Abstract
Subacute sclerosing panencephalitis (SSPE) is related to “defective” measles virus or vaccination, though an association with parainfluenza viruses has been reported. SSPE is characterized by a slow, erratic course and elevated cerebrospinal fluid measles titers. An immunocompetent, vaccinated infant, with onset of symptoms in parainfiuenza virus season and a catastrophic course is described. Cerebrospinal fluid titers were negative, but postmortem brain had typical SSPE lesions. Patient brain-derived RNA, subjected to reverse transcription followed by polymerase chain reaction yielded polymerase chain reaction products with measles virus but not parainfluenza virus genes. The sequenced fragment revealed multiple mutations, typical for SSPE. SSPE can thus present in infants, with short latency and no cerebrospinal fluid antibodies. Viral genomic analysis may be diagnostic, permitting early therapy.
The incidence of subacute sclerosing panencephalitis (SSPE), a fatal neurodegenerative disease most commonly associated with prior measles infection [1, 2], has significantly decreased in the United States [3]. Since the introduction of measles vaccines, age of onset and vaccine-associated cases have increased [3]. Cases associated with parainfluenza virus (PIV), relatively common in eastern Europe and the Middle East [4—6], have recently been reported in this country [7]. New therapies, e.g., interferon, hold hope for affected individuals, making timely diagnosis important [8, 9]. We report an infant who presented in PIV season with no cerebrospinal fluid (CSF) measles antibodies and document the diagnostic utility of molecular analysis of the virus.
Materials and Methods
RNA Preparation. Reverse Transcription—Polymerase Chain Reaction and Sequencing
Frozen brain tissue specimens were ground in liquid nitrogen, and total cellular RNA was extracted. RNA samples from measles virus (MV), or PIV type 1—, 2—, or 3—infected Vero cells, were used as positive controls. Reverse transcription—polymerase chain reaction (RT-PCR) was performed as described [10], using published primers specific for measles matrix and fusion protein [11] genes. Three pairs of measles nucleocapsid protein gene-specific nested primers, as follows, were developed: MN7/MN8 (5′-CAATCTGGCCTTACCTTCGCAT-3′ / 5′-CTCCTTACCATCTCTTGCCCTA-3′); MV1/MV2(5′-GCTAAGAAGGTGGATAAA-3′ / 5′-TATGCTGGATCAAAGTAAG-3′), and MV3/MV4 (5′-TTGGAGAGAAAATGGTTGGA-3′ / 5′-CAGAGCAGAGGGTATGAT-3′). Also developed was a primer pair, as follows, recognizing conserved PIV-1, 2, and 3 nucleocapsid gene: PIN1/PIN2 (5′-AATGCTGATGTCAAGTATGT-3′ / 5′-ATCCTGTCTGAATGCTTCTAA-3′)- A 410-bp fragment of the matrix gene PCR product derived from two brain specimens was sequenced along with a wild-type isolate.
Other Procedures
Magnetic resonance (MR) images (5 mm) were obtained on a 0.5-T magnet. CSF MV IgG and IgM antibody titers were performed by MRL (Cypress, CA); aliquots were cultured for viruses, including PIV. Brain specimens were stained with hematoxylin and eosin and Luxol fast blue. Measles antigen immunofluorescence was performed, as well as herpesvirus and cytomegalovirus immunohistochemical stains by using avidin-biotin methodology, and electron microscopy.
Patient Report
A 22-month-old girl developed clumsiness and falling in December. Over the subsequent 2 weeks, focal and generalized myoclonic seizures developed, occurring hundreds of times daily. There was no prior trauma, travel, drug ingestion, seizures, or abnormal movements, and birth, infancy, and physical and neurological development had been normal. No excess or unusual infections had occurred, and the child was apparently immunologically normal. A febrile illness with rash had occurred at the age of 5 weeks, but there was no recent infection. The measles—mumps—rubella vaccine had been administered at 18 months. Initial physical and neurological examinations were normal, except for numerous 1 to 2-second episodes of head drop, right arm jerk, or falling, without postictal fatigue. Blood counts, chemistry, and liver function tests were normal. A brain magnetic resonance imaging (MRI) scan showed focal areas of increased signal on T2-weighted images in the corona radiata, left greater than right. Electroencephalogram (EEG) was abnormal with slow background and multifocal epileptiform discharges, prominent in left central leads. The patient was referred to Childrens Hospital Los Angeles, where somnolence, hypotonia, hyperreflexia, and decreased right arm use were noted. Encephalopathy and loss of language and social skills followed, probably compounded by large clonazepam doses. Video-EEG showed almost continuous myoclonic seizures. A second MRI scan, 3 weeks later, demonstrated progression of the corona radiata abnormalities (Fig 1a—c). CSF studies are found in the Table.
