Abstract
The present study was undertaken to determine whether ethanol influences on the agonist-induced vascular smooth muscle contraction and, if so, to investigate the related mechanism. The measurement of isometric contractions using a computerized data acquisition system was combined with molecular experiments. Ethanol significantly inhibited thromboxane A2 mimetic-induced contraction with intact endothelial function, but there was no relaxation on thromboxane A2 mimetic U-46619-induced contraction irrespective of endothelium suggesting that the pathway such as Rho-kinase activation, Ca2+ entry or thin filament regulation was not affected. In addition, ethanol didn’t decrease thromboxane A2 mimetic-induced increase of phospho-myosin phosphatase targeting subunit protein 1 (pMYPT1) or pERK1/2. Interestingly, ethanol didn’t inhibit significantly phorbol ester-induced contraction in denuded muscles suggesting that thin filament regulation is less important on the ethanol-induced regulation in the muscle than endothelial NO synthesis. In conclusion, this study provides the evidence and possible related mechanism concerning the effect of ethanol on the agonist-dependent contraction in rat aortic rings with regard to endothelial function.
Keywords: Ethanol, Phorbol ester, Rho-kinase, Thromboxane A2 mimetic, Vasodilation
INTRODUCTION
Moderate alcohol consumption is associated with reduced risk of certain cardiovascular events (Stampfer et al., 1988). However, chronic ethanol consumption is associated with cardiovascular dysfunctions independent of other known risk factors (Altura and Altura, 1982). Enhanced vascular reactivity to vasoconstrictor agents or the impairment of vascular relaxation contributes to the cardiovascular complications associated with chronic ethanol consumption (Pinardi et al., 1992; Kähönen et al., 1999). However, the arteries of ethanol-treated rats have also displayed unchanged (Sahna et al., 2000; Tirapelli et al., 2007) or increased (Hatake et al., 1994) responses to vasodilators. The reasons for these differences are not entirely clear, but contributing factors may include differences in experimental design, ethanol administration protocols, the duration of chronic treatment with ethanol (Sahna et al., 2000), and the blood vessel studied (Tirapelli et al., 2007) controversially suggesting that chronic or high ethanol consumption is pro-hypertensive and acute or low ethanol consumption is somewhat protective.
It is generally accepted that the initiation of smooth muscle contractility is predominantly controlled by a Ca2+-dependent increase in myosin light chain 20 kDa (MLC20) phosphorylation (Somlyo and Somlyo, 1994). However, other pathways have now been described that may regulate the contractility of smooth muscle by regulating the phosphorylation of MLC20 independently of a rise in intracellular Ca2+ (Uehata et al., 1997; Somlyo and Somlyo, 1998;Sakurada et al., 2003). The phosphorylation of MLC20 promotes the interaction of actin and myosin II, and the contraction of smooth muscle. The degree of MLC20 phosphorylation or contraction does not always parallel the Ca2+ concentration. The extent of MLC20 phosphorylation or force of contraction induced by agonist stimulation is usually higher than that caused by an increase in the Ca2+ concentration, a finding explained by so-called Ca2+ sensitization (Somlyo and Somlyo, 1994). Thus, an additional mechanism of regulation that modulates the levels of MLC20 phosphorylation and degree of contraction has been proposed. Subsequent studies have revealed that inhibition of MLC phosphatase by Rho-kinase (Kitazawa et al., 1991) or thin filament regulation including activation of protein kinase C (PKC), mitogen-activated protein kinase kinases (MEK) and extracellular signal regulated kinase (ERK) 1/2, and phosphorylation of the actin binding protein caldesmon (Wier and Morgan, 2003) may be the major pathway in Ca2+ sensitization.
In various smooth muscles, thromboxane A2 mimetic has been shown to induce contraction, which may be due to enhancement of Ca2+ sensitivity. Thromboxane A2 mimetic has been known to induce contraction in blood vessel preparations leading to either thick or thin filament regulation. Thick or myosin filament regulation encompasses both Ca2+ activation and Ca2+ sensitization as it involves both activation of MLCK by Ca2+-calmodulin and regulation of MLCP activity. On the other hand, thin or actin filament regulation includes the possible disinhibition of actin-myosin interactions by phosphorylation of caldesmon, possibly by PKC, MEK or ERK that are translocated during their activation (Gerthoffer et al., 1996). It is possible that thromboxane A2 mimetic-induced contrac-tions involve the participation of the RhoA/Rho-kinase pathway (Tsai and Jiang, 2006). Therefore, the aim of the present study was to elucidate the possible role of thick filament regulation such as Rho-kinase inhibition or thin filament regulation on MEK inhibition in ethanol-induced controversial regulation of isolated rat aortae using RhoA/Rho-kinase activator such as thromboxane A2 mimetic or MEK activator such as phorbol ester.
