iHBMEC; Schleicher & Schuell, Dassel, Germany) or polyvinylidene difluoride membrane (for pHB-MEC; Biorad Laboratories). Blots were blocked in PBS containing 0.05% Tween 20 (PBST) and 5% skim dried milk for 2 hr at room temperature. After three washes with PBST buffer, blots were incubated overnight at 4℃, with 1:100 diluted C219 monoclonal antibody (Calbiochem, Darmstadt, Germany) in PBST containing 5% skim dried milk. After three washes with PBST buffer, blots were incubated with 1:5,000 diluted immunoglobulins-peroxidase conjugated goat anti mouse secondary antibody (Jacson ImmunoResearch Laboratories) in PBST containing 5% skim dried milk for 1 hr at room temperature. P-gp was detected with an enhanced chemiluminescence system (Amersham Biosciences, Buckinghamshire, UK) and exposed on Fuji medical X-ray film.
Cellular uptake studies
The confluent cytokine-treated iHBMEC and pHBMEC cells were washed three times with 1 ml extracellular fluid (ECF) buffer consisting of 122 mM NaCl, 25 mM NaHCO3, 3 mM KCl, 1.4 mM CaCl2, 1.2 mM MgSO4, 0.4 mM K2HPO4, 10 mM D-glucose and 10 mM Hepes (pH 7.4) at 37℃, and preincubated for 30 min at 37℃. Uptake was initiated by applying 200 μl ECF buffer containing 10 μM Rhodamin123 (Rh123; Sigma-Aldrich, St. Louis, MO, USA) at 37℃ in the presence or absence of inhibitors. After 1 hr, removing the applied solution terminated uptake and cells were immersed in ice-cold PBS. The cells were then solubilized in 0.2 N NaOH. Intracellular fluorescence intensity was measured using a Spectro-fluorimeter (Labsystems, Hants, UK), at excitation wavelength 485 nm and emission wavelength 538 nm. Intracellular Rh123
fluorescence intensity was corrected on the basis of protein content as assessed by the BCA protein assay kit (Sigma-Aldrich, St. Louis, MO, USA).
