Abstract
Background. Metabolomics studies can quantitatively detect the dynamic metabolic response of living systems. Objective. To detect urinary metabolomics after hepatic ischemia/reperfusion (I/R) injury induced by the Pringle maneuver using gas chromatography-mass spectrometry (GC-MS). Methods. Male Sprague-Dawley rats (N = 80) were randomly divided into 4 groups (n = 20/group): sham operation, day 1, day 3, and day 5. Rats in the day 1, day 3, and day 5 groups underwent the Pringle maneuver. Serum alanine transaminase (ALT) and total bilirubin (TBIL) were measured, and hematoxylin and eosin (HE) staining of the liver tissue was performed. GC-MS was used to detect urinary metabolomics. Results. Compared with the sham group, the serum ALT and TBIL levels at day 1 were significantly elevated (P < 0.01) and then decreased and reached close to normal levels at day 5. GC-MS detected 7 metabolites which had similar changes as those of liver tissue revealed by histological examination. Significant differences in lactic acid, pyruvic acid, alanine, serine, and glycerol-3-phosphate were found among the groups (P < 0.001). Principle component analysis showed that 7 metabolites distinguished the day 1 and day 3 groups from the sham group. Conclusions. Noninvasive urinary metabolomic analysis is a potential means for the early detection and diagnosis of hepatic I/R injury.
1. Introduction
The Pringle maneuver is a procedure that is frequently adopted in liver transplantation and liver resection [1, 2]. It causes hepatic ischemia/reperfusion (I/R) injury [3], which contributes to liver damage and multiple organ failure [2, 4–6]. Conventional monitoring approaches can only reflect hepatic changes within several days or even several weeks of injury, and they fail to reflect the instantaneous changes of the liver after hepatic I/R. For example, the specificity and sensitivity of current enzymatic assays of serum alanine transaminase (ALT) or lactate dehydrogenase (LDH) are not high enough to detect early stages of liver parenchymal injury, assess the magnitude of parenchymal cell necrosis, or to predict hepatic recovery. There is a need to explore novel sensitive biomarkers that can detect early stages of liver parenchymal cell injury so that hepatic I/R injury can be identified and managed at an early stage.
Previous study has tried to identify novel biomarkers of acute hepatic I/R injury using dual-platform proteomic/degradomic approaches, and it was found that hepatic proteins, including argininosuccinate synthase (ASS) and estrogen sulfotransferase (EST-1), were degraded in the liver and rapidly released into circulation during I/R injury [7]. As novel sensitive and specific biomarkers of acute liver ischemic injury with potential clinical application, ASS and EST-1 have attracted extensive attention, and an ELISA kit for the detection of ASS has been developed.
Proteomics studies the overall expression of proteins, and metabolomics characterizes and quantifies their end products, that is, the entire metabolite pool that exists within a cell, tissue, or biofluid under certain conditions [8]. The study of metabolomics is able to quantitatively detect the dynamic metabolic response of living systems under pathophysiological conditions [9]. Metabolomics studies mainly adopt two types of techniques, traditional nuclear magnetic resonance spectroscopy, and newly developed mass spectrometry and chromatography. The metabolomics approach has been applied to study renal I/R injury. In 2005, Serkova et al. [10] used nuclear magnetic resonance spectroscopy to study metabolomics after renal transplant-induced renal I/R injury and found a significantly decreased level of polyunsaturated fatty acids (PUFAs) and elevated levels of allantoin in the renal tissue and increased trimethylamine N-oxide (TMAO) and allantoin levels in blood after ischemic injury. A high-performance liquid chromatography coupled with mass spectrometry-based metabolomics approach was recently used to explore serum changes after I/R-induced kidney injury and the protective effect of L-carnitine on acute kidney injury [11].