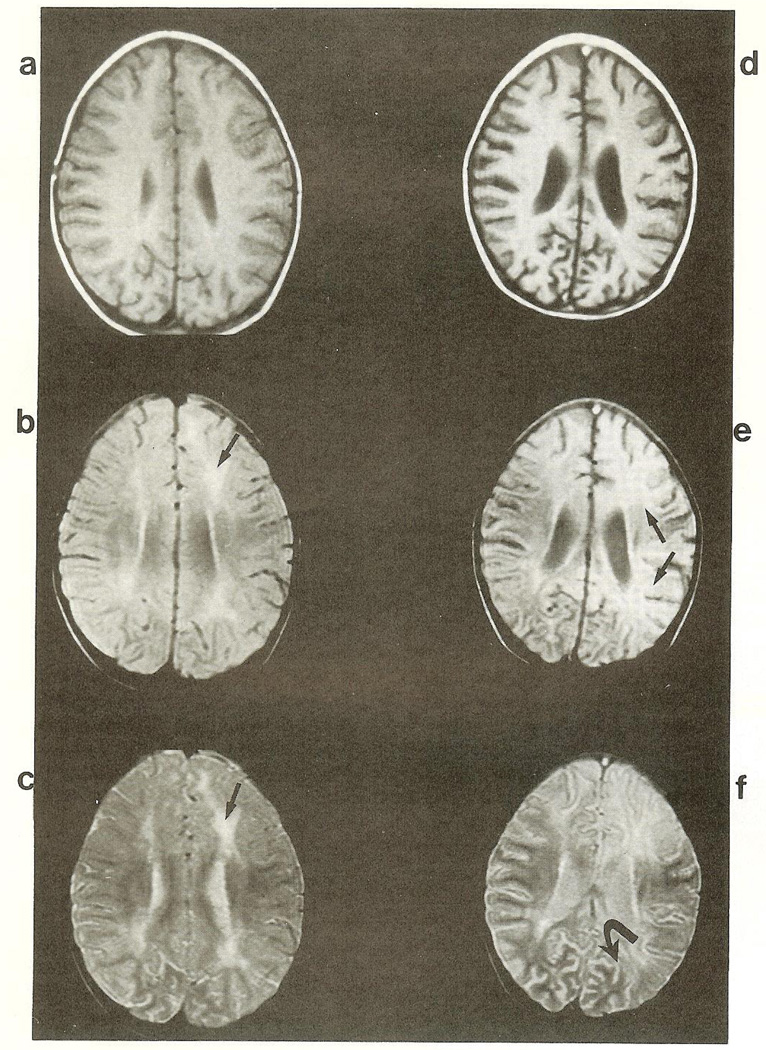
Fig 1.
Axial magnetic resonance imaging (MR1) studies. T1 (TR 28 msec/TE 483 msec) (a, d), intermediate (TR 48 msec/TE 2,500 msec) (b, e). and T2 (TR 96 msec/2,500 msec) (c, f) weighted images through the corona radiata and bodies of the lateral ventricles, (a—c) Study obtained 3 weeks after presentation. Focal areas of increased signal are present (arrows). The lateral ventricles and cerebral sulci are normal in size, (d—f) MR1 done 2 months after presentation. The focal areas of increased signal in the corona radiata have progressed and involve large areas of the cortex (arrows). The lateral ventricles and cerebral sulci (curved arrow) are enlarged, suggesting diffuse atrophy.
Laboratory Investigation of Patients with Subacute Sclerosing Panencephalitis
| Source | Test | Result | Comments |
|---|---|---|---|
| CSF: I | Cell count | 4 white/0 red | nl |
| Glucose/protein | 56/23 (mg/dl) | nl | |
| Oligoclonal bands | 2 | nl = 0 | |
| IgG synthesis index | 7.24 | nl < 0.7 | |
| CSF: II | Cell count | 1 white/0 red | nl |
| Glucose/protein | 79/27 (mg/dl) | nl | |
| IgG synthesis index | 6.05 | nl < 0.7 | |
| CSF: III | Cell count | 1 white/0 red | nl |
| Glucose/protein | 79/22 (mg/dl) | nl | |
| Measles titers: IgM/IgG | <1 : 1/1 :4 | nl < 1 : 20/<1 :8 | |
| Serum | Measles titers: IgM/IgG | <1 : 20/>1 : 32 | Immunity, remote |
All three CSF specimen: viral cultures, negative; brain paramyxovirus culture, negative.
nl = normal
Adrenocorticotropic hormone (150 U/m2/day) was given for 2 weeks. The child remained stuporous, with frequent myoclonus. By the third month, persistent left gaze preference and right hemiplegia were noted. An MRI scan was obtained (Fig 1d—f). The infant died of aspiration pneumonia 3.5 months after illness onset.