Statistical analysis
Experiments were performed at least in triplicate, and the results are expressed as means ± SEM. Statistical significance of differences among means of several groups was determined by one-way analysis of variance with Dunnett's post-hoc test and p<0.05 was considered statistically significant.
RESULTS
Effect of cytokines in the expression of P-gp mRNA in iHBMEC and pHBMEC cells
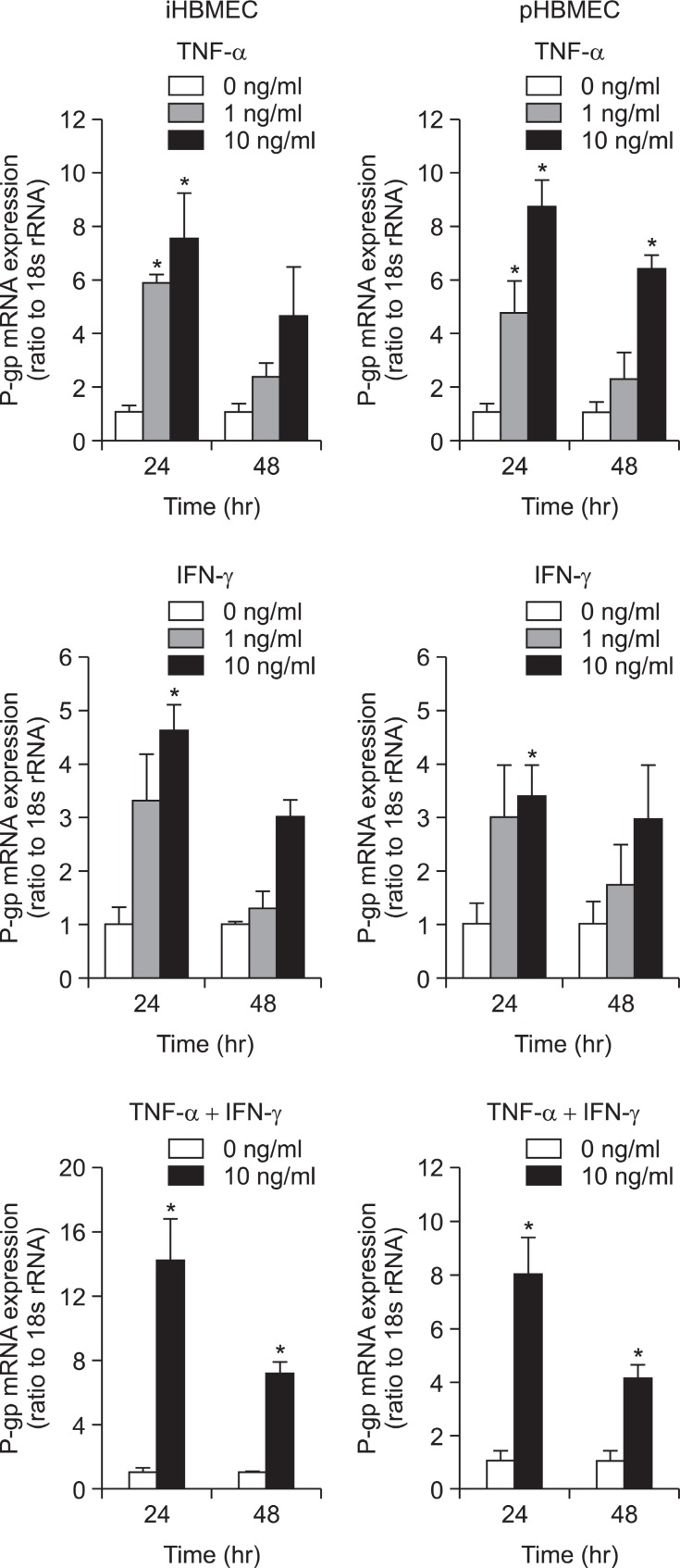
To investigate whether the expression levels of P-gp mRNA were affected by proinfl ammatory cytokines, TNF-α and IFN-γ, in iHBMEC and pHBMEC cells, a quantitative real-time PCR analysis was performed. When we pre-treated TNF-α for 24 hr on the cells, a significant increase of P-gp mRNA expression was induced (Fig. 1). It was only at 48 hr treatment of high concentration of TNF-α that p-gp mRNA expression was increased in primary cell lines (Fig. 1). P-gp mRNA expression was significantly up-regulated by IFN-γ in both cell lines after 24 hr treatment of high concentration of IFN-γ, but there were no significant changes of P-gp mRNA expression after 48 hr treatment of IFN-γ on both cell lines (Fig. 1). The incubation of TNF-α (10 ng/ml) and IFN-γ (10 ng/ml) for 48 hr on both cell lines caused a significant increase in P-gp mRNA expression (Fig. 1). However, the synergy effect by both cytokines was not big.
Fig. 1. Effect of TNF-α or/and IFN-γ on P-gp mRNA expression in HBMEC cells. Real time reverse transcription polymerase chain reaction (RT-PCR) analysis of P-gp mRNA expression in iHBMEC and pHBMEC without and with pre-treatment with two different concentration of TNF-α, IFN-γ or both cytokines together (10 ng/ml) for 24 hr or 48 hr. *p<0.05; significantly different from 0 ng/ml treatment.
