Abstract
Resveratrol (trans-3,5,4’-trihydroxystilbene) has received considerable attention recently for the potential neuroprotective effects in neurodegenerative disorders where heme oxygenase-1 (HO-1) and sirtuin 1 (SIRT1) represent promising therapeutic targets. Resveratrol has been known to increase HO-1 expression and SIRT1 activity. In this study, the effects of resveratrol and trans-3,5,4’-trimethoxystilbene (TMS), a resveratrol derivative, on cytotoxicity caused by glutamate-induced oxidative stress, HO-1 expression, and SIRT1 activation have been investigated by using murine hippocampal HT22 cells, which have been widely used as an in vitro model for investigating glutamate-induced neurotoxicity. Resveratrol protected HT22 neuronal cells from glutamateinduced cytotoxicity and increased HO-1 expression as well as SIRT1 activity in a concentration-dependent manner. Cytoprotec-tion afforded by resveratrol was partially reversed by the specific inhibition of HO-1 expression by HO-1 small interfering RNA and the nonspecific blockage of HO-1 activity by tin protoporphyrin IX, but not by SIRT1 inhibitors. Surprisingly, TMS, a resveratrol derivative with methoxyl groups in lieu of the hydroxyl groups, and trans-stilbene, a non-hydroxylated analog, failed to protect HT22 cells from glutamate-induced cytotoxicity and to increase HO-1 expression and SIRT1 activity. Taken together, our findings suggest that the cytoprotective effect of resveratrol was at least in part associated with HO-1 expression but not with SIRT1 activation and, importantly, that the presence of hydroxyl groups on the benzene rings of resveratrol appears to be necessary for cytoprotection against glutamate-induced oxidative stress, HO-1 expression, and SIRT1 activation in HT22 neuronal cells.
Keywords: Resveratrol, trans-3, 5, 4'-Trimethoxystilbene, Heme oxygenase-1, Sirtuin 1, Oxidative stress, Neuroprotection
INTRODUCTION
Although the production of reactive oxygen species and their detoxification are normal physiological processes, an imbalance between the production of reactive oxygen species and their removal may lead to oxidative stress (Steele et al., 2007). It is now well established that oxidative stress plays a crucial role in the development of the most common neurodegenerative diseases, namely Alzheimer's disease and Parkinson's disease (Floyd and Hensley, 2002). Although whether the oxidative stress is a cause or consequence of the neurodegenerative diseases remains to be elucidated, a growing body of evidence suggests that oxidative stress directly initiates and progresses to neuronal cell death (Pocernich et al., 2011). Therefore, a key therapeutic intervention would be to reduce cellular levels of oxidative stress and/or to enhance cellular resistance to oxidative stress. Because natural antioxidants, such as polyphenols, have been shown to prevent neuronal cell death via scavenging of reactive oxygen species and/or enhancing the cellular antioxidant system, they may be thought to be potential candidate neuroprotective agents (Kelsey et al., 2010). Among the natural polyphenols, resveratrol (trans-3,5,4’-trihydroxystilbene; RSV) that is present in a variety of plants is well known as one of neuroprotective antioxidants (Albani et al., 2010; Robb and Stuart, 2010; Sun et al., 2010; Richard et al., 2011; Li et al., 2012). Studies have shown that RSV is neuroprotective against oxidative stress (Darvesh et al., 2010; Hall et al., 2010). However, the mechanisms of actions involved in the antioxidant-related neuroprotective effects of RSV are not fully understood. RSV has been reported to have the capacity to chelate metal ions and to directly quench reactive oxygen species that contribute to oxidative damage (Martin et al., 2002; Ndiaye et al., 2005; Sun et al., 2008). While both of these physiochemical properties are known to contribute to the overall neuroprotective effects of RSV, RSV can also have the capacity to activate or inhibit various cellular signaling pathways and numerous additional regulatory molecules involved in cellular protection against oxidative stress (Sun et al., 2010). Interestingly, recent studies have shown that RSV can attenuate neuronal cell death caused by oxidative stress, indirectly through induction and/or activation of antioxidant/cytoprotective enzymes, including sirtuin 1 (SIRT1) and heme oxygenase-1 (HO-1) (Chen et al., 2005; Albani et al., 2009).
SIRT1 is a class III protein deacetylase and there are seven members of the sirtuin class of enzymes in mammals; SIRT1 to SIRT7 (Srivastava and Haigis, 2011). Sirtuins regulate a number of intracellular pathways via the activation of transcription factors and enzymes responsive to nutrient availability (Zhang et al., 2011). SIRT1 is the major sirtuin activated through both calorie restriction and RSV (Borra et al., 2005). SIRT1 has been shown to promote cell survival by inhibiting apoptosis or cellular senescence induced by stresses, including DNA damage and oxidative stress (Hasegawa et al., 2008; Hasegawa and Yoshikawa, 2008; Albani et al., 2009). HO-1 is a rate-limiting enzyme in the degradation of pro-oxidant free heme to produce free iron, carbon monoxide and biliverdin, which is further converted to the antioxidant bilirubin by biliverdin reductase (Pae et al., 2010). HO-1 can be induced by various oxidative-inducing agents, including heavy metals, UV radiation, cytokines, and endotoxin (Pae et al., 2008). Recently, numerous in vitro and in vivo studies have shown that the induction of HO-1 is an important cytoprotective mechanism against oxidative injury (Jazwa and Cuadrado, 2010; Wu et al., 2011a).
Attempts to design the analogues of RSV with more potent biological activities have yielded many new stilbenes with different aryl substituents, of which trans-3,5,4'-trimethoxystilbene (TMS) is a primary candidate. It has been demonstrated that TMS is more potent than RSV as an anticancer and anti-inflammatory agent in various cell systems (Belleri et al., 2005; Pan et al., 2008; Yang et al., 2009; Alex et al., 2010; Deng et al., 2011; Hasiah et al., 2011). However, whether TMS, similarly to RSV, would also exert antioxidant/cytoprotective effects remains to be investigated. Particularly, the effects of RSV on HO-1 expression and SIRT1 activation in neuronal cells are not compared with those of TMS. In the present study, we have investigated whether RSV and TMS would attenuate oxidative stress-induced cellular injury in neuronal cells and have further explored whether HO-1 expression and/or SIRT1 activation would be involved in these cytoprotective actions of RSV and TMS. For this end, we used HT22 neuronal cells (an immortalized mouse hippocampal cell line) which have been widely used as an in vitro model for elucidating the mechanism of oxidative stress-induced neurotoxicity (Behl, 1998). HT22 cells have been known to lack functional ionotropic glutamate receptor (Kulawiak and Szewczyk, 2012), thus excluding excitotoxicity as a cause for cell death induced by glutamate (Glu). In HT22 cells, Glu at high concentrations induces oxidative stress and subsequently cell death by inhibiting cysteine uptake, which results in the depletion of intracellular cysteine that is primarily associated to the cellular synthesis of the antioxidant glutathione (Behl, 1998; Kulawiak and Szewczyk, 2012).