MATERIALS AND METHODS
Tissue preparation
Male Sprague-Dawley rats were housed under standard laboratory conditions with free access to food and water. The housing conditions and experimental protocols were approved by the Institutional Animal Care and Use Committee and the approval number for animal protocol was approximately fifty. Male Sprague-Dawley rats, weighing 320-350 g, were anesthetized by sodium pentobarbital (50 mg/kg i.p.) followed by cervical dislocation, in agreement with procedures approved by the Institutional Animal Care and Use Committee. The thoracic aorta was quickly removed and immersed in oxygenated (95% O2/5% CO2) physiological saline solution composed of (mM): 115.0 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgCl2, 25.0 NaH-CO3, 1.2 KH2PO4, and 10.0 dextrose (pH 7.4). The aorta was cleaned of all adherent connective tissue, and the endothelium was removed by gentle abrasion with a cell scraper.
Contraction measurements
Sometimes care was taken to avoid rubbing the endothelial surface of the vessels that had intact endothelium. Two stainless-steel triangles were inserted through each vessel ring. Each aortic ring was suspended in a water-jacketed organ bath (10 ml) maintained at 37℃ and aerated with a mixture of 95% O2 and 5% CO2. One triangle was anchored to a stationary support, and the other was connected to an isometric force transducer (Grass FT03C, Quincy, Mass., USA). The rings were stretched passively by imposing the optimal resting tension, 2.0 g, which was maintained throughout the experiment. Each ring was equilibrated in the organ bath solution for 60 min before the experiment involving the contractile response to 50 mM KCl addition. Isometric contractions were recorded using a computerized data acquisition system (PowerLab/8SP, ADInstruments, Castle Hill, NSW, Australia).
After completion of the control KCl dose-response curve, vessel rings were pre-incubated with ethanol. Ethanol was applied 30 min before the addition of thromboxane A2 mimetic or phorbol ester. The absence of the endothelium was verified by the lack of relaxation after the addition of acetylcholine (1 μM) to precontracted ring segments.
Western blot analysis
Muscle strips were quick frozen by immersion in a dry ice/acetone slurry containing 10% trichloroacetic acid (TCA) and 10 mM dithiothreitol (DTT). Muscles were stored at -80℃ until used. Tissues were brought to room temperature in a dry ice/acetone/ TCA/DTT mixture. Then samples were homogenized in a buffer containing 20 mM mops, 4% SDS, 10% glycerol, 10 mM DTT, 20 mM β-glycerophosphate, 5.5 μM leupeptin, 5.5μM pepstatin, 20 KIU aprotinin, 2 mM Na3VO4, 1 mM NaF, 100μM ZnCl2, 20 μM 4-(2-aminoethyl) benzenesulphonyl fluoride (AEBSF) and 5 mM EGTA. Protein-matched samples (modified Lowry protein assay, DC Protein Assay Kit, Bio-Rad) were electrophoresed on SDS-PAGE (Protogel, National Diagnostics), transferred to PVDF membranes and subjected to immunostaining and densitometry, as above, using the appropriate antibody. The success of protein matching was confirmed by Naphthol Blue Black staining of the membrane and densitometry of the actin band. Any mismatch of lane loading was corrected by normalization to actin staining. Each set of samples from an individual experiment was run on the same gel and densitometry was performed on the same film.
Chemicals and antibodies
The drugs and chemicals were reagent grade unless otherwise indicated and obtained from the following source. U-46619, KCl, acetylcholine and nicardipine were purchased from Sigma Co. (St. Louis, MO, USA). DTT, TCA and acetone were obtained from Fisher Scientific (Hampton, NH, USA) and enhanced chemiluminescence (ECL) from Pierce (Rockford, IL, USA). The antibody against phospho-myosin phosphatase targeting subunit protein 1 (phospho-MYPT1) at Thr855 (1:1,000) or MYPT1 was purchased from Cell Signaling Technology (Danvers, MA, USA) or Upstate Biotechnology (Lake Placid, NY, USA) to check the level of RhoA/Rho-kinase activity (Wooldridge et al., 2004; Wilson et al., 2005) or MEK activity. Anti-mouse IgM (goat) and anti-rabbit IgG (goat), conjugated with horseradish peroxidase, were used as secondary antibodies (1:2,000, 1:2,000, respectively, Upstate, Lake Placid, NY). Acetylcholine was dissolved in deionized water.