Effect of cytokines on P-gp protein expression in iHBMEC and pHBMEC cells
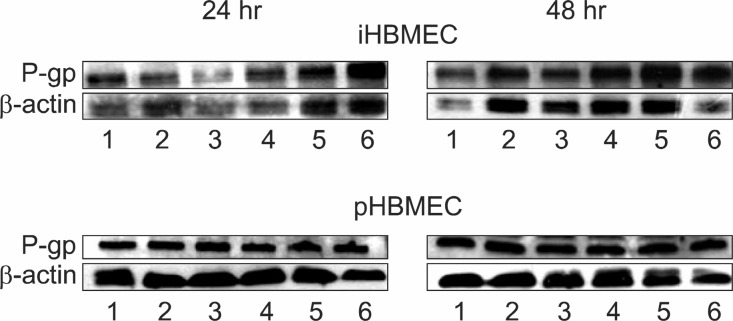
To evaluate changes of P-gp protein expression by pro-inflammatory cytokines, we carried out Western blot experiments using an anti P-gp monoclonal antibody C219. As shown in Fig. 2, TNF-α or IFN-γ had no effects on the level of P-gp expression whatever the concentration of cytokine or the duration of treatment in iHBMEC cells. Also, we did not
Fig. 2. Effect of TNF-α or/and IFN-γ on P-gp protein expression in HBMEC cells. Western blot analysis of P-gp expression was performed in iHBMEC and pHBMEC. The cells were treated 1 and 10 ng/ml TNF-α, IFN-γ or both cytokines together (10 ng/ml) for 24 hr or 48 hr. Cell lysate was separated on 8% polyacrylamide gell and transferred onto a polyvinylidene difluoride (PVDF) membrane.The immunodetection of the P-gp protein was performed using the specific monoclonal antibody C219. The blot was developed by using Western Blot Luminescence Reagent. Lane 1: control, Lane 2: TNF-α 1 ng/ml, Lane 3: TNF-α 10 ng/ml, Lane 4: IFN-γ 1 ng/m, lLane 5: IFN-γ 10 ng/ml Lane 6: TNF-α 10 ng/ml + IFN-γ 10 ng/ml.
observe an increased P-gp protein expression after TNF-αand/or IFN-γ treatment in pHBMEC cells (Fig. 2). However, treatment of both cytokines together led to an increase of P-gp protein expression in iHBMEC cells (Fig. 2).
Effect of cytokines on Rh123 accumulation in iHBMEC and pHBMEC cells
To test whether cytokines induced changes of P-gp functional activity, Rh123 accumulation study was performed in iHBMEC and pHBMEC cells. Rh123 is a well-known fluorescent substrate for P-gp, and its enhanced uptake by cells reflects decreased P-gp activity of efflux transport. Also, Rh123 was accumulated significantly by treatment of verapamil, well known substrate of P-gp, in both cell lines (data not shown).
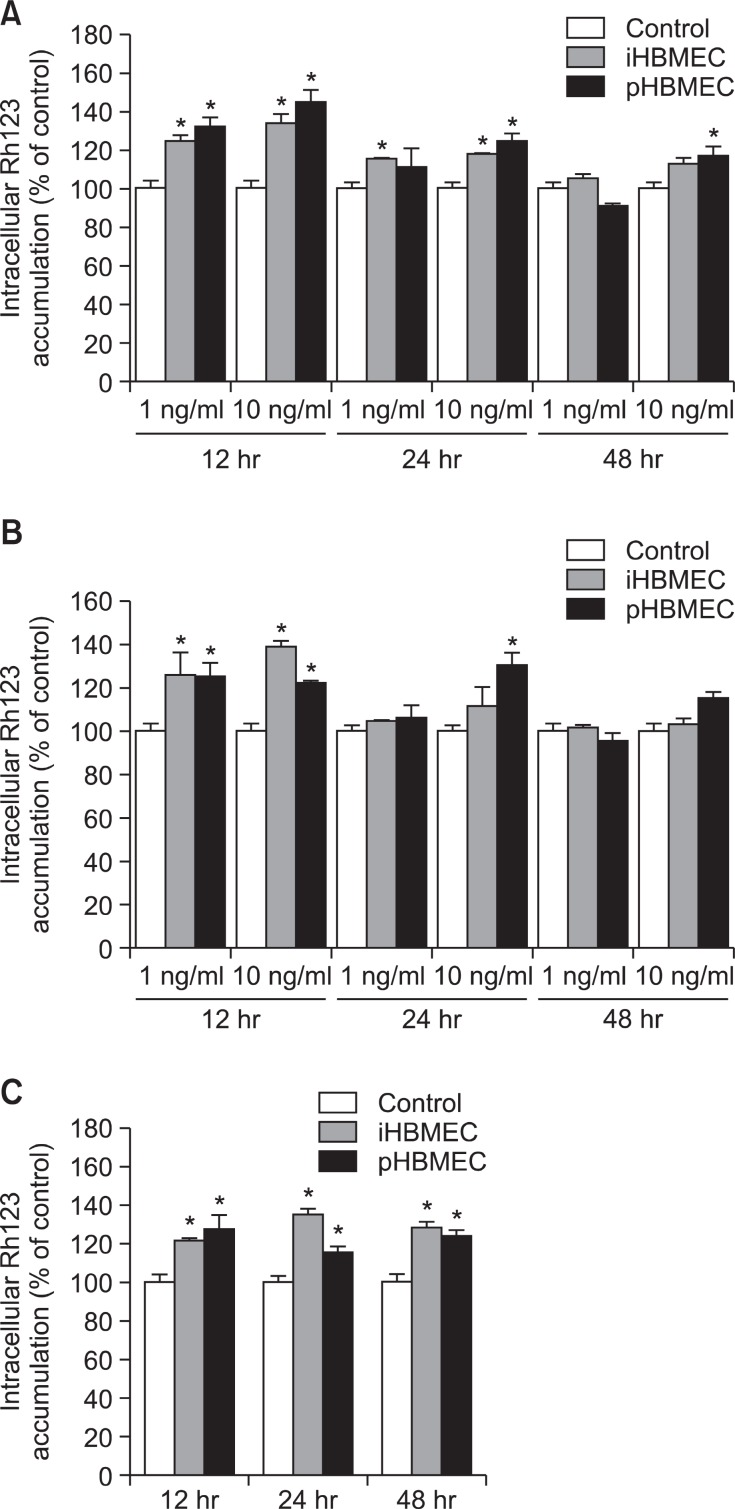
TNF-α presented significant increase of accumulation of Rh123 at 12 and 24 hr pre-treatment in iHBMEC cells, suggesting that the efflux was decreased by TNF-α and/or IFN-γ (Fig. 3A). TNF-α did not alter Rh123 efflux at 48 hr pre-treatment in iHBMEC cells. In pHBMEC cells, P-gp activity decreased continuously for 48 hr by TNF-α (Fig. 3A).
Fig. 3. Effect of TNF-α or/and IFN-γ on P-gp efflux activity in HBMEC cells. The cells treated with 1 and 10 ng/ml TNF-α, (A) IFN-γ (B) or both cytokines together (10 ng/ml; C) for 12, 24 or 48 hr. Intracellular uptake of Rh123 after 1 hr was determined as nanomoles per milligram protein and normalized to vehicle-treated controls. Verapamil was used as a positive control in untreated cells. Results are represented as means ± SEM (n=3). *p<0.05; significantly different from control.