MATERIALS AND METHODS
Reagents and antibodies
RSV, trans-stilbene (TS), Dulbecco’s modified Eagle’s medium (DMEM), hemin, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT), NADPH, glucose-6-phosphate dehydrogenase, glucose-6-phosphate, sirtinol, nicotinamide, tin protoporphyrin IX (SnPP), and glutamate were purchased from Sigma-Aldrich (St. Louis, MO, USA). TMS was obtained from Wako Pure Chemical Industries (Osaka, Japan). Antibodies against HO-1, SIRT1 and β-actin and small interfering RNA (siRNA) products against HO-1 and SIRT1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All other reagents used were of analytical grade.
Cell culture
Mouse hippocampal HT22 cells (kind gift of Prof. Youn-Chul Kim at Wonkwang University, Iksan, Korea) were maintained at 5×105 cells per 1 ml in DMEM medium supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA, USA), penicillin G (100 units/ml), streptomycin (100 mg/ml), and L-glutamine (2 mM) and were incubated at 37℃ in a humidified atmosphere containing 5% CO2 and 95% air.
MTT assay
Cell viability was determined by a modified MTT reduction assay. MTT is a pale yellow substance that is reduced by living cells to yield a dark blue formazan product. This process requires active mitochondria, and even fresh dead cells do not reduce significant amounts of MTT. HT22 cells were cultured in a 96-well flat-bottom plate at concentration of 5×105 cells/ml. After 12 h of preconditioning, the cells were treated with various concentrations of RSV, TMS, or TS for 24 h. Thereafter, culture medium was aspirated and 100 μl of MTT dye (1 mg/ml in phosphate-buffered saline) was added; the cultures were incubated for 4 h at 37℃. The formazan crystals produced through dye reduction by viable cells were dissolved using acidified isopropanol (0.1 N HCl). Index of cell viability was calculated by measuring the optical density of color pro-duced by MTT dye reduction at 570 nm.
Western blot analysis
Proteins isolated from cells were separated by 10% SDS-PAGE denaturing gels and transferred to nitrocellulose membranes. After blocking, the membranes were incubated with antibodies against HO-1 (1:1,000 dilution), SIRT1 (1:500 dilu-tion), or β-actin (1:1,000 dilution) at 4℃ overnight. Afterward, membranes were incubated with HRP-conjugated secondary antibodies for 1 h at room temperature. Specific bands were detected using enhanced chemiluminescence detection system (Amersham Biosciences Inc., Piscataway, NJ, USA), and the membranes were exposed to X-ray film.
HO activity assessment
HO activity was determined at the end of each treatment as described previously (Son et al., 2010). Briefly, harvested cells were subjected to three cycles of freeze-thawing before addition to a reaction mixture consisting of phosphate buffer (1 ml final volume, pH 7.4) containing magnesium chloride (2 mM), NADPH (0.8 mM), glucose-6-phosphate (2 mM), glucose-6-phosphate dehydrogenase (0.2 Units), rat liver cytosol as a source of biliverdin reductase, and the substrate hemin (20 μM). The reaction mixture was incubated in the dark at 37℃ for 1 h and was terminated by the addition of 1 ml of chloroform. After being vigorously vortexed and centrifuged, the extracted bilirubin in the chloroform layer was measured by the difference in absorbance between 464 and 530 nm (ε=40 mM-1 · cm-1). The absorbance values recorded for each sam-ple were normalized to μg of protein and the values were presented as the percentage of the control value.
SIRT1 activity assessment
SIRT1 activity was measured based on an enzymatic reaction
using the fluorometric SIRT1 assay kit (Sigma-Aldrich) according to the manufacturer's instructions. The substrate that is deacetylated by SIRT1 contains an acetylated lysine side chain, which reacts with the developing solution in the kit and leads to the release of a highly fluorescent substrate. The fluorescence intensity was measured at an excitation wavelength of 335 nm and emission wavelength of 444 nm. It is directly proportional to the deacetylation activity of the SIRT1 enzyme. The fluorescence values recorded for each sample were normalized to μg of protein and the values were presented as the percentage of the control value.
HO-1 and SIRT1 siRNA transfection
HT22 cells were transiently transfected with siRNA targeting to HO-1 or SIRT1 by Lipofectamine 2,000TM (Invitrogen, Paisley, UK) according to the manufacturer's protocol. The knockdown efficiency of HO-1 or SIRT1 was determined by Western blot using anti-HO-1 or anti-SIRT1. After 24 h of transfection, the cells were pretreated with 10 μM RSV for 12 h. Then, the cells were stimulated with culture medium supplemented with Glu (2 mM) for 24 h.
Statistical analysis
Data are expressed as means ± SE. One-way analysis of variance procedures were used to assess significant differences among treatment groups. For each treatment showing a statistically significant effect, the Newman-Keuls test was used for comparisons of multiple group means. The criterion for statistical significance was set at p<0.05.