Results
The Table summarizes salient serology and CSF analyses. An MRI scan from the first month shows focal increased signal in the corona radiata (see Fig 1a—c). Images obtained 6 weeks later show progression of bihemispheric white matter lesions (see Fig 1d—f) and marked cerebral and cerebellar atrophy (see Fig 1d).
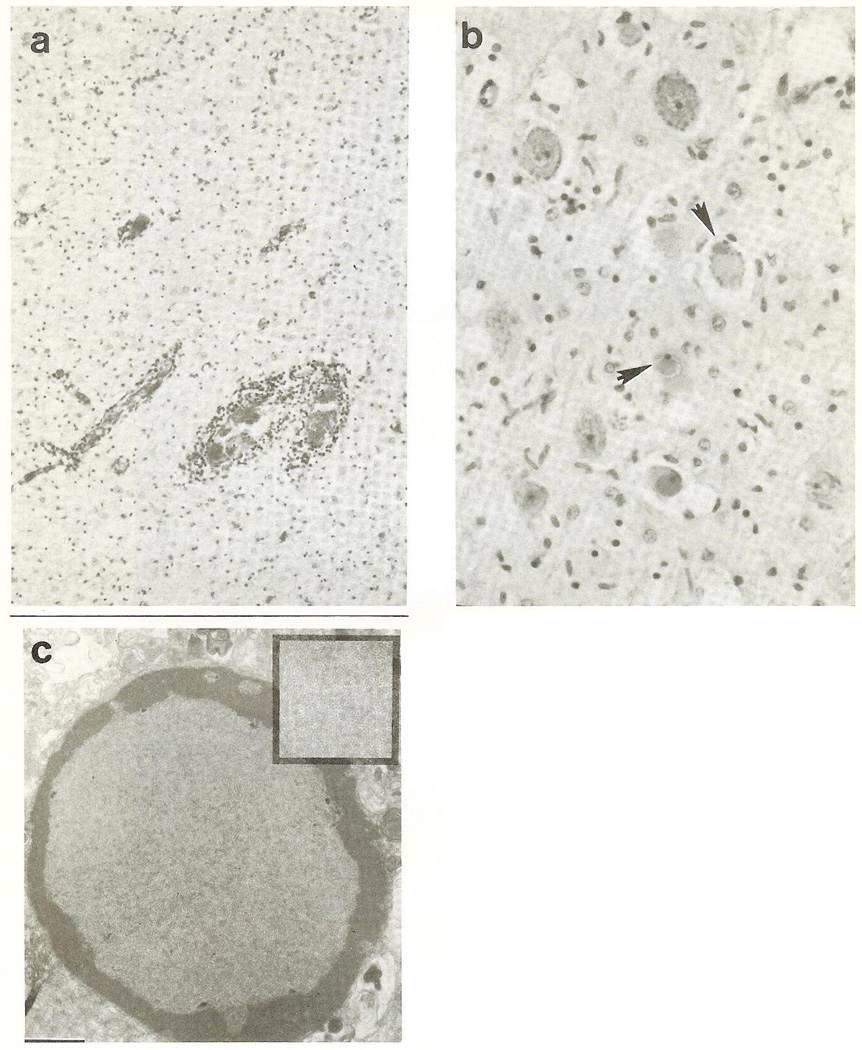
Autopsied brain weighed 915 gm (normal, 1,064 gm,). The cortical band was soft and pale, and the white matter discolored and reduced. Deep gray nuclei, amygdaloid and hippocampal formations, and tectum and tegmentum were softened and congested. The leptomeninges contained a lymphocytic infiltrate. Diffuse neuronal loss and gliosis were particularly severe in the cerebral and cerebellar cortex, hippocampi, and in diencephalic, brainstem, and cerebellar nuclei. Demyelination, axonal degeneration, microglia proliferation, hypertrophied astrocytes, and foamy macrophages were present throughout the white matter (Fig 2a). Intranuclear eosinophilic inclusions (Cowdry A) were observed in cortical neurons, oligodendrocytes, and astrocytes (Fig 2b). Measles, cytomegalovirus, and herpesvirus immunocytochemistry was negative. Electron microscopic examination revealed the intranuclear inclusions to contain helical, 14-nm diameter nucleocapsid tubules characteristic of paramyxovirus (Fig 2c).