IFN-γ also decreased P-gp activity at 12 hr pre-treatment in both cell lines (Fig. 3B). However, we could not see the change of Rh123 accumulation at 24 hr and 48 hr IFN-γ pre-treatment in both cell lines except 24 hr pre-treatment of high concentration of IFN-γ (Fig. 3B).
When the cells were pre-treated with both cytokines together, P-gp activity was decreased for 48 hr in both cell lines (Fig. 3C). However, the synergy effect by both cytokines was not big.
DISCUSSION
The purpose of this study is to investigate specific effects on P-gp expression and functionality of representative pro-inflammatory cytokines, IFN-γ as well as TNF-α in human brain microvascular endothelial cells. It has been reported that inflammation has profound effects on the BBB by release of the inflammatory mediators such as TNF-α, IL-1β, and IFN-γ (Huber et al., 2001). Also, the alteration of transporter at the BBB such as P-gp by pro-inflammatory cytokines has been demonstrated in diverse in vitro and in vivo studies. Especially, several studies showed that TNF-α altered P-gp expression and transport activity in the brain and in brain capillary endothelial cells (Théron et al., 2003; Hartz et al., 2004; Hartz et al., 2006; Bauer et al., 2007; Yu et al., 2007). Théron et al. reported that mdr1a and mdr1b mRNAs was increased by TNF-α in immortalized rat brain capillary endothelial GPNT cells. Whereas, P-gp protein levels did not change and the activity of P-gp was decreased in a time dependent manner for 96 hr after TNF-α treatment in GPNT cells (Théron et al., 2003). In contrast, TNF-α induced an increase of P-gp expression and activity in another rat cerebral microvascular endothelial cell line, RBE4 (Yu et al., 2007). In another study, proinflammatory stimuli (lipopolysaccharide, LPS; TNF-α; endothelin-1, ET-1) up to 6 hr decreased P-gp transport activity with no change in protein expression followed by delayed increases in expression and activity of P-gp in isolated rat brain capillary (Hartz et al., 2004; Hartz et al., 2006; Bauer et al., 2007). According to these studies, the effects of TNF-α on action of P-gp are discussed controversially and it is not clear from these studies whether the change of P-gp activity by TNF-α is increase or decrease. Also, these studies were performed in animal models, so it is still not clear to effects of TNF-α on P-gp in humans. In our study, it showed that TNF-α pre-treatment (24 hr) induced increase of P-gp mRNA in both cell lines like the results in animal model (Fig. 1).