RESULTS
Effects of pretreatment with RSV or TMS on Glu-induced cytotoxicity
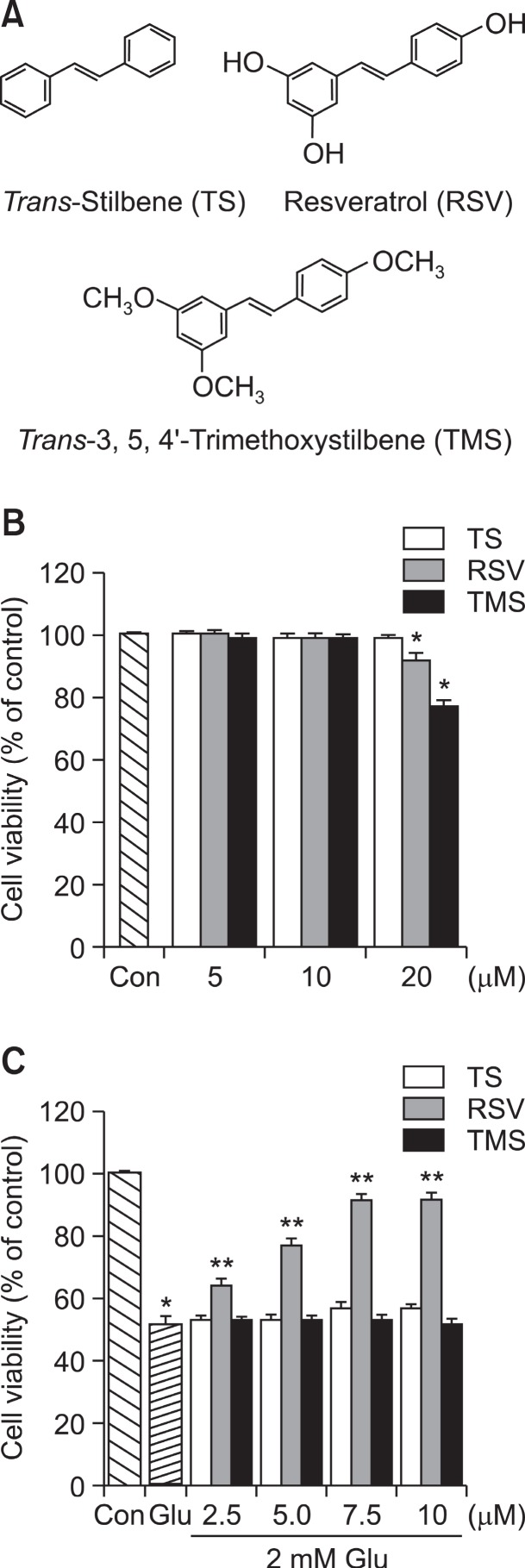
HT22 neuronal cells were treated with different concentrations of each of three stilbene compounds shown in Fig. 1A, and cell viability was performed after 24 h incubation with TS, RSV, or TMS. As shown in Fig. 1B, no cytotoxic sign was observed up to 10 μM of each compound. However, the cell viability was significantly reduced at 20 μM of RSV and TMS. Therefore, maximum concentration was limited to 10 μM of each compound in all subsequent experiments. As previously reported (Jeong et al., 2008), incubation of a high dose of Glu (2 mM) for 24 h markedly reduced the viability of HT22 cells (Fig. 1C). Interestingly, pretreatment with RSV for 12 h protected HT22 cells against Glu-induced cytotoxicity in a concentration-dependent manner (Fig. 1C). However, no significant cytoprotection against Glu-induced cytotoxicity was observed when either TMS or TS was pretreated for 12 h, similarly to RSV (Fig. 1C). It should be noted that the cytoprotective effect of RSV was much higher when HT22 cells were pre-incubated with RSV for 12 h prior to Glu treatment than when RSV was added simultaneously with Glu (not shown).
Fig. 1. Effects of TS, RSV and TMS on cell viability and Glu-induced cytotoxicity. (A) Chemical structures of TS, RSV and TMS. (B) HT22 cells were incubated for 24 h with TS, RSV or TMS at indicated concentrations. (C) HT22 cells were pretreated for 12 h with TS, RSV or TMS at indicated concentrations. Then, the cells were stimulated with culture medium supplemented with 2 mM Glu for 24 h. MTT assay for cell viability was performed as described under Materials and Methods. Data are expressed as means ± SE from 3 and 4 experiments. *p<0.05 with respect to the untreated control group (Con). **p<0.05 with respect to Glu-treated group.
Effects of RSV on HO-1 expression and SIRT1 activation
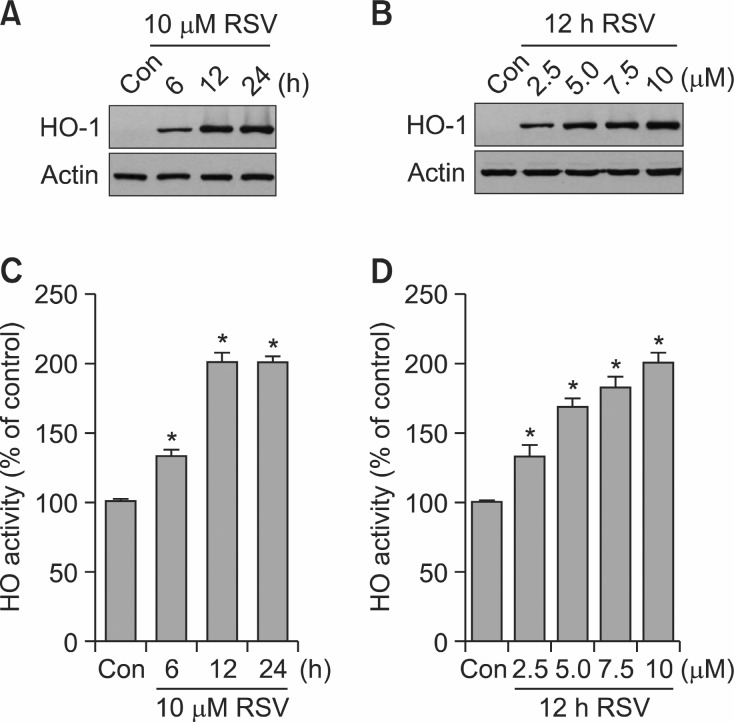
Incubation of HT22 neuronal cells with RSV at non-cytotoxic concentrations (2.5-10 μM) produced time- and concentration-dependent increases in HO-1 expression, with its maximum expression after exposure to 10 μM of RSV for 12 h (Fig. 2A and 2B). HO-1 expression by RSV in HT22 cells was associated with elevated HO activity levels (Fig. 2C, D). Similarly, stimulation of HT22 cells with increasing concentrations of
Fig. 2. Effects of RSV on HO-1 expression and HO activity. (A, C) HT22 cells were incubated with or without 10 μM RSV for indicated time periods. (B, D) HT22 cells were incubated for 12 h with or without RSV at indicated concentrations. Western blot analysis for HO-1 expression (A, B) and HO activity assessment (C, D) were performed as described under Materials and Methods. Blots shown are representative of three independent experiments. Data are expressed as means ± SE from 3 and 4 experiments. *p<0.05 with respect to the untreated control group (Con).
