Abstract
Rotavirus and hepatitis A virus (HAV) spread by the fecal-oral route and infections are important in public health, especially in developing countries. Here, two antigenic epitopes of the HAV polyprotein, domain 2 (D2) and domain 3 (D3), were recombined with rotavirus VP7, generating D2/VP7 and D3/VP7, cloned in a baculovirus expression system, and expressed in Spodoptera frugiperda 9 (Sf9) insect cells. All were highly expressed, with peak expression 2 days post-infection. Western blotting and ELISA revealed that two chimeric proteins were antigenic, but only D2/VP7 was immunogenic and elicited neutralizing antibody responses against rotavirus and HAV by neutralization assay, implicating D2/VP7 as a multivalent subunit-vaccine Candidate for preventing both rotavirus and HAV infections.
Keywords: Rotavirus, Hepatitis A virus, Recombinant chimera protein
INTRODUCTION
Human infections by rotavirus and hepatitis A virus (HAV) are important public health problems worldwide, especially in developing countries. Although their clinical manifestations are different, both viruses are transmitted by a fecal-oral route. The viruses shed by a susceptible individual’s feces can spread to contaminate food or water (Nwachuku and Gerba, 2006). As a major cause of diarrhea-related illness and death, rotavirus is responsible for 2 million hospitalizations and more than 600,000 deaths worldwide among children younger than 5 years of age (Parashar et al., 2003). Reoviridae, a member of the family Reoviridae, consists of 11 segments of a double-stranded RNA genome that encodes for 6 structural and 6 non-structural proteins (Offit and Blavat, 1986; Estes and Cohen, 1989). The outer capsid is composed of 2 glycoproteins, VP7 and VP4, are involved in protective immunity. Initially, VP7 was thought to be the most important antigen in stimulating the synthesis of neutralizing antibodies (Offit and Blavat, 1986; Estes and Cohen, 1989).
HAV belongs to the picornavirus family and causes hepatitis A in humans. Children are primarily affected by HAV infection, but its severity increases with age (Gust, 1992). The HAV genome is a 7.5-kb positive-stranded RNA, coding for a single 250 kD polyprotein that is further processed into structural and non-structural proteins (Totsuka and Moritsugu, 1999). Like all picornaviral genomes, the HAV genome can be divided into three regions; P1, P2, and P3. The P1 region encodes structural proteins, such as 1A (also referred as to VP4), 1B (VP2), 1C (VP3), and 1D (VP1), whereas the others encode nonstructural proteins, i.e. 2A-2C in the P2 region and 3A-3D in the P3 region (Totsuka and Moritsugu, 1999). Previously, 42 antigenic peptides were identified across the HAV polyprotein, using a total of 237 synthetic peptides spanning the entire polyprotein and convalescent antisera from HAV-infected patients (Khudyakov et al., 1999). Among them, two antigenic epitopes, including the second (D2) and third (D3) domains, are known to be able to induce a virus-neutralizing antibody (Ab) response (Khudyakov et al., 1999). The D2 domain at position 767-842 amino acids (aa) contains the C-terminal part of the VP1 protein and the entire P2A protein. The D3 domain at position 1403-1456 aa contains the C-terminal part of the P2C and the N-terminal half of the P3A protein (Khudyakov et al., 1999).
In this study, two defined antigenic epitopes of the HAV polyprotein, D2 and D3 were recombined with rotavirus structural protein VP7. The resultant recombinant viral proteins were designated D2/VP7 and D3/VP7, respectively, and expressed in Spodoptera frugiperda 9 (Sf9) insect cells using a baculovirus expression system.
MATERIALS AND METHODS
Ethics statement
The study was performed according to protocol (number 2009-13) approved by the Human Subjects Institutional Review Board (IRB) of Chung-Ang University College of Medicine, Seoul, Korea.