However, P-gp protein expression was not changed by TNF-α exposure (10 ng/ml) (Fig. 2). In addition, we observed decrease of P-gp activity by TNF-α treatment, with more Rh123 taken up by the cells (Fig. 3A). These controversial findings may be due to inter-species and P-gp substrate differences used in experiment as well as dose- and time-dependent effects of TNF-α on P-gp expression and activity. Recently, in human cell line as another in vitro BBB model, hCMEC/D3 cells, TNF-α treatment for 72 hr induced P-gp protein expression but did not significantly alter P-gp activity (Poller et al., 2010). However, in that report different concentration of TNF-α compared to our study was used and incubation time was longer than our study. The signaling pathways responsible for increasing effect of P-gp mRNA levels have been mapped in isolated rat brain capillaries (Hartz et al., 2004; Hartz et al., 2006; Bauer et al., 2007). These reports showed that TNF-α induced the release of ET-1 from endothelium and the nitric oxide synthase (NOS), protein kinese C (PKC) and nuclear factor-κB (NFκB) were involved in the down-stream regulation pathway. The results of the increase of P-gp mRNA by TNF-α in our cell may be caused by this regulation pathway, however, further studies about that are needed. While the increase of P-gp mRNA by TNF-α, P-gp activity was decreased (Fig. 3A). This is consistent with the in vitro finding of previous studies (Théron et al., 2003; Bauer et al., 2007). These results suggest the implication of another regulation mechanism rather than a transcriptional one as the change of P-gp activity. This decrease of activity with a similar level of protein may explain either by an interference of TNF-α with P-gp cellular distribution through trafficking of the transporter away from the cell surface, or by the change of transporter activity through modification of the transporter or its immediate microenvironment in the plasma membrane. Indeed, there is direct evidence for rapid trafficking of P-gp between intracellular sites and the canalicular membrane in hepatocytes (Kipp et al., 2001). However, these finding was also found out in animal model, so, it is still not clear to what extent these pathways operate in human.
The effects of IFN-γ were also examined in iHBMEC and pHBMEC cells. IFN-γ (10 ng/ml) exposure for 24 hr also induced up-regulation of P-gp mRNA but had no effect P-gp protein expression (Fig. 1 and Fig. 2). IFN-γ also decreased P-gp activity in both cell lines (Fig. 3B). Previously, the effects of IFN-γ on the expression and functionality of P-gp have been examined in various cultured cells. In human colon carcinoma cell lines (LoVo, HT115, SW480, and LS174T), IFN-γ caused a reduction in P-gp gene expression after 48 or 72 hr treatment (Walther and Stein, 1994). In contrast, in human macrophages, IFN-γ up-regulated P-gp expression and activity time and dose-dependently (Puddu et al., 1999). Also, up-regulation by IFN-γ of P-gp expression was also observed in rat primary hepatocytes (Akazawa et al., 2002). Whereas, in another human colon carcinoma cell line, Caco-2 cell, IFN-γ induced up-regulation of both P-gp mRNA and protein expression but did not affect P-gp activity (Belliard et al., 2004; Dixit et al., 2005). Transcription factor for IFN-γ have been known to signal transducer and activator of transcription-1 (STAT-1). In previous study, it has been reported that IFN-γ decreased expression of ABCA1 through the JAK/STAT1 signaling pathway (Hao et al., 2009). However, IFN-γ has been shown to enhance TNF-induced NF-κB nuclear translocation, and