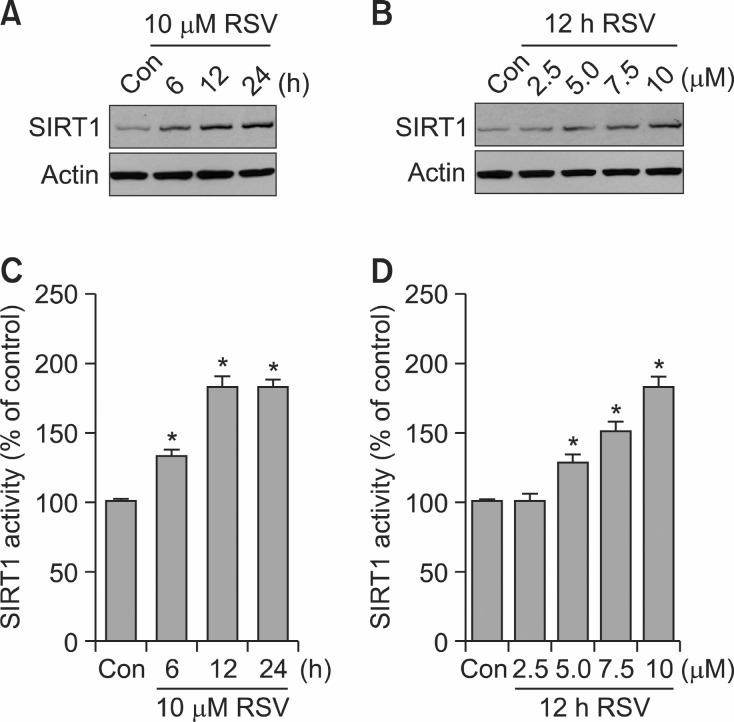
RSV led to time- and concentration-dependent increases in SIRT1 expression and activity (Fig. 3). Obviously, a marked increase in SIRT1 expression and activity was observed after exposure to 10 μM of RSV for 12 h (Fig. 3).
Fig. 3. Effects of RSV on SIRT1 expression and activity. (A ,C) HT22 cells were incubated with or without 10 μM RSV for indicatedtime periods. (B, D) HT22 cells were incubated for 12 h with or without RSV at indicated concentrations. Western blot analysisfor SIRT1 expression (A, B) and SIRT1 activity assessment (C, D) were performed as described under Materials and Methods. Blots shown are representative of three independent experiments. Data are expressed as means ± SE from 3 and 4 experiments. *p<0.05 with respect to the untreated control group (Con).
Effects of TMS on HO-1 expression and SIRT1 activation
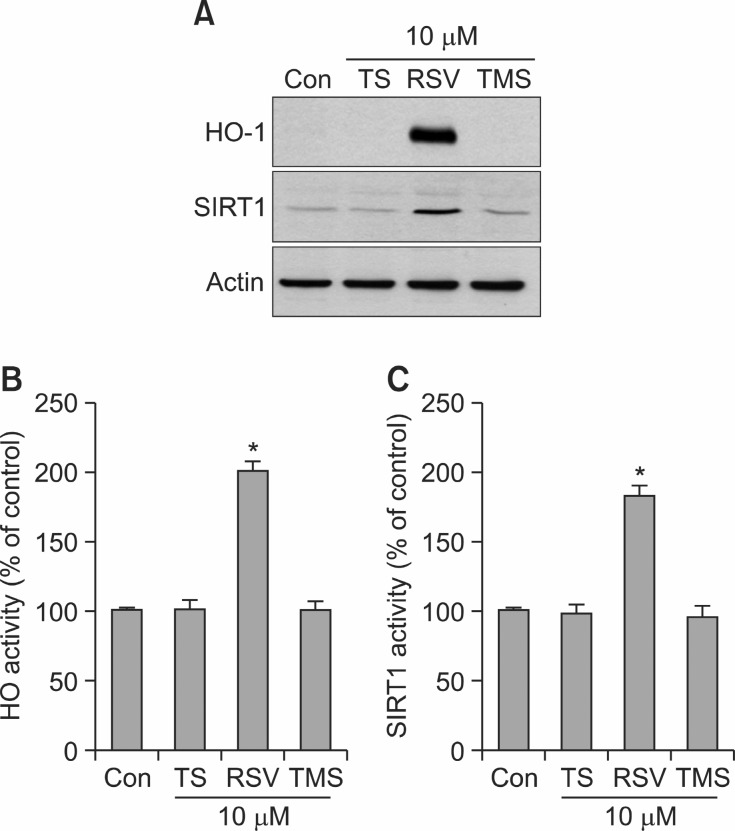
Because it has been demonstrated that TMS exhibits more potent anti-inflammatory and anticancer activities than RSV (Belleri et al., 2005; Pan et al., 2008; Yang et al., 2009; Alex et al., 2010; Deng et al., 2011; Hasiah et al., 2011), the effects of the three stilbene compounds shown in Fig. 1A on HO-1 expression and SIRT1 activation were compared in HT22 neuronal cells. Surprisingly, TS (10 μM) and TMS (10 μM) had no significant effect on HO-1 expression (Fig. 4A) and HO activity (Fig. 4B) under our experimental conditions. Also, TS (10 μM) and TMS (10 μM) failed to induce SIRT1 expression (Fig. 4A) and to increase SIRT1 activity (Fig. 4C).
Fig. 4. Effects of TS, RSV and TMS on the protein levels of HO-1 and SIRT1 and the enzymatic activities of HO-1 and SIRT1. (A-C) HT22 cells were incubated for 12 h with or without TS, RSV or TMS at 10 μM. Western blot analysis for protein levels of HO-1 and SIRT1 (A) and assessment for the activities of HO (B) and SIRT1(C) were performed as described under Materials and Methods. Blots shown are representative of three independent experiments. Data are expressed as means ± SE from 3 and 4 experiments.*p<0.05 with respect to the untreated control group (Con).