Currently, gas chromatography-mass spectrometry (GC-MS) is used to detect a variety of inherited metabolic disorders. Urine samples are convenient to collect, and urease pretreatment-gas chromatography-mass spectrometry (UP-GC-MS) can detect as many as 175 metabolites including amino acids, organic acids, monosaccharides, disaccharides, sugar alcohols, porphyrins, pyrimidine, and nucleic acids via a single injection of sample. In addition, plasma levels of volatile organic acids are not stable, and the MS-MS approach may provide false-negative results. GC-MS can detect the urinary metabolites of volatile acids, thereby indirectly reflecting blood levels. In addition, GC-MS has been used to study urinary metabolomics for the prediction of gastric cancer metastasis [12] and for the discovery of potential cancer biomarkers and early-stage biomarkers for diabetic kidney disease [13, 14]. However, a metabolomic approach has not been used to study hepatic I/R injury. This study aimed to detect urinary metabolomics that can reflect early-stage hepatic I/R injury using an UP-GC-MS approach.
2. Methods
2.1. Animals and Grouping
Male SPF-grade Sprague-Dawley rats weighing 220–250 g (N = 80) were used in this study. This study was approved by the Institutional Review Boards (IRBs) of Nanfang Medical University, and all animals were treated in accordance with the Declaration of Helsinki. Rats were randomly divided into 4 groups (n = 20 per group): a sham operation group and days 1, 3, and 5 after Pringle maneuver groups. Rats in the days 1, 3, and 5 groups underwent a Pringle maneuver, and specimens were collected on days 1, 3, and 5 afterwards. The Pringle maneuver was performed as follows. After anesthesia and skin disinfection, laparotomy was performed. The lower edge of the liver was dissociated, and the hepatic artery, portal vein, and common bile duct were located and clipped by vascular clips for 30 minutes. The vascular clips were then removed, and the abdomen was closed. In the sham group, rats underwent laparotomy, and the surgical field was covered by warm saline-soaked gauze for 30 minutes following the closure of the abdomen.
2.2. Sample Collection
Samples were collected from the rats in the sham group and days 1, 3, and 5 groups on day 0, 1, 3, and 5, respectively. Rats were placed in metabolic cages to collect urine samples, and approximately 4 mL of urine per rat was obtained. The collected urine samples were stored in a refrigerator at −20°C, and urine sample detection was completed within one week after sample collection. Twelve hours after urine sample collection, the rats underwent laparotomy, and approximately 3 mL of venous blood was collected from the inferior vena cava. The blood sample was centrifuged at 3500 rpm for 15 minutes, and the serum was collected. The samples were stored in a refrigerator at −70°C and analyzed within 2 days of collection. The rats were killed after venous blood samples were collected, and the left lobe of the liver was excised and rinsed with 0.9% NaCl solution. The specimen was fixed in 10% neutral formalin for 48 hours, and then sectioning and hematoxylin and eosin (HE) staining were performed.
2.3. HE Staining
Fixed liver tissue was dehydrated with ethanol, and then the tissue was embedded in paraffin. Tissue blocks were cut into 5 μm slices, dewaxed with xylene, subjected to gradient alcohol hydration, and then HE-stained. The samples were cleared in xylene and mounted in neutral gum for observation under a light microscope (type DX51, Olympus, Japan).
2.4. Detection of Serum Biomarkers
Serum ALT and total bilirubin (TBIL) levels were measured at the Department of Laboratory, Nanfang Hospital, using an automatic biochemical analyzer (AU5400, Japan).