Viruses and cells
Human rotavirus Wa G1P[8] and HAV CAU-H3 strains (isolated from a fecal specimen of a patient diagnosed with an acute form of hepatitis A at Kangnam St. Mary’s Hospital, The Catholic University of Korea School of Medicine, Korea, in 2002) were used as a viral genomic template for cDNA synthesis. Rotavirus Wa and cytopathic variant HM175 HAV were separately used for the infection of rotavirus and HAV and neutralization assay. MA104 cells and FRhK-4 cells were obtained from the Korean Cell Line Bank (Seoul, Korea) and used for cultivation of rotavirus and HAV, respectively. MA104 cells were grown in Minimum Essential Medium alpha (MEM-alpha; Gibco BRL Life Technologies, Grand Island, NY, USA) containing 5% fetal bovine serum (FBS; Gibco BRL Life Technologies) at 37℃ in present of 5% CO2. FRhK-4 cells were grown in DMEM (DMEM; Gibco BRL Life Technologies) containing 10% fetal bovine serum (FBS; Gibco BRL Life Technologies) at 37℃ in present of 5% CO2. Sf9 cells were obtained from the Korean Cell Line Bank (Seoul, Korea) and used for the production of baculoviruses. In general, the cells were grown and maintained in TNM-FH medium (Gibco BRL Life Technologies, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; Gibco BRL) at 27℃ in a 250 ml spinner flask with shaking at 90 rpm. Cellular density and viability were determined by standard microscopic observations, using a hemocytometer after trypan blue staining.
Reagents and animals
All enzymes used in this study were purchased from New England Biolabs (Beverly, MA, USA). For the expression of recombinant rotaviral proteins, both a baculovirus transfer vector (pBlueBac4.5/V5-His) and a baculovirus expression system (Bac-N-Blue™) were employed (Invitrogen, Carlsbad, CA, USA). Plasmids were isolated using a plasmid minprep kit (iNtRON Biotechnology, Seoul, Korea). Rabbit serum against the rotavirus Wa strain was produced using New Zealand white female rabbits (6-8 weeks of age; Samtako, Osan, Ko-rea), as previously described (Lee et al., 1995). Goat polyclonal to hepatitis A virus (HM175 strain) was purchased from Abcam (Abcam, Cambridge, UK). Human anti-HAV sera from HAV-infected patients were obtained from the Kangnam St. Mary’s Hospital (Seoul, Korea).
Construction of the recombinant rotavirus proteins carry-ing the HAV antigenic epitopes
Complementary DNAs (cDNAs) corresponding to rotaviral structural protein VP7 was amplified by reverse transcription-polymerase chain reaction (RT-PCR) using viral RNAs from the rotavirus Wa strain. Similarly, the 231-bp D2 and 165-bp D3 cDNA fragments were amplified using viral RNAs from the HAV CAU-H3 strain. Recombination events between D2 and VP7 and between D3 and VP7 were introduced via a XhoI restriction site (Fig. 1A). The resultant recombinant proteins were designated D2/VP7 and D3/VP7, respectively. They were cloned into the pBlueBac4.5 baculovirus transfer vector to produce the recombinant baculoviruses according to the manufacturer’s description (Invitrogen). All recombinant cassettes were confi rmed by DNA nucleotide sequencing using a BigDye terminator cycle sequencing kit (Applied Biosystems, Foster City, CA, USA) and an automatic DNA sequencer (model 3730; Applied Biosystems). Two recombinant baculoviruses were produced by mixing both Bac-N-Blue linear baculovirus DNAs and the baculovirus transfer vectors with the Cellfectin®II reagent in serum-free medium as per the manufacturer’s instructions (Invitrogen). Each recombinant virus was purified using a standard plaque assay (King et al., 2007).
Fig. 1. (A) Schematic representation of the two recombinant rotavirus proteins carrying antigenic epitopes of the HAV polyprotein. Recombinant gene cassettes were recombined in frame with a short peptide linker of Leu-Glu-Pro-Gly (LEPG). DNA regions of the vector plasmid are indicated by the filled circles (Օ ). (B) Expression of two recombinant rotavirus proteins using a baculovirus expression system in Sf9 cells. Whole cell lysates of infected Sf9 cells with individual recombinant baculoviruses were resolved by 10% SDS-PAGE. (C) Western blot analysis was performed using anti-V5 antibody and HRP-conjugated goat ani-mouse antibody.