Fig 2.
(a) Photomicrograph revealing white matter demyelination and gliosis. Perivascular lymphocytic infiltrates are seen. (Hematoxylin and eosin-Luxol fast blue {H&E-LFB} 100×.) (b) Cranial nerve III nucleus neurons exhibiting Cowdry type A intranuclear eosinophilic inclusions (arrowheads). (H&E-LFB, 200 ×.) (c) Intranuclear mass of viral particles in an oligodendrocyte. Bar = 1 µm. Helical viral nudeocapsids are seen in greater detail in the inset.
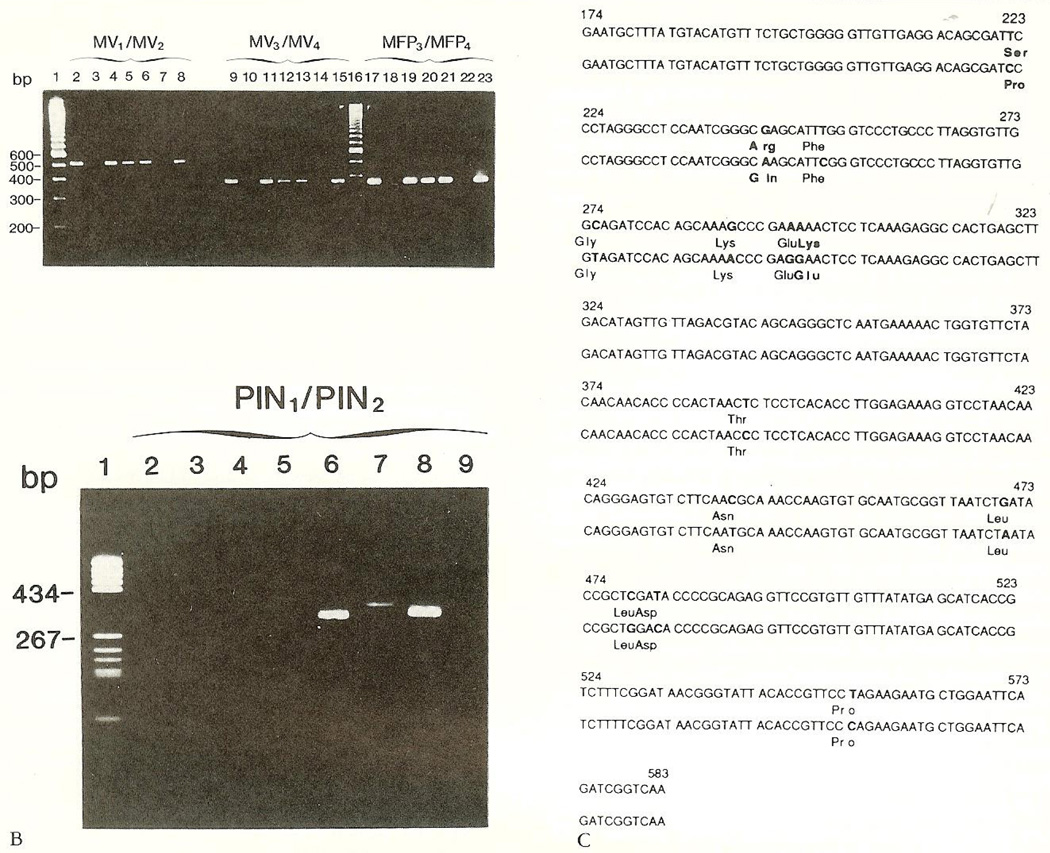
Patient brain-tissue samples (SSPE1, SSPE2) yielded PCR products of expected sizes with measles specific primers MV1/MV2 (Fig 3A, lanes 2 and 4), MV3/ MV4 (lanes 9 and 11), and MFP3/MFP4 (lanes 17 and 19). These comigrated with samples derived from a patient with progressive MV encephalitis (lanes 5, 12, and 20) and with wild-type (lanes 8, 15, and 23) and vaccine MV (lanes 6, 13, 21). No PCR products were obtained with PIV-derived consensus primers (Fig 3B). Patient-derived sequences of the 410-bp matrix gene fragment differed from the wild-type by 12 bases. Eleven mutations were transitions [T(U)-C]; one was a transversion. Three mutations resulted in amino acid substitutions; nine were “silent” (Fig. 3C).