2.5. GC/MS
A gas chromatograph/mass spectrometer (type Q1000, Japan Electronics Co., Japan) was used for urine sample detection. Urine samples were thawed at room temperature and homogenized using a vortex. Urease was added to remove urea, and then an internal quality standard heptadecanoic acid was added. After adding anhydrous ethanol, the samples were mixed well and centrifuged to remove protein. The supernatant was vacuum-dried, and the residues were derivatized with N,O-bis-trimethylsilyl-trifluoroacetamide (BSTFA)-trimethylchlorosilane (TMCS) in a constant temperature drying oven at 90°C for 1 hour. Nonsplit stream sampling was used, and sample injection volume was 1 μL. The sample was injected into a small volume sample injection vessel for GC-MS analysis, and the conditions were as follows. The temperature of sample injection was 260°C. The solvent was delayed for 2.5 minutes. The temperature was initialized at 60°C for 2 minutes and then gradually raised to 220°C at a rate of 17°C/min and further raised to 325°C at a rate of 15°C/min. The temperature was kept at 325°C for 10 minutes. The interface temperature was 220°C, and the ion source temperature was 200°C. Ionization mode was EI, ionizing voltage was 70 eV, and the quadrupole temperature was 150°C. Helium was used as the carrier gas with a flow rate of 1 mL/min. Scanning mode was full-scan mode, and the mass spectrometry scan range frequency was 50–650 m/z.
2.6. Statistical Analysis
Data were presented as median (interquartile range) and tested using the Kruskal-Wallis (K-W) test. Once a significant result was revealed by the K-W test, the Mann-Whitney U test was used for post hoc pair wise comparisons. Principle component analysis (PCA) was implemented to verify the distribution of variables for study groups, as well as confirm the time trend of variable changes. The significance level α was set at 0.05. When multiple comparisons were needed, the significance level was adjusted to 0.01. All analyses were performed using SAS statistical software (version 9.1.3, SAS Inc., Cathy, NY, USA).
3. Results
3.1. HE Staining
Typical images of HE-stained liver tissues are shown in Figure 1. The liver tissues in different groups were scored according to the liver damage evaluation criteria reported in the literature [15]. In the sham group, the liver sinusoids were normal, and no congestion was found (0 points). The liver cells had a cord-like arrangement and clear boundaries. No obvious liver cell necrosis was noticed (0 points). A very small amount of vacuolization was observed (1 point). The total score of the sham group was 1 point. In the day 1 group, a small amount of congestion was present in the hepatic sinusoids (1 point), and a moderate amount of vacuolization was observed (3 points). The nuclei of the liver cells were darkly stained and pyknotic, the cytoplasm was lightly stained, and the liver cells were swollen, compressing the expanded hepatic sinusoids. Approximately 30% of the cells had signs of necrosis (2 points). The total score of the day 1 group was 6 points. In the day 3 group, the liver sinusoids were obviously dilated, which were surrounded by more normal liver cells. However, necrosis of sporadic liver cells was seen (1 point). Some vacuoles were observed (2 points); congestion was present (1 point), and it could be seen that damaged cells were in recovery. The total score of the day 3 group was 4 points. In the day 5 group, the liver sinusoidal expansion was normal, and the liver cell arrangement was cord-like, but a small amount of congestion was still present (1 point). Generally, the liver cells were normal appearing, and necrosis of sporadic liver cells was seen (1 point). A very small number of vacuoles were seen (1 point). The total score of the day 5 group was 3 points.
Figure 1.
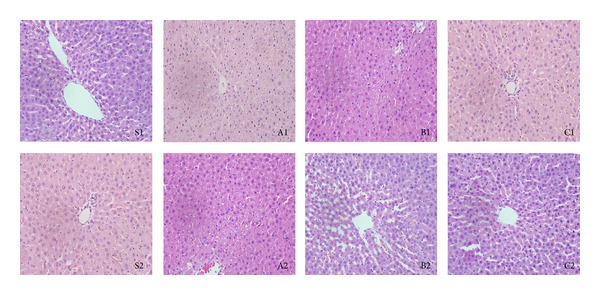
Hematoxylin and eosin staining results by group. S1 and S2, sham group; A1 and A2, day 1 group; B1 and B2, day 3 group; C1 and C2, day 5 group. In the sham group, the liver sinusoids were normal, and no congestion was found (0 point). S1 and S2 indicate cord-like arrangement of liver cells, no obvious liver cell necrosis, and a very small amount of vacuolization. A1 and A2 show a small amount of congestion in the hepatic sinusoids, moderate amount of vacuolization, darkly stained and pyknotic nuclei of the liver cells, lightly stained cytoplasm, and swollen liver cells compressing the expanded hepatic sinusoids. B1 and B2 show that the injury was alleviated; the liver sinusoids were dilated, and some vacuoles were observed, which were surrounded by more normal liver cells. C1 and C2 show normal liver sinusoidal expansion, cord-like liver cell arrangement, basically normal liver cells, and no obvious congestion.