Protein separation and Western blot analysis
Protein separation was carried out as previously described by Laemmli (Laemmli, 1970). Briefly, each recombinant protein sample was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by staining with Coomassie Brilliant Blue R-250 (Bio-Rad, Hercules, CA, USA). For Western blot, the resolved proteins were blotted onto Immun-BlotTM polyvinylidene fluoride (PVDF) membrane (Bio-Rad) using a wet transfer system (Bio-Rad). Membranes were blocked in 5% (w/v) non-fat dried milk solution overnight at 4℃. The primary antibodies (Abs) were prepared in TBS-T (20 mM Tris-HCl, 500 mM NaCl, 0.1% Tween20, pH 7.9) at the following dilutions: anti-V5 Ab, 1:10,000; rabbit antiserum against the rotavirus Wa strain, 1:5,000; anti-HAV sera, 1:5,000. The blocked membranes were incubated with the primary Ab for 1 hr and washed 4 times with TBS-T. The appropriate secondary Abs conjugated with peroxidase were used after dilution by 1:10,000. Protein bands were visualized by an enhanced chemiluminescence (ECL) method, as previously described (Penna and Cahalan, 2007).
Kinetic of the recombinant baculovirus synthesis in Sf9 cells
Sf9 cells at a density of 1×106 cells/ml were seeded in a 6-well plate. The cultures were simultaneously infected with the recombinant baculoviruses of D2/VP7 at a multiplicity of infection (MOI) of 10 plaque forming units per cell (pfu/cell) (Jiang et al., 1998; Roldão et al., 2007) and harvested every 24 hrs during the experimental period of 10 days after the initial virus infection. Cells were clarified by centrifugation at 2,500×g for 5 min and lysed using RIPA buffer (RIPA cell lysis buffer; AMRESCO Inc., Solon, OH, USA). The supernatants were harvested after centrifugation at 14,000×g for 15 min at 4℃ and stored frozen at 20℃ until use.
Rabbit immunization
New Zealand white female rabbits were immunized intramuscularly
with 50 μg of whole-cell lysis vaccination containing the recombinant D2/VP7 protein in 1×phosphate buffered saline (PBS) with complete Freund’s adjuvant on day 0, followed with incomplete Freund’s adjuvant on day 28 after the primary immunization. Blood was collected from the ear vein on 0 and 28 days after the primary boost, 10 days after the first boost. Sera were prepared by centrifugation at 1,500×g for 10 min, aliquoted, and stored at -80℃ until use.
Enzyme-linked immunosorbant assay (ELISA)
Purified viral antigens from the rotavirus Wa strain or HAV protein (Abcam) were diluted in a coating buffer (0.1 M Na2CO3, 0.1 M NaHCO3, pH 9.4) and coated in a 96-well microplate (0.1 μg/well) overnight at 4℃. After washing 3 times with 200 μl of PBS containing 0.05% Tween20 (PBS-T), plates were blocked in 5% (w/v) non-fat dried skimmed milk in PBS-T. The hyper immune sera were diluted from 1:10 to 1:640 and incubated for 1 hr at 37℃. After washing with PBS-T, 100 μl of peroxidase-conjugated goat anti-rabbit IgG (diluted 1:20,000 in PBS-T containing 5% non-fat skimmed milk) was added to each well and incubated for 1 hr at 37℃. Finally, the plates were washed and developed in the presence of ortho-phenylenediamine (Sigma, St. Louis, MO, USA) for 5 min. The reaction was then stopped by the addition of 50 μl of 1M H2SO4 per well. The optical density at 492 nm was measured using a Infinite® 200 PRO NanoQuant microplate reader (Tecan, Männedorf, Switzerland). Rabbit anti-human rotavirus Wa polyclonal Ab and Goat polyclonal to hepatitis A virus (HM175 strain; Abcam) were used as positive controls.