Roles of HO-1 expression and SIRT1 activation by RSV in cytoprotection
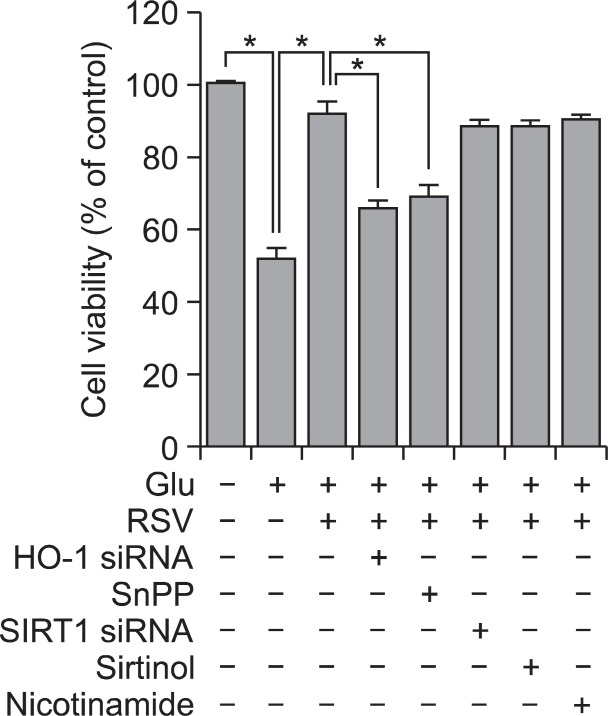
In order to explore a potential involvement of HO-1 activa-tion in the observed cytoprotection mediated by RSV, we used HO-1 siRNA to down-regulate endogenous HO-1 synthesis and SnPP to inhibit HO-1 activity. The cytoprotective effect of RSV on Glu-induced cytotoxicity was partially (but not completely) abolished in HT22 cells either transfected transiently with HO-1 siRNA or treated with SnPP (Fig. 5). We next tested whether SIRT1 activation could be also involved in cytopro-tection afforded by RSV against Glu-induced cytotoxicity, and
Fig. 5. Effects of inhibitors for HO-1 and SIRT1 on RSV-mediated cytoprotection. HT22 cells and the cells transiently transfected with either HO-1 siRNA or SIRT1 siRNA were pretreated with 10 μM RSV for 12 h, and then stimulated with 2 mM Glu for 24 h in the presence or absence of 20 μM SnPP, 5 μM sirtinol or 10 mM nicotinamide. MTT assay for cell viability was performed as described under Materials and Methods. Data are expressed as means ± SE from 3 and 4 experiments. *p<0.05.
found that three different SIRT1 inhibitors (i.e., SIRT1 siRNA for down-regulation of endogenous SIRT1 synthesis, sirtinol for specific inhibition of SIRT1 activity, and nicotinamide for nonspecific inhibition of activities of sirtuins) all failed to reverse RSV-mediated cytoprotection (Fig. 5). Inhibitors alone had no significant effect on cell viability (data not shown).
DISCUSSION
The present study demonstrates that the natural polyphenol RSV protected HT22 neuronal cells from Glu-induced cytotoxicity. Other authors have reported the direct cytotoxic effect of RSV, although at micromolar concentrations (Delmas et al., 2011; Huang and Zhu, 2011). In line with these findings, RSV reduced the viability of HT22 cells only at concentrations above 20 μM under our experimental conditions. However, at lower concentrations, RSV appeared to be predominantly cytoprotective according to our results. This study also demonstrates that RSV seems to exert a cytoprotective effect, at least under our experimental conditions, by inducing HO-1 expression but not by activating SIRT1 pathway. We have also assessed whether TMS, a derivative of RSV with methoxyl groups in lieu of the hydroxyl groups, and TS, a non-hydroxylated analog, could be also cytoprotective against Glu-induced cytotoxicity, similarly to RSV. According to our results, neither TMS nor TS reduced Glu-induced cytotoxicity in HT22 cells, thus implicating that the presence of hydroxyl groups on the benzene rings of the RSV structure appears to be associated with its cytoprotective activity.
It has been previously reported that oxidative stress plays a crucial role in induction of cell death by Glu treatment in HT22 neuronal cells (Behl, 1998; Kulawiak and Szewczyk, 2012). It is therefore conceivable that the known antioxidant effects of RSV may contribute to the increased resistance of HT22 cells against Glu-induced cytotoxicity. However, the observed effects might not be solely due to the antioxidant properties of RSV itself, because pretreatment of the cells with RSV significantly attenuated Glu-induced cytotoxicity. Alternatively, it is most likely that RSV might up-regulate and/or activate antioxidant/cytoprotective proteins, including SIRT1 and HO-1 (Chen et al., 2005; Albani et al., 2009), which could contribute to the attenuation of Glu-induced cytotoxicity.
The physiological mechanism(s) by which RSV exerts antioxidant/cytoprotective actions is still the subject of debate. Several studies suggest that the beneficial effects of RSV directly or indirectly depend on its roles in activating SIRT1, a key regulator of cell defense and survival under various stress conditions, such as oxidative stress (Wang et al., 2011a; Chong et al., 2012). Indeed, RSV has been shown to prevent cytotoxicity triggered by H2O2 or 6-hydroxydopamine, two well-known oxidative stress inducers, in SK-N-BE neuronal cells, and this protective pathway involved SIRT1 activation (Albani et al., 2009). Moreover, RSV has been reported to have SIRT1-dependent neuroprotective effects both in vitro and in vivo, mainly against oxidative stress inducers (Kim et al., 2007; Ho et al., 2010; Shindler et al., 2010; Wang et al., 2011b; Wu et al., 2011b). In HT22 neuronal cells, RSV significantly increased SIRT1 expression and activity, as the ability of RSV to activate SIRT1 has been examined in other cell lines (Kao et al., 2010; Xia et al., 2011), and we therefore examined whether the cytoprotection afforded by RSV could account for its activation of SIRT1. Surprisingly, the cytoprotective effect of RSV against Glu-induced cytotoxicity in HT22 cells was independent of SIRT1 activation, because specific and nonspecific SIRT1 inhibitors failed to reverse the protective effect of RSV. Our results suggest that the cytoprotective effect of RSV might not be due to SIRT1 activation but could rather be attributed to other antioxidant/cytoprotective proteins.