Statistical analysis
The data were expressed as mean ± standard error of the mean (SEM). Student’s unpaired t test was used to determine the statistical significance of the means between two groups using SPSS 12.0 (SPSS Inc., Chicago, Illinois, USA). p values<0.05 were regarded as statistically significant.
RESULTS
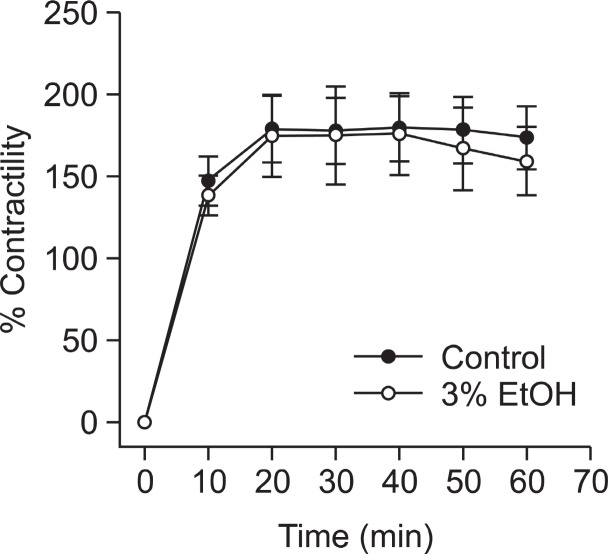
Effect of ethanol on phorbol ester-induced contraction of denuded aortae
The absence of the endothelium was verified by the lack of relaxation after the addition of acetylcholine (1 μM) to precontracted ring segments. Ethanol didn’t inhibit significantly phorbol ester-induced contraction regardless of endothelial function (Fig. 1) suggesting that thin filament regulation might
Fig. 1. Effect of pretreated ethanol on phorbol 1213-dibutyrate (PDBu)-induced vascular contraction in denuded ring. Ethanol was applied 30 min before the addition of phorbol ester. Developed tension is expressed as a percentage of the maximum contraction to 50 mM KCl. Data are expressed as means of 3-5 experiments with vertical bars showing SEM.
be of little importance.
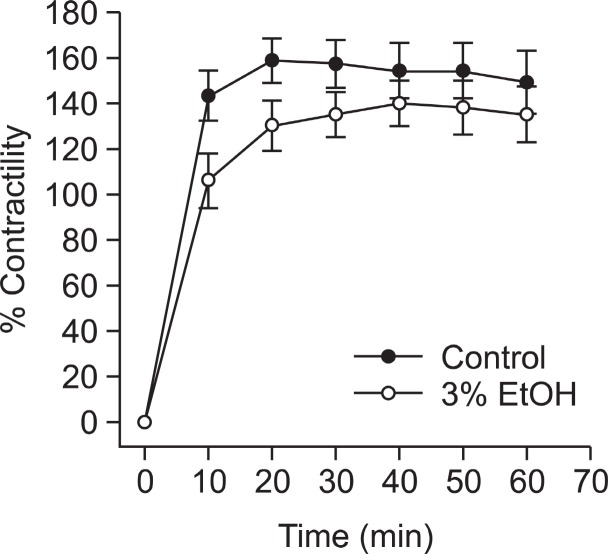
Effect of ethanol on thromboxane A2-induced contraction of denuded aortae
Ethanol didn’t inhibited Rho-kinase activator thromboxane A2-induced contraction without endothelial function (Fig. 2) suggesting that Rho-kinase might not be inhibited in the regulation of vascular contractility.
Fig. 2. Effect of pretreated ethanol on U46619-induced contraction in denuded muscle. Ethanol was applied 30 min before the addition of thromboxane A2 mimetic. Developed tension is expressed as a percentage of the maximum contraction to 50 mM KCl. Data are expressed as means of 3-5 experiments with vertical bars showing SEM.