Virus neutralization assay
The neutralization Ab titer of rabbit anti-D2/VP7 polyclonal serum was evaluated using neutralization test. Briefly, monolayer cells of each MA104 or FRhK-4 cell line was completely prepared in 96 well plates. The rabbit serum against D2/VP7 was inactivated in 56℃ for 30 min and then serially diluted in culture medium for each cell as describe above by two fold from 1:10 to 1:640. 50 μl of 50% Tissue Culture Infective Dose (TCID50) of each rotavirus Wa strain or HAV HM175/18f strain was mixed with 50 μl of each serum dilution and incubated in 37℃ for 1 hr, respectively. After 1 hr incubation, 100 μl of the virus-antibody mixture was then inoculated onto the monolayer cells and incubated in 2 hr at 37℃, 5% CO2. Finally, plates were blotted and overlaid with 200 μl of culture medium and incubated at 37°C, 5% CO2 for five days. Result of 50% serum neutralized destination was calculated by Reed-Muench method (Reed, 1938).
RESULTS
Expression of the recombinant rotavirus proteins carrying two antigenic epitopes of the HAV polyprotein in Sf9 cells using a baculovirus expression system
The schematic diagrams of the two recombinant rotavirus proteins with the HAV antigenic epitopes are shown in Fig. 1A. The recombinant gene cassettes were confirmed by PCR, which produced approximately 1,215-bp and 1,149-bp sized amplicons, corresponding to D2/VP7 and D3/VP7, respectively (data not shown). DNA sequencing demonstrated that all recombinant gene cassettes were fused in frame with a short peptide linker of Leu-Glu-Pro-Gly (LEPG), due to the artificially incorporated restriction sites. As expected, the experimental expression of the D2/VP7 and D3/VP7 recombinant proteins using a baculovirus expression system in Sf9 cells demonstrated approximately 44.3 kDa and 41.9 kDa proteins on 10% SDS PAGE (Fig. 1B). Since all the recombinant proteins contained the C-terminal V5 epitope tag, the identity of those proteins was confirmed by Western blot using anti-V5 Ab. Two recombinant proteins were highly expressed in Sf9 insect cells, especially for D3/VP7 (Fig. 1C). These results imply that recombinant rotavirus proteins containing the D2 and D3 antigenic epitopes of the HAV polyprotein are expressed in Sf9 insect cells using a baculovirus expression system.
Kinetic of recombinant baculovirus synthesis in Sf9 cells
To examine the kinetic of the recombinant baculovirus synthesis in Sf9 cells (Roldão et al., 2007), recombinant baculoviruses of D2/VP7 were infected at an MOI of 10 pfu/cell, and its expression was monitored by Western blot using anti-V5 Ab. Expression of D2/VP7 from the infected Sf9 cells peaked at 2 days post-infection and gradually declined, resulting in no protein detection at 10 days post-infection (Fig. 2).
Fig. 2. Kinetics of recombinant baculovirus production of D2/VP7. Cells were harvested every 24 hrs during the experimental period of 10 days after the initial viral infection. Aliquots of infected Sf9 cells were clarified by low-speed centrifugation, lysed with RIPA buffer, and analyzed by Western blot. Lanes 1-10: Cells from 1 to 10 days post-infection.

Antigenicity of D2/VP7 and D3/VP7 against rotavirus and HAV
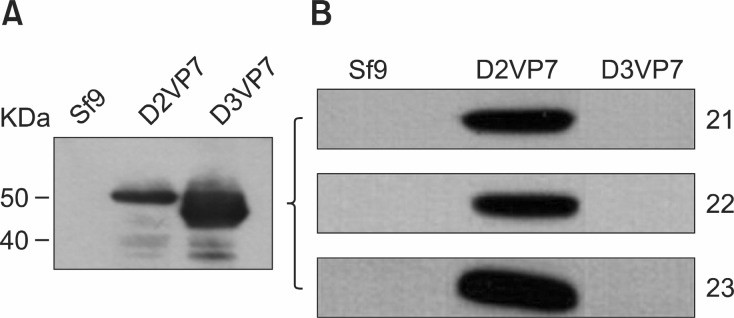
To examine whether or not our recombinant proteins are immunogenic, antisera obtained from rotavirus-infected rabbits and HAV-infected human patients were analyzed by Western blot. The results demonstrated that two recombinant proteins, D2/VP7 and D3/VP7, were able to react with the rabbit
antiserum against the rotavirus Wa strain (Fig. 3A). However, D2/VP7 reacted positively to antisera from 3 HAV-infected human patients, unlike D3/VP7 (Fig. 3B). These results imply that D2/VP7 is immunogenic against both rotavirus and HAV.