thus lead to persistent activation of NF-κB (Cheshire and Baldwin, 1997). Dixit et al. suggested that IFN-γ induced release of NO and activated NFκB, which may enhance transcription of mdr gene in Caco2 cells (Dixit et al., 2005). Until now there has been no evidence about the transcriptional control of P-gp by STAT1. Further studies about regulation of P-gp by STAT1 in our cells are needed. The increase of P-gp gene expression by IFN-γ in our study may be explained by the effect of NF-κB activation as shown in TNF-α, the results regarding P-gp activity are discordant. Previous report suggested that a modification of the localization of P-gp transporter by IFN-γ could explain the absence of correlation between the up-regulation of P-gp expression and unmodifi ed P-gp activity (Dixit et al., 2005). Therefore, this decrease of activity with a similar level of protein in our human BBB cells may be also explained a modification of P-gp cellular distribution by IFN-γ. As the results of TNF-α pretreatment, this evidence, taken together, indicates that P-gp expression and its activity are modulated by some inflammatory cytokines and the responses differ from one cell type to another. Until now little was known about the modulation of P-gp activity by IFN-γ. So, our data provide first evidence that IFN-γ modulated P-gp expression and activity in human brain microvascular endothelial cells.
Previous studies showed that IFN-γ and TNF-α could act in synergy on the function of other cell line (Paludan, 2000). Therefore, to learn whether such synergistic or antagonist effect between these two cytokines exists, P-gp mRNA and P-gp protein expression and rh123 transport activity were examined. Our results were not found synergistic effect between IFN-γ and TNF-α at activity of P-gp but at expression level (Fig. 1 and Fig. 2). This synergistic cytokine effect has been known to act via interactions between transcription factor for IFN-γ, STAT1, and for TNF-α, NF-κB (Paludan, 2000). IFN-γ can induce the expression of both TNF-α receptor (Schmitz et al., 1999). In previous study, IFN-γ has been shown to lead to activation of NF-κB, which is induced by TNF-α (Cheshire and Baldwin, 1997). In addition, the synergy between these cytokines may be due to their ability to induce the expression of interferon-regulatory factor-1 (IRF-1) (Ohmori et al., 1997).
In summary, the present findings show down-regulation of P-gp transport activity without altering P-gp protein levels by TNF-α as well as IFN-γ in HBMEC cells. Therefore, for the efficacy of pharmacotherapy of CNS diseases, the observed alterations in P-gp transporter activity during neuro-inflammation needs to be considered. Furthermore, the numerous mechanisms involved in these processes (such as transcriptional and translational regulations, mRNA stability, phosphorylation state of the protein and changes of it localization in cell surface) needs to be explored.
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (KRF-2008-532-E00031), and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2011-0030701).
References
- 1.Akazawa Y. Kawaguchi H. Funahashi M. Watanabe Y. Yamaoka K. Hashida M. Takakura Y. Effect of interferons on P-glycoprotein-mediated rhodamine-123 efflux in cultured rat hepatocytes. J. Pharm. Sci. (2002);91:2110–2115. doi: 10.1002/jps.10199. [DOI] [PubMed] [Google Scholar]
- 2.Bauer B. Hartz A. M. Miller D. S. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol. Pharmacol. (2007);71:667–675. doi: 10.1124/mol.106.029512. [DOI] [PubMed] [Google Scholar]