3.2. Serum Levels of ALT and TBIL
Table 1 shows the serum levels of ALT and TBIL in the groups. Compared with the sham group, the serum ALT and TBIL levels in the day 1 group were significantly elevated (P < 0.01). The serum ALT and TBIL levels in the day 3 group were significantly decreased compared with the day 1 group (P < 0.01), but their levels were still higher than that in the sham group (P < 0.01). Serum ALT and TBIL levels in the day 5 group were significantly decreased as compared to the day 1 and day 3 groups (P < 0.01), and levels in the day 5 group were not significantly different from that in the sham group.
Table 1.
Comparisons of serum ALT and TBIL levels by group.
| Sham (n = 20) | Day 1 (n = 20) | Day 3 (n = 20) | Day 5 (n = 20) | P value1 | |
|---|---|---|---|---|---|
| ALT (IU/L) | 46.01 (45.40, 47.60) | 138.41 (123.47, 147.87)† | 59.75 (48.29, 64.44)†, ‡ | 43.59 (39.82, 46.74)‡,¶ | 0.003* |
| TBIL (IU/L) | 0.83 (0.75, 0.87) | 3.16 (2.96, 3.35)† | 1.66 (1.48, 1.80)†, ‡ | 0.84 (0.77, 0.93)‡,¶ | <0.001* |
ALT: alanine transaminase; TBIL: total bilirubin.
1Data were presented as median (interquartile range) and tested by Kruskal-Wallis test; Mann-Whitney U test was used for post hoc tests.
*indicates significant difference among groups, P < 0.05.
†indicates significant difference from sham group, P < 0.01.
‡indicates significant difference from day 1 group, P < 0.01.
¶indicates significant difference from day 3 group, P < 0.01.
3.3. Urinary Metabolites in the Groups
GC-MS detected 170 metabolites in the urine samples, and values of 163 were 0. The remaining 7 metabolites were detected in all the rats included in this study. Table 2 shows the levels of the 7 urinary metabolites with meaningful results (nonzero) including lactic acid, pyruvic acid, alanine, serine, threonine, glycerol-3-phosphate, and oleic acid. Significant differences in lactic acid, pyruvic acid, alanine, serine, and glycerol-3-phosphate were found among the groups (P < 0.001). Lactic acid levels were increased significantly in the day 1 group compared with the sham group (P < 0.001), declined in the day 3 group (P < 0.001), and returned to a level that was not significantly different from the sham group at day 5. Pyruvic acid levels were increased significantly in the day 1 group compared with the sham group (P < 0.001), reached a peak at day 3, and returned to zero at day 5. Alanine levels were decreased significantly in the day 1 group compared with the sham group (P < 0.001), increased in the day 3 group, and in the day 5 group were not significantly different from the sham group. Serine levels were decreased significantly in the day 1 group compared with the sham group (P < 0.001) and returned to a level that was not significantly different from that in the sham group at day 3. Glycerol-3-phosphate levels were increased significantly in the day 1 group compared with the sham group (P < 0.001), decreased in the day 3 group (P < 0.001), and returned to a level that was not significantly different from the sham group at day 5.
Table 2.