Fig. 3. Western blot analyses using anti-sera from either (A) rotavirus-infected rabbits or (B) HAV-infected human patients. The Western XP marker (Invitrogen, USA) is shown at the left. Numbers (21, 22 and 23) are patient serum numbers.
Induction of neutralizing antibodies and immunity in vaccinated rabbit
Since the recombinant protein D2/VP7 appears to be antigenic for both rotaviral and HAV specific antibodies, rabbits were immunized with D2/VP7, and the production of virus-specific antibodies using ELISA and neutralization assay were investigated. In this study, titers were defined as the highest dilution factor of the rabbit sera that gave a positive result. As expected, the ELISA results showed that D2/VP7 could produced the rotavirus-specific Abs (Fig. 4A) and the HAV Abs (Fig. 4B), compared to the positive control. The neutralizing Ab titers of rabbit anti-D2/VP7 polyclonal serum to the rotavirus and the HAV were 1:320 and 1:160, respectively. These results imply that D2/VP7 elicited potent neutralizing Ab responses against rotavirus and HAV in vivo and may be used as an experimental candidate of an immunogen to prevent both rotavirus and HAV infections.
Fig. 4. Production of the rotavirus-specific antibodies after experimental immunization of D2/VP7 against rabbits. Rabbit serum antibody response to rotavirus Wa strain was examined using ELISA. Rabbit serum antibody response to rotavirus Wa strain (A) and hepatitis a HM175 strain (B) was examined using ELISA (see Materials and Methods). The sera were serially diluted from 1:10 to 1:640 and analyzed at 492 nm. Negative rabbit serum (♦); Immunized rabbit serum (■); Rabbit serum against rotavirus Wa or goat anti HAV HM175 (▲). OD values are indicated at the right.
DISCUSSION
In this study, we constructed and expressed the two recombinant rotavirus proteins containing antigenic epitopes of the HAV polyprotein in Sf9 insect cells using a baculovirus expression system. Among them, D2/VP7 was able to induce rotavirus and HAV funtional antibodies in vivo, showing sufficient immunogenicity against both rotavirus and HAV. D3VP7 did not react with antisera from HAV-infected human patients could concluded that domain D3 epitope is not immune dominant in Korean populations.
Historically, most vaccines against viral diseases were developed based on either attenuated or inactivated live viruses. However, either unexpected failure to inactivate the virulent viral strain or genetic reversion of an attenuated vaccine strain to a more virulent strain can be a serious public health problem (Melnick, 1989; Plotkin, 2005). For such reasons, several recombinant subunit vaccines for viral infections have been developed and have provided successful and effective protection, even with a single viral protein or peptide in some cases. Nonetheless, those vaccines often required more doses at higher concentrations of antigen to achieve the same protective effect as an inactivated or attenuated viral vaccine, suggesting that they are not costeffective. The cost of vaccine is one of the most important considerations in developing novel vaccines, especially in veterinary fields (Rogan and Babiuk, 2005; Plotkin, 2008).
The incidence of hepatitis A and rotavirus infection are closely related to socioeconomic status and environmental conditions. A person who has infected those viruses can easily spread on to others via the fecal and oral route or fomites (Schlindwein et al., 2010). HAV is the most common cause of acute infectious hepatitis, which estimates 1.5 million of clinical cases every year (WHO, 2000). Currently, four inactivated vaccines against HAV are available worldwide. However, none of the vaccines are approved for children less than one year of age (Jiang et al., 1998; Martin and Lemon, 2006; Roldão et al., 2007).