HO-1 has been reported to be one of other key mediators of antioxidant/cytoprotective actions of RSV (Bastianetto and Quirion, 2010; Ren et al., 2011). In our study, we found that RSV induced HO-1 expression and increased the ensuring HO activity in HT22 neuronal cells. Importantly, we have shown here that the specific down-regulation of HO-1 cellular synthesis by using HO-1 siRNA partially (but not completely) reduced the ability of RSV to prevent Glu-induced cytotoxicity, indicating that HO-1 expression may be at least partly responsible for cytoprotective actions of RSV in HT22 cells. This result was further confirmed by using the nonspecific HO inhibitor SnPP; SnPP, similarly to HO-1 siRNA, partially abrogated the cytoprotective effect of RSV. It should be noted that RSV, together with HO-1 pathway, might also activate other antioxidant/cytoprotective pathways, and overall cytoprotection afforded by RSV could be achieved by virtue of the concerted actions of the multiple pathways being activated. In this regard, it has been previously demonstrated that RSV protected HT22 cells from Glu-induced cytotoxicity by inducing the expression of the mitochondrial antioxidant superoxide dismutase 2 (Fukui et al., 2010). The importance of the HO-1 pathway in physiology and pathophysiology has been confirmed by many experi-mental studies. It has been reported that the human HO-1-deficient case described in the literature and HO-1-deficient mice exhibit increased susceptibility to oxidative stress (Pae et al., 2008). The beneficial effects of HO-1 expression have been attributed to several factors, including the degradation of pro-oxidant heme, formation of biliverdin and/or bilirubin with their antioxidant properties, as well as the release of carbon monoxide, which has cytoprotective and anti-inflammatory effects (Pae et al., 2008). Although the exact mechanisms involved in cytoprotective actions of the HO-1 system have not been fully elucidated, one or more of the HO-1 reaction products may mediate the cytoprotective actions of RSV under our experimental conditions. Interestingly, it has been demonstrated that pretreatment of rats with RSV had neuroprotective effect on cerebral ischemia/reperfusion injury, which is likely exerted by up-regulated HO-1 expression to ameliorate oxidative damage, reduce neuronal apoptosis, protect ischemic penumbra, reduce infarct size, reduce reperfusion damage of brain tissue (Ren et al., 2011). Our results, together with previous reports (Bastianetto and Quirion, 2010; Ren et al., 2011), suggest that HO-1 may play an important role in neuroprotection of RSV against the damage from oxidative stress.
In our study, the biological properties of RSV have been compared with those of TMS, one of the well-known analogues of RSV. In particular, the cytoprotective activity was investigated in vitro by measuring the ability to increase HO-1 expression and SIRT1 activity. In HT22 cells, TMS, unlike RSV, failed to increase HO-1 expression as well as SIRT1 activation. Moreover, TMS also failed to reduce Glu-induced cytotoxicity; this is, presumably, because it was inactive in increasing HO-1 expression. Similarly, TS, of which chemical structure lacks three hydroxyl groups, had no significant effects on HO-1 expression and SIRT1 activation. These findings suggest that the presence of hydroxyl groups in the benzene rings of RSV may be required at least in part for HO-1 expression and SIRT1 activation.
In summary, the results of the present study demonstrate that: (i) pretreatment of HT22 neuronal cells with RSV can confer a marked protection against Glu-induced cytotoxicity, which appears to be associated at least in part with HO-1 expression; (ii) the cytoprotective activity of RSV was independent of its activation of SIRT1; and (iii) TMS failed to protect HT22 cells from Glu-induced cytotoxicity and to increase HO-1 expression and SIRT1 activation, suggesting that the hydroxyl groups on the benzene rings of RSV was necessary for cytoprotective activity, HO-1 expression, and SIRT1 activation. Although we are not concluding that HO-1 is the only pathway by which RSV can be cytoprotective, we believe that HO-1 may be a unique candidate by which RSV can induce an endogenous cellular pathway that leads to building cellular resistance to oxidative stress. Further studies will be required in order to understand the exact mechanisms of overall cyto-protective actions of RSV.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2011-0030717).
References
- 1.Albani D. Polito L. Batelli S. De Mauro S. Fracasso C. Martelli G. Colombo L. Manzoni C. Salmona M. Caccia S. Negro A. Forloni G. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1-42) peptide. J. Neurochem. (2009);110:1445–1456. doi: 10.1111/j.1471-4159.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- 2.Albani D. Polito L. Signorini A. Forloni G. Neuroprotective properties of resveratrol in different neurodegenerative disorders. Biofactors. (2010);36:370–376. doi: 10.1002/biof.118. [DOI] [PubMed] [Google Scholar]
- 3.Alex D. Leong E. C. Zhang Z. J. Yan G. T. Cheng S. H. Leong C. W. Li Z. H. Lam K. H. Chan S. W. Lee S. M. Resveratrol derivative trans-3,5,4'-trimethoxystilbene, exerts an-tiangiogenic and vascular-disrupting effects in zebrafi sh through the downregulation of VEGFR2 and cell-cycle modulation. J. Cell Biochem. (2010);109:339–346. doi: 10.1002/jcb.22405. [DOI] [PubMed] [Google Scholar]