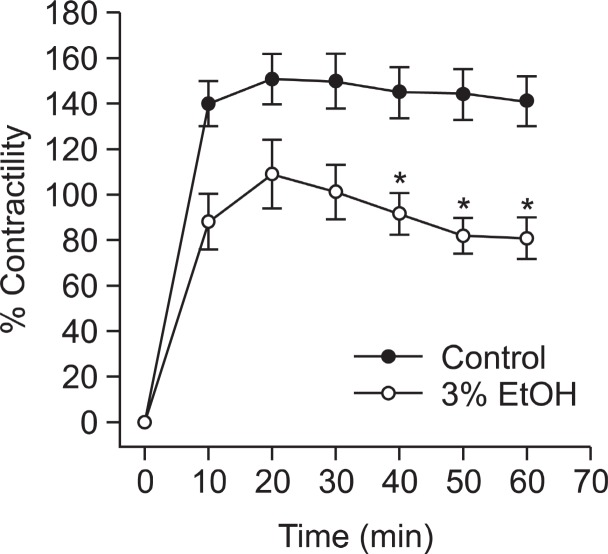
Effect of ethanol on thromboxane A2-induced contraction of intact aortae
Pretreated ethanol didn’t significantly inhibit Rho-kinase activator thromboxane A2 mimetic U-46619-induced contraction without endothelial function but it inhibited the contraction with endothelium (Fig. 3) suggesting that Rho-kinase activity might not be inhibited and other pathways than RhoA/Rho-kinase pathway might be involved in the thromboxane A2 mimetic U-46619-induced Ca2+ sensitization or the ethanol-induced Ca2+ desensitization such as Ca2+ entry or the phosphorylation of extracellular signal-regulated kinase (ERK), protein kinase C-potentiated inhibitory protein for protein phosphatase type 1 (CPI-17) or integrin-linked kinase (ILK) (Deng et al., 2001;
Fig. 3. Effect of pretreated ethanol on U46619-induced contraction in intact muscle. Ethanol was applied 30 min before the addition of thromboxane A2 mimetic. Developed tension is expressed as a percentage of the maximum contraction to 50 mM KCl. Data are expressed as means of 3-5 experiments with vertical bars showing SEM. *p<0.05, presence versus absence of ethanol.
Murányi et al., 2002).
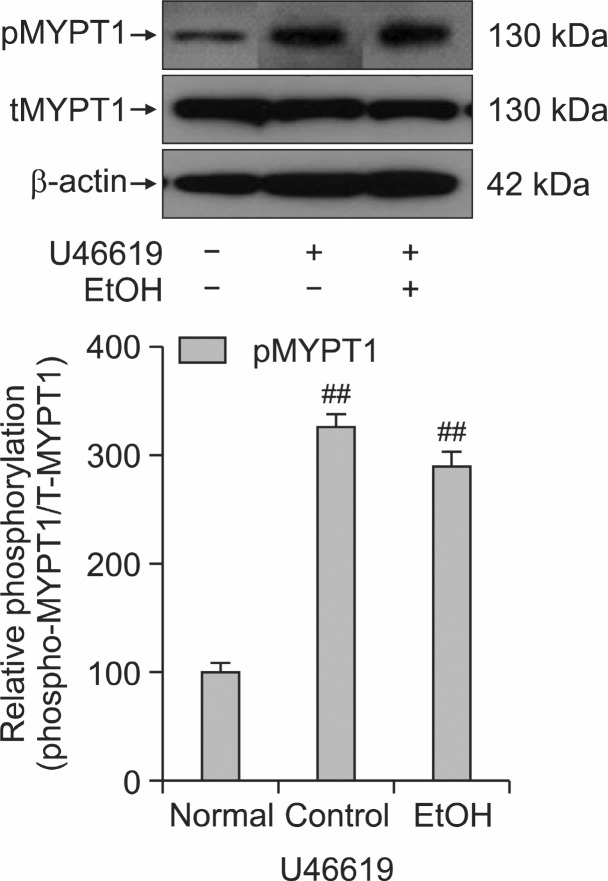
Effect of ethanol on the level of phospho-MYPT1 at Thr855 or phospho-ERK1/2
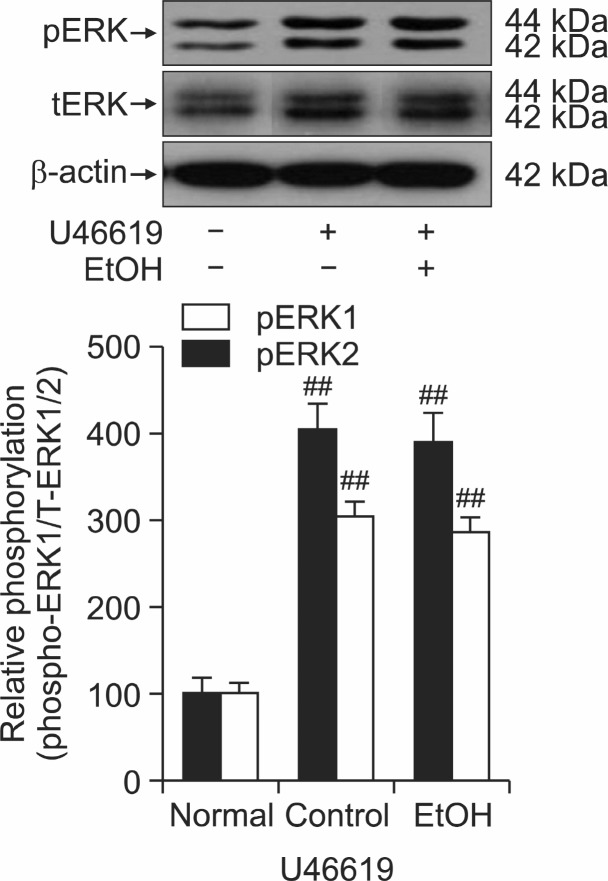
To confirm the role of ethanol in thick filament regulation of smooth muscle contractility, we measured the levels of myosin phosphatase targeting subunit protein 1 (MYPT1) and phospho-MYPT1 or ERK and phosphor-ERK in the muscles quick frozen after 30 min exposure to 0.1 μM U-46619. Interestingly, there was no significant decrease in the thromboxane A2-induced MYPT1 phosphorylation at Thr855 (Wilson et al., 2005; Tsai and Jiang, 2006) or ERK1/2 phosphorylation in quick frozen ethanol-treated rat aorta with endothelium compared to the vehicle treated rat aorta (Fig. 4, Fig. 5). Thus thick or myosin filament regulation including myosin phosphatase activation through RhoA/Rho-kinase or MEK inactivation might be rarely involved in decreased contractility in ethanol treated rat aorta.
Fig. 4. The phospho-MYPT1 protein levels in quick frozen ethanol-added rat aorta with endothelium compared to the vehicle-added rat aorta contracted with 0.1 μM U46619. Upper panel shows a typical blot and lower panel shows average densitometry results on the relative level of phospho-MYPT1/MYPT1. Data are expressed as means of 3-5 experiments with vertical bars showing SEM. ##p<0.01, presence versus absence of U46619. Normal, no addi-tion of U46619 or ethanol; Control, addition of only U46619.