Rotaviruses are considered the most important cause of severe viral gastroenteritis in humans and animals with high morbidity and mortality in developed and developing countries. Two rotavirus vaccines are currently available in the world market. RotaTeq®, a pentavalent human-bovine reassortment vaccine has been filed in more than 100 countries, including Australia, Canada, the European Union, Asia, and Latin America. The vaccine is recommended in a three-dose schedule at 2, 4, and 6 months of age. Rotarix®, a monovalent attenuated vaccine has been approved in 90 countries worldwide except in the United States and is routinely recommended as a two-dose schedule beginning at 6 weeks of age (Dennehy, 2008). Despite this potential for rotavirus vaccines to substantially reduce the risk of death from diarrhea, there are considerable challenges, such as the storage and shipment requirements, narrow window of administration, and high costs, to implementing their use in the poorest countries of the world (Santosham, 2010).
As part of national policies concerning immunization strategies for young children, HAV vaccine should be considered against the impact of other vaccine-preventable infections, such as hepatitis B, Haemophilus influenzae type b, measles, mumps, rubella, and varicella to reduce immunization program costs and increase compliance with published recommendations (Nolan et al., 2006; Beran, 2007). On the other hand, rotavirus is an important viral causes of microbial-related gastrointestinal disorders in human newborns and like reoviral infections may also cause neonatal hepatitis (Richardson et al., 1994). Looked at from that point of view of the logistics of vaccination programs and weakness of the current vaccines available, the chimeric protein D2/VP7 can be considered as a suitable multivalent vaccine candidate to prevent both rotavirus and HAV infections because of easier and more cost-effective production, and strong immunogenicity in vivo.
Based on the fact that recombinant baculoviruses have been widely used to produce xenogenic proteins in insect cells, we demonstrate that the recombinant baculovirus of D2/VP7 could be a model system to assemble rotavirus VP7 gene with other immunodominant viral epitopes in the future. Although it is generally accepted that protective immunity to virus is principally due to a neutralizing Ab. Further studies are needed to challenge test with D2/VP7 vaccinated animals for a blueprint for the protection and prevention of HAV and rotavirus infections.
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen 21 Program (No. PJ008325), Rural Development Administration, Republic of Korea.
References
- 1.Beran J. Bivalent inactivated hepatitis A and recombinant hepatitis B vaccine. Expert Rev. Vaccines. (2007);6:891–902. doi: 10.1586/14760584.6.6.891. [DOI] [PubMed] [Google Scholar]
- 2.Dennehy P. H. Rotavirus vaccines: an overview. Clin. Microbiol. Rev. (2008);21:198–208. doi: 10.1128/CMR.00029-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estes M. K. Cohen J. Rotavirus gene structure and function. Microbiol. Rev. (1989);53:410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gust I. D. Epidemiological patterns of hepatitis A in different parts of the word. Vaccine. (1992);10(Suppl 1):S56–58. doi: 10.1016/0264-410x(92)90544-t. [DOI] [PubMed] [Google Scholar]
- 5.Jiang B. Barniak V. Smith R. P. Sharma R. Corsaro B. Hu B. Madore H. P. Synthesis of rotavirus-like particles in insect cells: comparative and quantitative analysis. Biotechnol. Bioeng. (1998);60:369–374. doi: 10.1002/(sici)1097-0290(19981105)60:3<369::aid-bit14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 6.Khudyakov Y. E. Lopareva E. N. Jue D. L. Fang S. Spelbring J. Krawczynski K. Margolis H. S. Fields H. A. Antigenic epitopes of the hepatitis A virus polyprotein. Virology. (1999);260:260–272. doi: 10.1006/viro.1999.9813. [DOI] [PubMed] [Google Scholar]