- 3.Belliard A. M. Lacour B. Farinotti R. Leroy C. Effect of tumor necrosis factor-alpha and interferon-gamma on intestinal P-glycoprotein expression activity and localization in Caco-2 cells. J. Pharm. Sci. (2004);93:1524–1536. doi: 10.1002/jps.20072. [DOI] [PubMed] [Google Scholar]
- 4.Cheshire J. L. Baldwin A. S. Jr. Synergistic activation of NF-kappaB by tumor necrosis factor alpha and gamma interferon via enhanced I kappaB alpha degradation and de novo I kappaB-beta degradation. Mol. Cell Biol. (1997);17:6746–6754. doi: 10.1128/mcb.17.11.6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornford E. M. The blood /brain barrier a dynamic regulatory interface. Mol. Physiol. (1985);7:219–259. [Google Scholar]
- 6.Dixit S. G. Zingarelli B. Buckley D. J. Buckley A. R. Pauletti G. M. Nitric oxide mediates increased P-glycoprotein activity in interferon-{gamma}-stimulated human intestinal cells. Am. J. Physiol. Gastrointest. Liver Physiol. (2005);288:G533–G540. doi: 10.1152/ajpgi.00248.2004. [DOI] [PubMed] [Google Scholar]
- 7.Gijbels K. Van Damme J. Proost P. Put W. Carton H. Billiau A. Interleukin 6 production in the central nervous system during experimental autoimmune encephalomyelitis. Eur. J. Immunol. (1990);20:233–235. doi: 10.1002/eji.1830200134. [DOI] [PubMed] [Google Scholar]
- 8.Hao X. R. Cao D. L. Hu Y. W. Li X. X. Liu X. H. Xiao J. Liao D. F. Xiang J. Tang C. K. IFN-gamma down-regulates ABCA1 expression by inhibiting LXRalpha in a JAK/STAT signaling pathway-dependent manner. Atherosclerosis. (2009);203:417–428. doi: 10.1016/j.atherosclerosis.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 9.Hartz A. M. Bauer B. Fricker G. Miller D. S. Rapid regulation of P-glycoprotein at the blood-brain barrier by endothelin-1. Mol. Pharmacol. (2004);66:387–394. doi: 10.1124/mol.104.001503. [DOI] [PubMed] [Google Scholar]
- 10.Hartz A. M. Bauer B. Fricker G. Miller D. S. Rapid modulation of P-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-alpha and lipopolysaccharide. Mol. Pharmacol. (2006);69:462–470. doi: 10.1124/mol.105.017954. [DOI] [PubMed] [Google Scholar]
- 11.Huber J. D. Egleton R. D. Davis T. P. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. (2001);24:719–725. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- 12.Kallmann B. A. Hummel V. Lindenlaub T. Ruprecht K. Toyka K. V. Rieckmann P. Cytokine-induced modulation of cellular adhesion to human cerebral endothelial cells is mediated by soluble vascular cell adhesion molecule-1. Brain. (2000);123:687–697. doi: 10.1093/brain/123.4.687. [DOI] [PubMed] [Google Scholar]
- 13.Kipp H. Pichetshote N. Arias I. M. Transporters on demand: intrahepatic pools of canalicular ATP binding cassette transporters in rat liver. J. Biol. Chem. (2001);276:7218–7224. doi: 10.1074/jbc.M007794200. [DOI] [PubMed] [Google Scholar]
- 14.Konsman J. P. Vigues S. Mackerlova L. Bristow A. Blomqvist A. Rat brain vascular distribution of interleukin-1 type-1 receptor immunoreactivity: relationship to patterns of inducible cyclo-oxygenase expression by peripheral inflammatory stimuli. J. Comp. Neurol. (2004);472:113–129. doi: 10.1002/cne.20052. [DOI] [PubMed] [Google Scholar]
- 15.McCarron R. M. Wang L. Racke M. K. McFarlin D. E. Spatz M. Cytokine-regulated adhesion between encephalitogenic T lymphocytes and cerebrovascular endothelial cells. J. Neuroimmunol. (1993);43:23–30. doi: 10.1016/0165-5728(93)90071-6. [DOI] [PubMed] [Google Scholar]
- 16.Ohmori Y. Schreiber R. D. Hamilton T. A. Synergy between interferon-gamma and tumor necrosis factor-alpha in transcriptional activation is mediated by cooperation between signal transducer and activator of transcription 1 and nuclear factor kappaB. J. Biol. Chem. (1997);272:14899–14907. doi: 10.1074/jbc.272.23.14899. [DOI] [PubMed] [Google Scholar]