Fig. 5. The phospho-ERK1/2 protein levels in quick frozen ethanol-added rat aorta with endothelium compared to the vehicle-added rat aorta contracted with 0.1 μM U46619. Upper panel shows a typical blot and lower panel shows average densitometry results on the relative level of phospho-ERK1/2/ERK1/2. Data are expressed as means of 3-5 experiments with vertical bars showing SEM. ##p<0.01, presence versus absence of U46619. Normal, no addition of U46619 or ethanol; Control, addition of only U46619.
DISCUSSION
Some epidemiological studies have suggested a cardioprotective association and a lower risk of heart disease or stroke among those with light-to-moderate alcohol intake (Stampfer et al., 1988). On the other hand, ingesting excessive amounts of alcohol within a short period of time (binge drinking) has been related to an increased risk of myocardial infarction, stroke, and atrial fibrillation (McElduff and Dobson, 1997). When the endothelium to a region is completely damaged leading to the metabolic disease such as diabetes, ethanol-treated arterioles in the region dilate because of the unknown mechanism. Before the endothelium is removed, ethanol to the previously pro-hypertensive tissue is transiently beneficial because the arterioles are dilated. It was reported that the effect of ethanol included vasodilation suggesting other pathways (Nicoletti et al., 2008). This study has investigated whether the inhibition of RhoA/Rho-kinase or MEK activity contributes to the ethanol-induced controversial relaxation in rat aortae contracted with a RhoA/Rho-kinase or MEK activator such as thromboxane A2 or phorbol ester.
Two mechanisms are postulated for the development of
contraction and myosin light chain 20 kDa (MLC20) phosphorylation in smooth muscle. The first mechanism is that elevation in [Ca2+]i activates a Ca2+-calmodulin-dependent myosin light chain kinase (MLCK), which in turn catalyzes phosphorylation of MLC20, leading to cross bridge cycling and contraction (Murphy, 1982). A decrease in the concentration of intracellular Ca2+ inactivates MLCK and allows MLC phosphatase to dephosphorylate MLC20, resulting in muscle relaxation. A second mechanism of vascular smooth muscle contraction involves Rho-kinase (Amano et al., 1996), whose activity is dependent on activation of the small GTP-binding protein RhoA. The RhoA/Rho-kinase pathway plays a key role in the Ca2+ sensitization of smooth muscle contraction, which is mainly induced by MLC phosphatase inhibition (Noda et al., 1995; Somlyo and Somlyo, 2000).