Comparisons of meaningful urinary metabolites detected by GC-MS by group.
| Sham (n = 20) | Day 1 (n = 20) | Day 3 (n = 20) | Day 5 (n = 20) | P value2 | |
|---|---|---|---|---|---|
| Lactic acid (mg/dL) | 0.80 (0.76, 0.86) | 2.91 (2.55, 3.14)† | 1.60 (0.97, 1.94)†, ‡ | 0.71 (0.22, 0.92)‡,¶ | <0.001* |
| Pyruvic acid (mg/dL)1 | 0.13 (0, 0.26) | 0.14 (0.14, 0.14)† | 1.67 (1.67, 1.71)†, ‡ | 0 (0, 0)‡,¶ | <0.001* |
| Alanine (mg/dL) | 0.48 (0.45, 0.49) | 0.04 (0.02, 0.07)† | 0.15 (0.09, 0.25)†, ‡ | 0.43 (0.37, 0.52)‡,¶ | <0.001* |
| Serine (mg/dL) | 0.20 (0.19, 0.24) | 0.09 (0.05, 0.11)† | 0.15 (0.09, 0.21)‡ | 0.22 (0.15, 0.28)‡ | <0.001* |
| Threonine (mg/dL) | 0.11 (0.04, 0.15) | 0.06 (0.01, 0.11) | 0.11 (0.04, 0.13) | 0.1 (0.04, 0.14) | 0.311 |
| Glyceraldehyde 3-phosphate (mg/dL) | 0.02 (0, 0.06) | 0.12 (0.08, 0.16)† | 0.06 (0.05, 0.08)†, ‡ | 0.03 (0.01, 0.06)‡,¶ | <0.001* |
| Oleic acid1 (mg/dL) | 3.00 (0.30, 7.25) | 2.32 (0.70, 4.70) | 4.63 (1.05, 6.65) | 4.2 (1.1, 7.58) | 0.507 |
1Original value was too small to be meaningful in hundredth. Values reported in table were magnified through multiplying by 106 for pyruvic acid and by 103 by oleic acid.
2Data were presented as median (interquartile range) and tested by Kruskal-Wallis test; Mann-Whitney U test was used for post hoc tests.
*indicates significant difference among groups, P < 0.05.
†indicates significant difference from sham group, P < 0.01.
‡indicates significant difference from day 1 group, P < 0.01.
¶indicates significant difference from day 3 group, P < 0.01.
Post hoc pair wise comparisons revealed that the levels of five urinary metabolites among the seven detected urinary metabolites including lactic acid, pyruvic acid, alanine, serine, and glycerol-3-phosphate were significantly different between the sham group and the day 1 group (P < 0.01), indicating that the changes in the levels of those five urinary metabolites are related to hepatic I/R injury. Rats with hepatic I/R injury at day 1 had increased levels of lactic acid, pyruvic acid, and glycerol-3-phosphate and decreased levels of alanine and serine compared with rats in the sham group (P < 0.01). Furthermore, the post hoc pair wise comparisons showed that the levels of those five urinary metabolites were significantly different between day 1 group and day 5 group (P < 0.01), the groups with the most severe liver injury (with a liver damage score of 6 points) and the least severe liver injury (with a liver damage score of 3 points), respectively. When the liver injury was less severe at day 5, levels of lactic acid, pyruvic acid, and glycerol-3-phosphate were significantly reduced, and levels of alanine and serine were significantly increased compared with those at day 1 when the liver injury was most severe (P < 0.01), which indicates a relationship between the levels of those five urinary metabolites and the severity of liver injury.
3.4. PCA Analysis
The PCA analysis revealed 4 components that provided a good summary of the data, and these components accounted for 35.4%, 17.7%, 13.8%, and 11.5% of the total variance (Table 3). The first component consisted of alanine and serine with positive loading, as well as glycerol-3-phosphate and lactic acid with negative loadings. The second eigenvector had high positive loadings from the level of threonine and high negative loadings from the level of pyruvic acid. A high positive loading from the level of oleic acid was found in the third component. The fourth component shared some variables with the previous three components (data not shown).
Table 3.