- 17.Paludan S. R. Synergistic action of pro-inflammatory agents: cellular and molecular aspects. J. Leukoc. Biol. (2000);67:18–25. doi: 10.1002/jlb.67.1.18. [DOI] [PubMed] [Google Scholar]
- 18.Pardridge W. M. Recent advances in blood-brain barrier transport. Annu. Rev. Pharmacol. Toxicol. (1988);28:25–39. doi: 10.1146/annurev.pa.28.040188.000325. [DOI] [PubMed] [Google Scholar]
- 19.Perry V. H. Anthony D. C. Bolton S. J. Brown H. C. The blood-brain barrier and the inflammatory response. Mol. Med. Today. (1997);3:335–341. doi: 10.1016/S1357-4310(97)01077-0. [DOI] [PubMed] [Google Scholar]
- 20.Poller B. Drewe J. Krähenbühl S. Huwyler J. Gutmann H. Regulation of BCRP (ABCG2) and P-glycoprotein (ABCB1) by cytokines in a model of the human blood-brain barrier. Cell. Mol. Neurobiol. (2010);30:63–70. doi: 10.1007/s10571-009-9431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puddu P. Fais S. Luciani F. Gherardi G. Dupuis M. L. Romagnoli G. Ramoni C. Cianfriglia M. Gessani S. Interferon-gamma up-regulates expression and activity of P-glycoprotein in human peripheral blood monocyte-derived macrophages. Lab. Invest. (1999);79:1299–1309. [PubMed] [Google Scholar]
- 22.Schinkel A. H. Smit J. J. van Tellingen O. Beijnen J. H. Wagenaar E. van Deemter L. Mol C. A. van der Valk M. A. Robanus-Maandag E. C. te Riele H. P. Berns A. J. M. Borst P. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. (1994);77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 23.Schmitz H. Fromm M. Bentzel C. J. Scholz P. Detjen K. Mankertz J. Bode H. Epple H. J. Riecken E. O. Schulzke J. D. Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J. Cell Sci. (1999);112:137–146. doi: 10.1242/jcs.112.1.137. [DOI] [PubMed] [Google Scholar]
- 24.Spector R. Micronutrient homeostasis in mammalian brain and cerebrospinal fluid. J. Neurochem. (1989);53:1667–1674. doi: 10.1111/j.1471-4159.1989.tb09229.x. [DOI] [PubMed] [Google Scholar]
- 25.Stins M. F. Gilles F. Kim K. S. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J. Neuroimmunol. (1997);76:81–90. doi: 10.1016/s0165-5728(97)00036-2. [DOI] [PubMed] [Google Scholar]
- 26.Tamai I. Tsuji A. Transporter-mediated permeation of drugs across the blood-brain barrier. J. Pharm. Sci. (2000);89:1371–1388. doi: 10.1002/1520-6017(200011)89:11<1371::aid-jps1>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 27.Théron D. Barraud de Lagerie S. Tardivel S. Pélerin H. Demeuse P. Mercier C. Mabondzo A. Farinotti R. Lacour B. Roux F. Gimenez F. Influence of tumor necrosis factor-alpha on the expression and function of P-glycoprotein in an immortalised rat brain capillary endothelial cell line GPNT. Biochem. Pharmacol. (2003);66:579–587. doi: 10.1016/s0006-2952(03)00340-x. [DOI] [PubMed] [Google Scholar]
- 28.Uçeyler N. Valenza R. Stock M. Schedel R. Sprotte G. Sommer C. Reduced levels of antiinfl ammatory cytokines in patients with chronic widespread pain. Arthritis. Rheum. (2006);54:2656–2664. doi: 10.1002/art.22026. [DOI] [PubMed] [Google Scholar]
- 29.Walther W. Stein U. Influence of cytokines on mdr1 expression in human colon carcinoma cell lines: increased cytotoxicity of MDR relevant drugs. J. Cancer Res. Clin. Oncol. (1994);120:471–478. doi: 10.1007/BF01191800. [DOI] [PubMed] [Google Scholar]
- 30.Yu C. Kastin A. J. Tu H. Waters S. Pan W. TNF activates P-glycoprotein in cerebral microvascular endothelial cells. Cell Physiol. Biochem. (2007);20:853–858. doi: 10.1159/000110445. [DOI] [PubMed] [Google Scholar]