Phorbol ester is a classical Thr/Tyr kinase activator and is routinely included in extraction buffers to activate phosphorylation of proteins on Thr and Tyr residues by endogenous kinases including MEK or ERK (Fonseca et al., 2008; Han et al., 2010). On the other hand, previous studies examining the mechanisms of thromboxane A2 mimetic U46619-induced arterial contraction have reported variable findings with regard to the contraction related to Rho-kinase activation (Nobe and Paul, 2001; Tasaki et al., 2003; Wilson et al., 2005). Therefore, it was consistent with the possibility that the ethanol could decrease thromboxane A2 mimetic- or phorbol ester-induced contraction inhibiting kinases activity such as Rho-kinase or MEK activity.
The mechanisms by which Rho-kinase activation causes vascular contraction is an area of intense study, and several possibilities exist. For example, Rho-kinase phosphorylates myosin light chain phosphatase, resulting in decreased phos-phatase activity and a buildup of phosphorylated myosin light
chains (Somlyo and Somlyo, 2000; Pfitzer 2001). Rho-kinase has also been demonstrated to directly phosphorylate myosin light chains independently of myosin light chain kinase and phosphatase activity (Amano et al., 1996). Recently, a role for Rho-kinase in vascular contraction evoked by thromboxane A2 was established (Wilson et al., 2005; Tsai and Jiang, 2006). Because thromboxane A2-induced contraction was not comparably blocked by the ethanol, it seems likely that under our experimental conditions, Rho-kinase or MEK inactivation may not be the main pathway in the ethanol-induced relaxation (Fig. 1, Fig. 2).
The main finding of the present study is that ethanol significantly inhibit thromboxane A2-induced contraction with endothelial function (Fig. 2, Fig. 3), and ethanol didn’t decrease thromboxane A2-induced vasoconstriction without endothelial function compared to contraction in intact muscles (Fig. 3) suggesting some difference between the mechanisms in intact and denuded muscles. Therefore, we postulated that other pathways than RhoA/Rho-kinase pathway might be involved in thromboxane A2 mimetic U-46619-induced Ca2+ sensitization suggesting that ethanol might not inhibit Ca2+ entry (Davis et al., 2001; Low, 1996) or the phosphorylation of extracellular signal-regulated kinase (ERK), protein kinase C-potentiated inhibitory protein for protein phosphatase (CPI-17) or integrin-linked kinase (ILK) (Deng, et al., 2001; Murányi, et al., 2002). Furthermore, ethanol didn’t decrease thromboxane A2-induced phosphorylation of MYPT1 at Thr855 (Fig. 4) or ERK1/2(Fig. 5) suggesting no inhibition of Rho-kinase or MEK activity. Therefore, suffice it to say that Rho-kinase or MEK inhibition is not the major mechanism in ethanol-induced vasorelaxation at least in the intact and thromboxane A2-contracted muscle, and the above-mentioned other pathways than RhoA/Rho-kinase pathway might be involved in thromboxane A2 mimetic-induced Ca2+ sensitization.
In summary, our results indicate that the ethanol comparably attenuated not without endothelium but with endothelium in rat aortic rings contracted with thromboxane A2 suggesting the endothelial NO synthesis is more important than the inhibition of additional Ca2+ entry, the phosphorylation of MEK/ERK or RhoA/Rho-kinase pathway in the thromboxane A2-induced contraction or the ethanol-induced relaxation.