Results of principle component analysis.
| Eigenvalue | Proportion | Cumulative proportion | |
|---|---|---|---|
| Component 1 | 2.47729627 | 0.3539 | 0.3539 |
| Component 2 | 1.23676622 | 0.1767 | 0.5306 |
| Component 3 | 0.96223779 | 0.1375 | 0.6680 |
| Component 4 | 0.80457156 | 0.1149 | 0.7830 |
| Component 5 | 0.61566509 | 0.0880 | 0.8709 |
| Component 6 | 0.56651689 | 0.0809 | 0.9519 |
| Component 7 | 0.33694618 | 0.0481 | 1.0000 |
PCA results showed that 7 metabolites distinguished the day 1 group and the day 3 group from the sham group (Figure 2). The PCA score plot showed a similar changing trend of urinary metabolites as that presented in Table 2, and measurements in the day 1 group were distinct from that in the sham group. On the contrary, measurements in the day 3 and day 5 groups were closer to that in the sham group. Notably, compared to the measurements in the day 3 group, more measurements in the day 5 group overlapped with those in the sham group.
Figure 2.
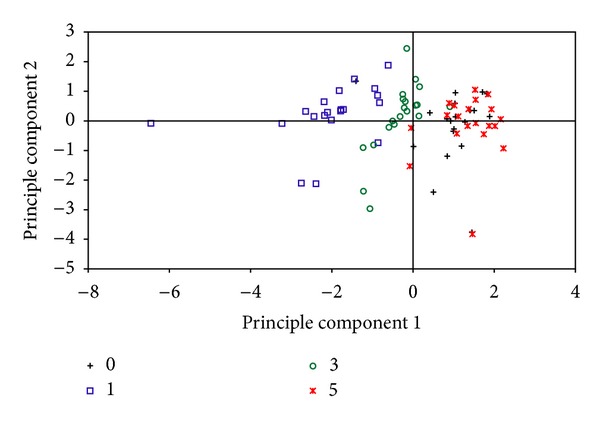
Scatter plot of principle component analysis (PCA) scores classified by the 7 metabolites in the rat urine samples.
4. Discussion
This study used GC-MS to assess urinary metabolomics in rat model of hepatic I/R injury induced by the Pringle maneuver. In addition, conventional histological observation was used to confirm hepatic I/R injury. GC-MS detected 7 urinary metabolites in rats after hepatic I/R injury, and 5 of them including lactic acid, pyruvic acid, alanine, serine, and glycerol-3-phosphate demonstrated significantly different levels between sham group (without hepatic I/R injury) and day 1 group (with hepatic I/R injury) and between groups with different liver injury severity (day 1 group and day 5 group), indicating that the changes in those five urinary metabolites are associated with liver injury. PCA analysis further confirmed that the 7 variables were enough to describe subsets with similar characteristics, and separated the day 1 and day 3 groups from the sham group. The aforementioned five urinary metabolites account for the leading percentage of total variance in PCA analysis, which further support an association between the changes in those five urinary metabolites and liver injury. These results indicate a significant potential of early diagnosis of hepatic I/R injury using noninvasive urinary metabolomic analysis.
The five detected urinary metabolites with significantly different levels between rats with and without hepatic I/R injury and between rats with different severity of liver injury play important roles in energy metabolism. The liver primarily uses lactate and alanine for gluconeogenesis [16]. In biological organisms, lactic acid is formed by reduction of pyruvic acid. Pyruvic acid is the first substrate of the gluconeogenic pathway and can then be used to generate glucose [17]. Transamination or deamination of alanine and serine enables the entrance of their carbon skeleton into gluconeogenesis directly as pyruvate. Glyceraldehyde 3-phosphate is an intermediate in both glycolysis and gluconeogenesis. We speculate that anaerobic conditions increase glycolysis, which induces the buildup of lactic acid in the muscles. The lactic acid diffuses into the blood stream, and the liver increases its synthesis of glucose by gluconeogenesis [18], resulting in consumption of alanine and serine. At the same time, increased glycolysis and gluconeogenesis result in an increase of the intermediate, glyceraldehyde 3-phosphate.