Fig 3.
Polymerase chain reaction (PCR)-amplified fragments of viral genes from brain RNA extracts. (A) Agarose gel electropboresis of PCR-amplified nucleocapsid protein (507 bp, lanes 2—8; 368 bp, lanes 9—15), and fusion protein (367 bp, lanes 17—23) gene fragments with measles virus (MV)-specific nested primers. Sample order for each primer set: current patient (SSPE1), a child with “encephalitis,” current patient (duplicate, SSPE2), patient with acquired immunodeficiency syndrome and progressive MV encephalitis, vaccine MV strain, reaction mix without template, wild-type MV strain. (B) Nudeocapsid protein gene fragments (323—330 bp) obtained with consensus parainfluenza virus (PIV) primers. RNA from PIV-1, 2, and 3 (lanes 6—8); reaction mixture without RNA (lane 9);patient-derived RNA (lanes 2—5) did not yield a signal. (C) Sequence of a 410-bp fragment of the matrix gene of a wild-type MV (top rows), compared with the patient’s strain (bottom rows). Base differences between the cDNA sequences of these strains and amino acid substitutions in the derived polypeptides are indicated in bold type.
Discussion
SSPE is characterized by progressive dementia and myoclonic seizures [1—3], most commonly subsequent to measles infection or vaccine. Age of onset in the United States, averaging 14 years, has been increasing, with an average latency of 12 years from antecedent measles infection and 7.73 years from vaccination [3]. SSPE incidence in the United States has decreased dramatically with the advent of measles vaccine, averaging 4.2 cases per year [3]. One-sixth of SSPE patients lack a history of prior measles infection or vaccination [3]. Recently, PIV-3 has been implicated in the etiology of SSPE-like encephalitis in infants and children in this country ([7], Patient 2). Worldwide, PIV is a significant cause of neurological diseases in children [12]. PIV antibody production in CSF of SSPE patients and elevated serum titers to PIV-1 have been reported [4, 5]. PIV causes a panencephalitis followed by white matter degeneration in infant mice [13] and is indistinguishable microscopically from MV. In North America, epidemics of PIV affecting infants and children occur infall and early winter, the time of onset of this infant’s illness.
The course of SSPE is typically stuttering and progressive [1—3], with 5% of patients surviving 3 months or less, and 20% of patients surviving for 4 years or longer [2]. Inflammation and gliosis (“sclerosis”) are associated with Cowdry A viral inclusions, and an etiological role has been established for a “defective” MV (reviewed in [1]). MV antigens and RNA are found in CSF and brain of patients [1, 11]. In immunocompetent hosts suspected of having SSPE, antibody production in the central nervous system is diagnostic [1].
Recent therapeutic successes for SSPE with interferon, and interferon—isoprinosine [9], have been reported, providing impetus for early diagnosis, particularly in cases with atypical history, course, and laboratory findings. This infant had illness onset in PIV season, 4 months after measles immunization. Atypical EEG and lack of CSF measles titers decreased the likelihood of measles-associated SSPE. Autopsied brain verified the presence of SSPE and of viral inclusions compatible with both MV and PIV [14]. RNA analysis confirmed the etiological role of MV: PCR products were obtained only with MV primers. Further, the sequenced fragment of measles matrix gene PCR product contained 12 mutations from “wild-type” MV, of which five were T(U)-C transitions, typical for SSPE [15]. Sequence information was insufficient to determine whether the patient’s SSPE strain was derived from a vaccine [16] or wild-type [15, 17].
In conclusion, this 22-month-old infant, with a history of a “rash” at 5 weeks, had SSPE without CSF measles antibodies. Robust central nervous system immunoglobiilin production and neuropathological lesions exclude progressive measles encephalitis, seen in immunocompromised individuals [1]. We speculate that measles infection in early infancy, during the presence of maternal antibodies, led to tolerance to the virus, permitting its survival and mutation. We suggest that PCR on CSF or biopsy specimens, and virus identification in similar cases, may permit early therapeutic intervention and improved outcome.
Acknowledgments
We thank Drs R.M. Baum for patient referral, O. Carter Snead III for intellectual interest, and Bing Cai for oligonudeotide synthesis and purification.