- 4.Bastianetto S. Quirion R. Heme oxygenase 1: another possible target to explain the neuroprotective action of resveratrol, a multifaceted nutrient-based molecule. Exp. Neurol. (2010);225:237–239. doi: 10.1016/j.expneurol.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Behl C. Effects of glucocorticoids on oxidative stress-induced hippocampal cell death: implications for the pathogenesis of Alzheimer's disease. Exp. Gerontol. (1998);33:689–696. doi: 10.1016/s0531-5565(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 6.Belleri M. Ribatti D. Nicoli S. Cotelli F. Forti L. Vannini V. Stivala L. A. Presta M. Antiangiogenic and vascular-targeting activity of the microtubule-destabilizing trans-resveratrol derivative 354'-trimethoxystilbene. Mol. Pharmacol. (2005);67:1451–1459. doi: 10.1124/mol.104.009043. [DOI] [PubMed] [Google Scholar]
- 7.Borra M. T. Smith B. C. Denu J. M. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. (2005);280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 8.Chen C. Y. Jang J. H. Li M. H. Surh Y. J. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem. Biophys. Res. Commun. (2005);331:993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- 9.Chong Z. Z. Shang Y. C. Wang S. Maiese K. SIRT1: new avenues of discovery for disorders of oxidative stress. Expert. Opin. Ther. Targets. (2012);16:167–178. doi: 10.1517/14728222.2012.648926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darvesh A. S. Carroll R. T. Bishayee A. Geldenhuys W. J. Van der Schyf C. J. Oxidative stress and Alzheimer's disease: dietary polyphenols as potential therapeutic agents. Expert Rev. Neurother. (2010);10:729–745. doi: 10.1586/ern.10.42. [DOI] [PubMed] [Google Scholar]
- 11.Delmas D. Solary E. Latruffe N. Resveratrol a phyto-chemical inducer of multiple cell death pathways: apoptosis autophagy and mitotic catastrophe. Curr. Med. Chem. (2011);18:1100–1121. doi: 10.2174/092986711795029708. [DOI] [PubMed] [Google Scholar]
- 12.Deng Y. H. Alex D. Huang H. Q. Wang N. Yu N. Wang Y. T. Leung G. P. Lee S. M. Inhibition of TNF-α-mediated endothelial cell-monocyte cell adhesion and adhesion molecules expression by the resveratrol derivative trans-354'-trimethoxystilbene. Phytother. Res. (2011);25:451–457. doi: 10.1002/ptr.3279. [DOI] [PubMed] [Google Scholar]
- 13.Floyd R. A. Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol. Aging. (2002);23:795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 14.Fukui M. Choi H. J. Zhu B. T. Mechanism for the protective effect of resveratrol against oxidative stress-induced neuronal death. Free Radic. Biol. Med. (2010);49:800–813. doi: 10.1016/j.freeradbiomed.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall E. D. Vaishnav R. A. Mustafa A. G. Antioxidant therapies for traumatic brain injury. Neurotherapeutics. (2010);7:51–61. doi: 10.1016/j.nurt.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasegawa K. Wakino S. Yoshioka K. Tatematsu S. Hara Y. Minakuchi H. Washida N. Tokuyama H. Hayashi K. Itoh H. Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem. Biophys. Res. Commun. (2008);372:51–66. doi: 10.1016/j.bbrc.2008.04.176. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa K. Yoshikawa K. Necdin regulates p53 acetylation via Sirtuin1 to modulate DNA damage response in cortical neurons. J. Neurosci. (2008);28:8772–8784. doi: 10.1523/JNEUROSCI.3052-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasiah A. H. Ghazali A. R. Weber J. F. Velu S. Thomas N. F. Inayat Hussain S. H. Cytotoxic and antioxidant effects of methoxylated stilbene analogues on HepG2 hepatoma and Chang liver cells: Implications for structure activity relationship. Hum. Exp. Toxicol. (2011);30:138–144. doi: 10.1177/0960327110368739. [DOI] [PubMed] [Google Scholar]
- 19.Ho D. J. Calingasan N. Y. Wille E. Dumont M. Beal M. F. Resveratrol protects against peripheral deficits in a mouse model of Huntington's disease. Exp. Neurol. (2010);225:74–84. doi: 10.1016/j.expneurol.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Huang X. Zhu H. L. Resveratrol and its analogues: prom-ising antitumor agents. Anticancer Agents Med. Chem. (2011);11:479–490. doi: 10.2174/187152011795677427. [DOI] [PubMed] [Google Scholar]
- 21.Jazwa A. Cuadrado A. Targeting heme oxygenase-1 for neuroprotection and neuroinflammation in neurodegenerative diseases. Curr. Drug Targets. (2010);11:1517–1531. doi: 10.2174/1389450111009011517. [DOI] [PubMed] [Google Scholar]
- 22.Jeong G. S. An R. B. Pae H. O. Chung H. T. Yoon K. H. Kang D. G. Lee H. S. Kim Y. C. Cudratricusxanthone A protects mouse hippocampal cells against glutamate-induced neurotoxicity via the induction of heme oxygenase-1. Planta Med. (2008);74:1368–1373. doi: 10.1055/s-2008-1081315. [DOI] [PubMed] [Google Scholar]
- 23.Kao C. L. Chen L. K. Chang Y. L. Yung M. C. Hsu C. C. Chen Y. C. Lo W. L. Chen S. J. Ku H. H. Hwang S. J. Resveratrol protects human endothelium from H(2)O(2)-induced oxidative stress and senescence via SirT1 activation. J. Atherocler. Thromb. (2010);17:970–979. doi: 10.5551/jat.4333. [DOI] [PubMed] [Google Scholar]
- 24.Kelsey N. A. Wilkins H. M. Linseman D. A. Nutraceutical antioxidants as novel neuroprotective agents. Molecules. (2010);15:7792–7814. doi: 10.3390/molecules15117792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D. Nguyen M. D. Dobbin M. M. Fischer A. Sananbenesi F. Rodgers J. T. Delalle I. Baur J. A. Sui G. Armour S. M. Puigserver P. Sinclair D. A. Tsai L. H. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. (2007);26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulawiak B. Szewczyk A. Glutamate-induced cell death in HT22 mouse hippocampal cells is attenuated by paxilline, a BK channel inhibitor. Mitochondrion. (2012);12:169–172. doi: 10.1016/j.mito.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Li F. Gong Q. Dong H. Shi J. Resveratrol, a neuropro-tective supplement for Alzheimer's disease. Curr. Pharm. Des. (2012);18:27–33. doi: 10.2174/138161212798919075. [DOI] [PubMed] [Google Scholar]