References
- 1.Altura B. M. Altura B. T. Microvascular and vascular smooth muscle actions of ethanol, acetaldehyde, and acetate. Fed. Proc. (1982);41:2447–2451. [PubMed] [Google Scholar]
- 2.Amano M. Ito M. Kimura K. Fukata Y. Chihara K. Nakano T. Matsuura Y. Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. (1996);271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 3.Davis M. J. Wu X. Nurkiewicz T. R. Kawasaki J. Gui P. Hill M. A. Wilson E. Regulation of ion channels by protein tyrosine phosphorylation. Am. J. Physiol. Heart Circ. Physiol. (2001);281:H1835–1862. doi: 10.1152/ajpheart.2001.281.5.H1835. [DOI] [PubMed] [Google Scholar]
- 4.Deng J. T. Van Lierop J. E. Sutherland C. Walsh M. P. Ca2+-independent smooth muscle contraction. a novel function for integrin-linked kinase. J. Biol. Chem. (2001);276:16365–16373. doi: 10.1074/jbc.M011634200. [DOI] [PubMed] [Google Scholar]
- 5.Fonseca B. D. Lee V. H. Proud C. G. The binding of PRAS40 to 14-3-3 proteins is not required for activation of mTORC1 signalling by phorbol esters/ERK. Biochem. J. (2008);411:141–149. doi: 10.1042/BJ20071001. [DOI] [PubMed] [Google Scholar]
- 6.Gerthoffer W. T. Yamboliev I. A. Shearer M. Pohl J. Haynes R. Dang S. Sato K. Sellers J. R. Activation of MAP kinases and phosphorylation of caldesmon in canine colonic smooth muscle. J. Physiol. (1996);495:597–609. doi: 10.1113/jphysiol.1996.sp021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han H. Du B. Pan X. Liu J. Zhao Q. Lian X. Qian M. Liu M. CADPE inhibits PMA-stimulated gastric carcinoma cell invasion and matrix metalloproteinase-9 expression by FAK/MEK/ERK-mediated AP-1 activation. Mol. Cancer Res. (2010);8:1477–1488. doi: 10.1158/1541-7786.MCR-10-0114. [DOI] [PubMed] [Google Scholar]
- 8.Hatake K. Wakabayashi I. Taniguchi T. Hishida S. Increased endothelium-dependent vascular relaxation in ethanol-fed rats. Alcohol. Clin. Exp. Res. (1994);18:1018–1023. doi: 10.1111/j.1530-0277.1994.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 9.Kähönen M. Karjala K. Hutri-Kähönen N. Wu X. Jaatinen P. Riihioja P. Hervonen A. Pörsti I. Influence of chronic ethanol consumption on arterial tone in young and aged rats. Am. J. Physiol. (1999);276:H464–471. doi: 10.1152/ajpheart.1999.276.2.H464. [DOI] [PubMed] [Google Scholar]
- 10.Kitazawa T. Masuo M. Somlyo A. P. G protein-mediated inhibition of myosin light-chain phosphatase in vascular smooth muscle. Proc. Natl. Acad. Sci. USA. (1991);88:9307–9310. doi: 10.1073/pnas.88.20.9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Low A. M. Role of tyrosine kinase on Ca2+ entry and refilling of agonist-sensitive Ca2+ stores in vascular smooth muscles. Can. J. Physiol. Pharmacol. (1996);74:298–304. [PubMed] [Google Scholar]
- 12.McElduff P. Dobson A. J. How much alcohol and how of-ten? Population based case-control study of alcohol consumption and risk of a major coronary event. BMJ. (1997);314:1159–1164. doi: 10.1136/bmj.314.7088.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murányi A. MacDonald J. A. Deng J. T. Wilson D. P. Haystead T. A. Walsh M. P. Erdodi F. Kiss E. Wu Y. Hartshorne D. J. Phosphorylation of the myosin phosphatase target subunit by integrin-linked kinase. Biochem. J. (2002);366:211–216. doi: 10.1042/BJ20020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy R. A. Myosin phosphorylation and crossbridge regulation in arterial smooth muscle. State-or-the-art review. Hyperten-sion. (1982);4:3–7. [PubMed] [Google Scholar]
- 15.Nicoletti P. Trevisani M. Manconi M. Gatti R. De Siena G. Zagli G. Benemei S. Capone J. A. Geppetti P. Pini L. A. Ethanol causes neurogenic vasodilation by TRPV1 activation and CGRP release in the trigeminovascular system of the guinea pig. Cephalalgia. (2008);28:9–17. doi: 10.1111/j.1468-2982.2007.01448.x. [DOI] [PubMed] [Google Scholar]
- 16.Nobe K. Paul R. J. Distinct pathways of Ca2+ sensitiza-tion in porcine coronary artery: effects of Rho-related kinase and protein kinase C inhibition on force and intracellular Ca2+. Circ. Res. (2001);88:1283–1290. doi: 10.1161/hh1201.092035. [DOI] [PubMed] [Google Scholar]