In this study, we constructed a rat model of hepatic I/R injury induced by the Pringle maneuver. Currently, there are various surgical approaches for liver transplantation and liver resection, and although the operative time has been shortened and the surgical approach improved [19–21], the Pringle maneuver is still a basic means to reduce intraoperative hemorrhage, and it may induce hepatic I/R injury. This is the reason we constructed a rat model of hepatic I/R injury induced by the Pringle maneuver. Our results showed that conventional examinations clearly outlined the trend of recovery after hepatic I/R injury. ALT and TBIL were increased most significantly in the day 1 group, indicating the highest severity of injury. Their levels were reduced in the day 3 group, and returned to normal in the day 5 group, showing a trend of recovery. The HE staining results of the liver tissue also confirmed hepatic I/R injury and the recovery trend, indicating that our animal model construction was successful.
Our urinary metabolomic analysis using GC/MS detected a changing trend of urinary metabolomics after hepatic I/R injury, which truly reflects the dynamic hepatic I/R injury process in rats. The hepatic I/R injury process reflected by the urinary metabolomics is consistent with the hepatic injury and recovery process indicated by serum enzymes and pathological findings. Urinary metabolomic analysis itself has many advantages. Urinary metabolomic analysis is noninvasive, sensitive, convenient, and rapid. Using GC/MS to detect urinary metabolomics, a lab technician can analyze approximately 150 samples per work day. Urine sample collection is noninvasive and is not limited by sample collection time or frequency. In addition, the urinary metabolomic analysis is very effective for monitoring the dynamic changing process of the liver. However, based on the results of this preliminary study, no conclusion can be drawn that the urinary metabolomic analysis is superior to conventional examination methods for hepatic I/R injury such as serological examinations. Further studies on using GC/MS to detect urinary metabolomics in hepatic I/R injury detection and monitoring may deepen our understanding of the value of urinary metabolomic analysis in early detection and diagnosis of hepatic I/R injury.
GC/MS data exhibit great variation within group, and study with a large sample size is warranted. During GC-MS, samples undergo derivatization to increase stability and volatility. Therefore, thermally instable substances may be destroyed and thus cannot be detected. The limitation of GC-MS analysis is that it is more useful for detection of volatile substances [22]. In future studies, other metabolomics techniques can be used to examine more metabolites related to hepatic I/R injury, and preoperative and postoperative human urine samples can be studied.
Conflict of Interests
The authors declare that there is no conflict of interests.
Acknowledgment
The authors want to thank Dr. Jie Mao for writing and editing assistance.