- 7.King L. A. Hitchman R. Possee R. D. Recombinant baculovirus isolation. Methods Mol. Biol. (2007);388:77–94. doi: 10.1007/978-1-59745-457-5_4. [DOI] [PubMed] [Google Scholar]
- 8.Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. (1970);227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 9.Lee J. Babiuk L. A. Harland R. Gibbons E. Elazhary Y. Yoo D. Immunological response to recombinant VP8* subunit protein of bovine roravirus in pregnant cattle. J. Gen. Virol. (1995);76:2477–2483. doi: 10.1099/0022-1317-76-10-2477. [DOI] [PubMed] [Google Scholar]
- 10.Martin A. Lemon S. M. Hepatitis A virus: from discovery to vaccines. Hepatology. (2006);43(2 Suppl 1):S164–172. doi: 10.1002/hep.21052. [DOI] [PubMed] [Google Scholar]
- 11.Melnick J. L. Virus vaccines: principles and prospects. Bull. World Health Organ. (1989);67:105–112. [PMC free article] [PubMed] [Google Scholar]
- 12.Nolan T. Bernstein H. Blatter M. M. Bromberg K. Guerra F. Kennedy W. Pichichero M. Senders S. D. Trofa A. Collard A. Sullivan D. C. Descamps D. Immunogenicity and safety of an inactivated hepatitis A vaccine administered concomitantly with diphtheria-tetanus-acellular pertussis and haemophilus influenzae type B vaccines to children less than 2 years of age. Pediatrics. (2006);118:e602–609. doi: 10.1542/peds.2005-2755. [DOI] [PubMed] [Google Scholar]
- 13.Nwachuku N. Gerba C. P. Health risks of enteric viral infections in children. Rev. Environ. Contam. Toxicol. (2006);186:1–56. doi: 10.1007/0-387-32883-1_1. [DOI] [PubMed] [Google Scholar]
- 14.Offit P. A. Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J. Virol. (1986);57:376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parashar U. D. Hummelman E. G. Bresee J. S. Miller M. A. Glass R. I. Global illness and deaths caused by rotavirus disease in children. Emerg. Infect Dis. (2003);9:565–572. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penna A. Cahalan M. Western Blotting using the Invitrogen NuPage Novex Bis Tris minigels. J. Vis. Exp. (2007);(7):264. doi: 10.3791/264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plotkin S. A. Vaccines: past present and future. Nat. Med. (2005);11(4 Suppl):S5–11. doi: 10.1038/nm1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plotkin S. A. New vaccination strategies. Bull. Acad. Natl. Med. (2008);192:511–518. [PubMed] [Google Scholar]
- 19.Reed L. J. A simple method of estimating fifty percent endpoints. Am. J. Epidemiol. (1938);27:493–497. [Google Scholar]
- 20.Richardson S. C. Bishop R. F. Smith A. L. Reovirus serotype 3 infection in infants with extrahepatic biliary atresia or neonatal hepatitis. J. Gastroenterol. Hepatol. (1994);9:264–268. doi: 10.1111/j.1440-1746.1994.tb01721.x. [DOI] [PubMed] [Google Scholar]
- 21.Rogan D. Babiuk L. A. Novel vaccines from biotechnology. Rev. Sci. Tech. (2005);24:159–174. [PubMed] [Google Scholar]
- 22.Roldão A. Vieira H. L. Charpilienne A. Poncet D. Roy P. Carrondo M. J. Alves P. M. Oliveira R. Modeling rotavirus-like particles production in a baculovirus expression vector system: Infection kinetics baculovirus DNA replication mRNA synthesis and protein production. J. Biotechnol. (2007);128:875–894. doi: 10.1016/j.jbiotec.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Santosham M. Rotavirus vaccine-a powerful tool to combat deaths from diarrhea. N. Engl. J. Med. (2010);362:358–360. doi: 10.1056/NEJMe0912141. [DOI] [PubMed] [Google Scholar]
- 24.Schlindwein A. D. Rigotto C. Simões C. M. Barardi C. R. Detection of enteric viruses in sewage sludge and treated wastewater effluent. Water Sci. Technol. (2010);61:537–544. doi: 10.2166/wst.2010.845. [DOI] [PubMed] [Google Scholar]
- 25.Totsuka A. Moritsugu Y. Hepatitis A virus proteins. Inter-virology. (1999);42:63–68. doi: 10.1159/000024967. [DOI] [PubMed] [Google Scholar]
- 26.WHO. Hepatitis A vaccines. Wkly. Epidemiol. Rec. (2000);75:38–44. [PubMed] [Google Scholar]