- 17.Noda M. Yasuda-Fukazawa C. Moriishi K. Kato T. Okuda T. Kurokawa K. Takuwa Y. Involvement of rho in GTP gamma S-induced enhancement of phosphorylation of 20 kDa myosin light chain in vascular smooth muscle cells: inhibition of phosphatase activity. FEBS Lett. (1995);367:246–250. doi: 10.1016/0014-5793(95)00573-r. [DOI] [PubMed] [Google Scholar]
- 18.Pfitzer G. Invited review: regulation of myosin phosphorylation in smooth muscle. J. Appl. Physiol. (2001);91:497–503. doi: 10.1152/jappl.2001.91.1.497. [DOI] [PubMed] [Google Scholar]
- 19.Pinardi G. Brieva C. Vinet R. Penna M. Effects of chronic ethanol consumption on alpha-adrenergic-induced contractions in rat thoracic aorta. Gen. Pharmacol. (1992);23:245–248. doi: 10.1016/0306-3623(92)90019-g. [DOI] [PubMed] [Google Scholar]
- 20.Sahna E. Kurcer Z. Ozturk F. Cengiz N. Vardi N. Birincioglu M. Olmez E. Effects of chronic ethanol consumption on alpha-adrenergic-induced contractions and endothelium-dependent relaxations in rat thoracic aorta. Pharmacol. Res. (2000);41:629–633. doi: 10.1006/phrs.1999.0629. [DOI] [PubMed] [Google Scholar]
- 21.Sakurada S. Takuwa N. Sugimoto N. Wang Y. Seto M. Sasaki Y. Takuwa Y. Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ. Res. (2003);93:548–556. doi: 10.1161/01.RES.0000090998.08629.60. [DOI] [PubMed] [Google Scholar]
- 22.Somlyo A. P. Somlyo A. V. From pharmacomechanical coupling to G-proteins and myosin phosphatase. Acta. Physiol. Scand. (1998);164:437–448. doi: 10.1046/j.1365-201X.1998.00454.x. [DOI] [PubMed] [Google Scholar]
- 23.Somlyo A. P. Somlyo A. V. Signal transduction and regulation in smooth muscle. Nature. (1994);372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- 24.Somlyo A. P. Somlyo A. V. Signal transduction by G-proteins rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J. Physiol. (2000);522:177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stampfer M. J. Colditz G. A. Willett W. C. Speizer F. E. Hennekens C. H. A prospective study of moderate alcohol consumption and the risk of coronary disease and stroke in women. N. Engl. J. Med. (1988);319:267–273. doi: 10.1056/NEJM198808043190503. [DOI] [PubMed] [Google Scholar]
- 26.Tasaki K. Hori M. Ozaki H. Karaki H. Wakabayashi I. Difference in signal transduction mechanisms involved in 5-hydroxytryptamine-and U46619-induced vasoconstrictions. J. Smooth Muscle Res. (2003);39:107–117. doi: 10.1540/jsmr.39.107. [DOI] [PubMed] [Google Scholar]
- 27.Tirapelli C. R. Leone A. F. Coelho E. B. Resstel L. B. Corrêa F.M. Lanchote V. L. Uyemura S. A. Padovan C. M. Olivei-ra A. M. Effect of ethanol consumption on blood pressure and rat mesenteric arterial bed, aorta and carotid responsiveness. J. Pharm. Pharmacol. (2007);59:985–993. doi: 10.1211/jpp.59.7.0011. [DOI] [PubMed] [Google Scholar]
- 28.Tsai M. H. Jiang M. J. Rho-kinase-mediated regulation of receptor-agonist-stimulated smooth muscle contraction. Pflugers Arch. (2006);453:223–232. doi: 10.1007/s00424-006-0133-y. [DOI] [PubMed] [Google Scholar]
- 29.Uehata M. Ishizaki T. Satoh H. Ono T. Kawahara T. Morishita T. Tamakawa H. Yamagami K. Inui J. Maekawa M. Naru-miya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. (1997);389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 30.Wier W. G. Morgan K. G. Alpha1-adrenergic signaling mechanisms in contraction of resistance arteries. Rev. Physiol. Biochem. Pharmacol. (2003);150:91–139. doi: 10.1007/s10254-003-0019-8. [DOI] [PubMed] [Google Scholar]
- 31.Wilson D. P. Susnjar M. Kiss E. Sutherland C. Walsh M. P. Thromboxane A2-induced contraction of rat caudal arterial smooth muscle involves activation of Ca2+ entry and Ca2+ sensitization: Rho-associated kinase-mediated phosphorylation of MYPT1 at Thr-855, but not Thr-697. Biochem. J. (2005);389:763–774. doi: 10.1042/BJ20050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wooldridge A. A. MacDonald J. A. Erdodi F. Ma C. Borman M. A. Hartshorne D. J. Haystead T. A. Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of Serine 695 in response to cyclic nucleotides. J. Biol. Chem. (2004);279:34496–34504. doi: 10.1074/jbc.M405957200. [DOI] [PubMed] [Google Scholar]