References
- 1.Asher DM. Slow viral infections of the human nervous system. In: Scheld WM, Whitley RJ, Durack DT, editors. Infections of the central nervous system. New York: Raven Press; 1991. pp. 145–149. [Google Scholar]
- 2.Risk WS, Haddad FS. The variable natural history of subacute sclerosing panencephalitis: a study of 118 cases from the Middle East. Arch Neurol. 1979;36:610–614. doi: 10.1001/archneur.1979.00500460044004. [DOI] [PubMed] [Google Scholar]
- 3.Dyken PR, Cunningham SC, Ward LC. Changing character of subacute sclerosing panencephalitis in the United States. Pediatr Neurol. 1989;5:339–341. doi: 10.1016/0887-8994(89)90045-3. [DOI] [PubMed] [Google Scholar]
- 4.Polna I, Cendrowski W, Niedzwiedzka E. Elevated parainfluenza type 1 antibody in patients with subacute sclerosing panencephalitis. J Neutol Sci. 1977;31:159–161. doi: 10.1016/0022-510x(77)90012-0. [DOI] [PubMed] [Google Scholar]
- 5.Abdelnoor AM, Dhib-Jalbut SS, Haddad FS. Different virus antibodies in serum and cerebrospinal fluid of patients suffering from subacute sclerosing panencephalitis. J Neuroimmunol. 1982;2:27–34. doi: 10.1016/0165-5728(82)90073-x. [DOI] [PubMed] [Google Scholar]
- 6.Wender M. Parainfluenza encephalitis with predominant clinical symptoms of status epilepticus. Pediatr Pol. 1980;55:257–258. [PubMed] [Google Scholar]
- 7.McCarthy VP, Zimmerman AW, Miller CA. Central nervous system manifestations of parainfluenza virus type 3 infections in childhood. Pediatr Neurol. 1990;6:197–201. doi: 10.1016/0887-8994(90)90063-7. [DOI] [PubMed] [Google Scholar]
- 8.Behan PO. Interferon in the treatment of subacute sclerosing panencephalitis. Lancet. 1981;8228:1059–1060. doi: 10.1016/s0140-6736(81)92228-5. [DOI] [PubMed] [Google Scholar]
- 9.Yalaz K, Anlar B, Oktem F, et al. Intraventricular interferon and oral inosiplex in the treatment of subacute sclerosing panencephalitis. Neurology. 1992;42:488–491. doi: 10.1212/wnl.42.3.488. [DOI] [PubMed] [Google Scholar]
- 10.Eggerding FA, Peters J, Lee RK, Inderlied CB. Detection of rubella virus gene sequences by enzymatic amplification and direct sequencing of amplified DNA. J Clin Mictobiol. 1991;29:945–952. doi: 10.1128/jcm.29.5.945-952.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godec MS, Asher DM, Swoveland PT, et al. Detection of measles virus genomic sequences in SSPE brain tissue by the polymerase chain reaction. J Med Virol. 1990;30:237–244. doi: 10.1002/jmv.1890300402. [DOI] [PubMed] [Google Scholar]
- 12.Assaad F, Gispen F, Kleemola M, et al. Neurological diseases associated with viral and Mycoplasma pneumonias infections. Bull WHO. 1980;58:297–311. [PMC free article] [PubMed] [Google Scholar]
- 13.Rorke LB, Gilden KH, Wroblewska Z, Wolinski JS. Experimental panencephalitis induced in suckling mice by parainfiuenza type 1 virus. I. Clinical and pathological features. J Neuropathol Exp Neurol. 1976;35:247–258. doi: 10.1097/00005072-197605000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Bouteille M, Fontaine C, Vedrenne C, Delarue J. Sur un cas d’encephalite subaigue a inclusions. Etude anatomoclinique et ultrastructurale. Rev Neurol. 1965;113:454–458. [Google Scholar]
- 15.Cattaneo R, Schmid A, Eschle D, et al. Biased hypermutation and other genetic changes in defective measles viruses in human brain infections. Cell. 1988;55:255–265. doi: 10.1016/0092-8674(88)90048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellini WJ, Englund G, Richardson CD, et al. Matrix genes of measles virus and canine distemper virus: cloning, nucleotide sequences, and deduced amino acid sequences. J Virol. 1986;58:408–416. doi: 10.1128/jvi.58.2.408-416.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curran MD, Rima BK. Nucleotide sequence of the gene encoding the matrix protein of a recent measles virus isolate. J Gen Virol. 1988;69:2407–2411. doi: 10.1099/0022-1317-69-9-2407. [DOI] [PubMed] [Google Scholar]