References
- 1.Man K, Fan S-T, Ng IOL, Lo C-M, Liu C-L, Wong J. Prospective evaluation of pringle maneuver in hepatectomy for liver tumors by a randomized study. Annals of Surgery. 1997;226(6):704–713. doi: 10.1097/00000658-199712000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irie T, Ito K, Ozasa H, et al. Splenic artery ligation: a protection against hepatic ischemia/reperfusion injury in partially hepatectomized rats. Hepatology Research. 2012;42(8):819–827. doi: 10.1111/j.1872-034X.2012.00989.x. [DOI] [PubMed] [Google Scholar]
- 3.Jaeschke H. Mechanisms of reperfusion injury after warm ischemia of the liver. Journal of Hepato-Biliary-Pancreatic Surgery. 1998;5(4):402–408. doi: 10.1007/s005340050064. [DOI] [PubMed] [Google Scholar]
- 4.Colletti LM, Burtch GD, Remick DG, et al. The production of tumor necrosis factor alpha and the development of a pulmonary capillary injury following hepatic ischemia/reperfusion. Transplantation. 1990;49(2):268–272. doi: 10.1097/00007890-199002000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB Journal. 1990;4(15):3355–3359. [PubMed] [Google Scholar]
- 6.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. American Journal of Physiology. 1991;260(3):G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 7.Svetlov SI, Xiang Y, Oli MW, et al. Identification and preliminary validation of novel biomarkers of acute hepatic ischaemia/reperfusion injury using dual-platform proteomic/degradomic approaches. Biomarkers. 2006;11(4):355–369. doi: 10.1080/13547500600775110. [DOI] [PubMed] [Google Scholar]
- 8.Fiehn O. Metabolomics—the link between genotypes and phenotypes. Plant Molecular Biology. 2002;48(1-2):155–171. [PubMed] [Google Scholar]
- 9.Nicholson JK, Lindon JC, Holmes E. ‘Metabonomics’: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29(11):1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- 10.Serkova N, Fuller TF, Klawitter J, Preise CE, Niemann CU. 1H-NMR-based metabolic signatures of mild and severe ischemia/reperfusion injury in rat kidney transplants. Kidney International. 2005;67(3):1142–1151. doi: 10.1111/j.1523-1755.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Yan S, Ji C, et al. Metabolomic changes and protective effect of L-carnitine in rat kidney ischemia/reperfusion injury. Kidney and Blood Pressure Research. 2012;35(5):373–381. doi: 10.1159/000336171. [DOI] [PubMed] [Google Scholar]
- 12.Hu J-D, Tang H-Q, Zhang Q, et al. Prediction of gastric cancer metastasis through urinary metabolomic investigation using GC/MS. World Journal of Gastroenterology. 2011;17(6):727–734. doi: 10.3748/wjg.v17.i6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Kloet FM, Tempels FWA, Ismail N, et al. Discovery of early-stage biomarkers for diabetic kidney disease using ms-based metabolomics (FinnDiane study) Metabolomics. 2012;8(1):109–119. doi: 10.1007/s11306-011-0291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva CL, Passos M, Câmara JS. Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. British Journal of Cancer. 2011;105(12):1894–1904. doi: 10.1038/bjc.2011.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury: modulating effects of FK506 and cyclosporine. Transplantation. 1993;55(6):1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabetic Medicine. 2010;27(2):136–142. doi: 10.1111/j.1464-5491.2009.02894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrett RH, Grisham CM. Principles of Biochemistry with a Human Focus. Pacific Grove, Calif, USA: Brooks/Cole, Thomson Learning; 2002. [Google Scholar]
- 18.Widmaier E. Vander's Human Physiology. Boston, Mass, USA: McGraw Hill; 2006. [Google Scholar]
- 19.Chen YW, Li CH, Zhang AQ, Yang SZ, Zhang WZ, Dong JH. Preserving hepatic artery flow during portal triad blood inflow occlusion reduces liver ischemia-reperfusion injury in rats. Journal of Surgical Research. 2012;174(1):150–156. doi: 10.1016/j.jss.2010.11.913. [DOI] [PubMed] [Google Scholar]
- 20.Hoekstra LT, Van Trigt JD, Reiniers MJ, Busch OR, Gouma DJ, Van Gulik TM. Vascular occlusion or not during liver resection: The continuing story. Digestive Surgery. 2012;29(1):35–42. doi: 10.1159/000335724. [DOI] [PubMed] [Google Scholar]
- 21.Helewski K, Kowalczyk-Ziomek G, Czecior E, et al. Protective effect of intermittent clamping of the portal triad in the rat liver on liver ischemia-reperfusion injury. Hepatitis Monthly. 2011;11(6):445–451. [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao H, Chen J, Shi Q, et al. Metabolomics-based study of clinical and animal plasma samples in coronary heart disease with blood stasis syndrome. Evidence-Based Complementary and Alternative Medicine. 2012;2012:6 pages. doi: 10.1155/2012/638723.638723 [DOI] [PMC free article] [PubMed] [Google Scholar]