- 28.Martin S. Andriambeloson E. Takeda K. Andriantsitohaina R. Red wine polyphenols increase calcium in bovine aortic en-dothelial cells: a basis to elucidate signalling pathways leading to nitric oxide production. Br. J. Pharmacol. (2002);135:1579–1587. doi: 10.1038/sj.bjp.0704603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ndiaye M. Chataigneau M. Lobysheva I. Chataigneau T. Schini-Kerth V. B. Red wine polyphenol-induced, endothelium-dependent NO-mediated relaxation is due to the redox-sensitive PI3-kinase/Akt-dependent phosphorylation of endothelial NO-synthase in the isolated porcine coronary artery. FASEB J. (2005);19:455–457. doi: 10.1096/fj.04-2146fje. [DOI] [PubMed] [Google Scholar]
- 30.Pae H. O. Kim E. C. Chung H. T. Integrative survival response evoked by heme oxygenase-1 and heme metabolites. J. Clin. Biochem. Nutr. (2008);42:197–203. doi: 10.3164/jcbn.2008029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pae H. O. Son Y. Kim N. H. Jeong H. J. Chang K. C. Chung H. T. Role of heme oxygenase in preserving vascular bioactive NO. Nitric Oxide. (2010);23:251–257. doi: 10.1016/j.niox.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Pan M. H. Gao J. H. Lai C. S. Wang Y. J. Chen W. M. Lo C. Y. Wang M. Dushenkov S. Ho C. T. Antitumor activity of 3,5,4'-trimethoxystilbene in COLO 205 cells and xenografts in SCID mice. Mol. Carcinog. (2008);47:184–196. doi: 10.1002/mc.20352. [DOI] [PubMed] [Google Scholar]
- 33.Pocernich C. B. Lange M. L. Sultana R. Butterfield D. A. Nutritional approaches to modulate oxidative stress in Alzheimer's disease. Curr. Alzheimer Res. (2011);8:452–469. doi: 10.2174/156720511796391908. [DOI] [PubMed] [Google Scholar]
- 34.Ren J. Fan C. Chen N. Huang J. Yang Q. Resveratrol pretreatment attenuates cerebral ischemic injury by upregulating expression of transcription factor Nrf2 and HO-1 in rats. Neurochem. Res. (2011);36:2352–2362. doi: 10.1007/s11064-011-0561-8. [DOI] [PubMed] [Google Scholar]
- 35.Richard T. Pawlus A. D. Iglésias M. L. Pedrot E. Waffo-Teguo P. Mérillon J. M. Monti J. P. Neuroprotective properties of resveratrol and derivatives. Ann. N. Y. Acad. Sci. (2011);1215:103–108. doi: 10.1111/j.1749-6632.2010.05865.x. [DOI] [PubMed] [Google Scholar]
- 36.Robb E. L. Stuart J. A. trans-Resveratrol as a neuroprotectant. Molecules. (2010);15:1196–1212. doi: 10.3390/molecules15031196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shindler K. S. Ventura E. Dutt M. Elliott P. Fitzgerald D. C. Rostami A. Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J. Neuroophthalmol. (2010);30:328–339. doi: 10.1097/WNO.0b013e3181f7f833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Son Y. Lee J. H. Kim N. H. Surh N. Y. Kim E. C. Chung H. T. Kang D. G. Pae H. O. Dilinoleoylphosphatidylcholine induces the expression of the anti-inflammatory heme oxygenase-1 in RAW264.7 macrophages. Biofactors. (2010);36:210–215. doi: 10.1002/biof.87. [DOI] [PubMed] [Google Scholar]
- 39.Srivastava S. Haigis M. C. Role of sirtuins and calorie restriction in neuroprotection: implications in Alzheimer's and Parkinson's diseases. Curr. Pharm. Des. (2011);17:3418–3433. doi: 10.2174/138161211798072526. [DOI] [PubMed] [Google Scholar]
- 40.Steele M. Stuchbury G. Münch G. The molecular basis of the prevention of Alzheimer's disease through healthy nutrition. Exp. Gerontol. (2007);42:28–36. doi: 10.1016/j.exger.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Sun A. Y. Wang Q. Simonyi A. Sun G. Y. Botanical phenolics and brain health. Neuromolecular Med. (2008);10:259–274. doi: 10.1007/s12017-008-8052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun A. Y. Wang Q. Simonyi A. Sun G. Y. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol. Neu-robiol. (2010);41:375–383. doi: 10.1007/s12035-010-8111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J. Zhang Y. Tang L. Zhang N. Fan D. Protective effects of resveratrol through the up-regulation of SIRT1 expression in the mutant hSOD1-G93A-bearing motor neuron-like cell culture model of amyotrophic lateral sclerosis. Neurosci. Lett. (2011a);503:250–255. doi: 10.1016/j.neulet.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 44.Wang R. H. Kim H. S. Xiao C. Xu X. Gavrilova O. Deng C. X. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia oxidative damage, and insulin resistance. J. Clin. Invest. (2011b);121:4477–4490. doi: 10.1172/JCI46243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu M. L. Ho Y. C. Yet S. F. A central role of heme oxygenase-1 in cardiovascular protection. Antioxid. Redox. Signal. (2011a);15:1835–1846. doi: 10.1089/ars.2010.3726. [DOI] [PubMed] [Google Scholar]
- 46.Wu Y. Li X. Zhu J. X. Xie W. Le W. Fan Z. Jankovic J. Pan T. Resveratrol-activated AMPK/SIRT1/autophagy in cellular models of Parkinson's disease. Neurosignals. (2011b);19:163–174. doi: 10.1159/000328516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xia L. Ding F. Zhu J. H. Fu G. S. Resveratrol attenuates apoptosis of pulmonary microvascular endothelial cells induced by high shear stress and proinfl ammatory factors. Hum. Cell. (2011);24:127–133. doi: 10.1007/s13577-011-0031-2. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y. T. Weng C. J. Ho C. T. Yen G. C. Resveratrol analog-3,5,4'-trimethoxy-trans-stilbene inhibits invasion of human lung adenocarcinoma cells by suppressing the MAPK pathway and decreasing matrix metalloproteinase-2 expression. Mol. Nutr. Food. Res. (2009);53:407–416. doi: 10.1002/mnfr.200800123. [DOI] [PubMed] [Google Scholar]
- 49.Zhang F. Wang S. Gan L. Vosler P. S. Gao Y. Zigmond M. J. Chen J. Protective effects and mechanisms of sirtuins in the nervous system. Prog. Neurobiol. (2011);95:373–395. doi: 10.1016/j.pneurobio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]